Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Lindsay Dong and Version 2 by Ivan Šoša.
Autopsies are still needed for the determination and correction of causes of death, even in “clear-cut” cases. Moreover, post mortem sample handling and analysis are challenges that need to be addressed, as they can produce variability in the findings; for this reason, validation with biomarkers is of key importance.
- blood-based biomarkers
- clotting
- inflammation
- post mortem
1. Introduction
Biomarkers found in bodily fluids may represent the active disease process or the patient’s reaction to that disease [1]. Moreover, they can act as an alternative measure of outcomes to assess the efficacy of therapy. According to common wisdom, a biomarker is a protein, enzyme, or cytokine with discriminatory value in clinical care [2,3][2][3]. A variety of molecules have been evaluated, and although post mortem biomarkers and a multimarker strategy are best investigated in the light of sudden cardiac death and agonal cardiac function [4[4][5],5], their significant potential in relation to peripheral vasculature is yet to be addressed [1,6][1][6]. All biomarkers must meet certain criteria to constitute a surrogate endpoint, or to be able to predict a clinically relevant endpoint, such as the loss of vision or a decrease in quality of life. In addition, the effect of a proposed treatment on the surrogate must capture the effect of the treatment on the clinically relevant endpoint [7,8][7][8].
This information should be considered in the context of the fact that autopsies face a number of challenges; for example, the lack of regulation for governmental funding for hospital-based autopsies, or hospitals rejecting autopsies requested by families [9]. In any case, autopsy numbers have fallen significantly worldwide [10[10][11][12][13][14],11,12,13,14], and the accessibility of post mortem healthcare is uneven [15]. It is necessary to improve these statistics and also to address the major problem of discrepancies between clinical diagnosis and initial autopsy findings regarding the panel of clinical biomarkers.
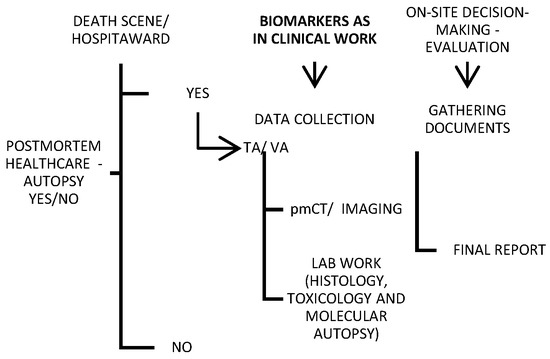
2. Traditional Post Mortem Healthcare
Despite its discrepancies with clinical records, autopsy remains the gold standard as the ultimate diagnostic procedure [21,22][16][17]. Although these discrepancies have decreased significantly over time, in 2010 their rate remained high [23][18]; in the “post-COVID” era, the rate has reached an unprecedented 42% [24][19]. This renders between one in two and one in three autopsies superfluous. Our knowledge about normal circulation stems entirely from thorough post mortem dissection [25][20]. More than 40 years ago, in a series of 500 clinical autopsies, vascular disorders were found to account for 25.2% of anatomopathological diagnoses [26][21]. These figures were more or less the same in osteoarthritis/rheumatoid arthritis research from 2015 [27][22]. Data from the Eurostat indicate the same phenomenon: diseases of the circulatory system are the main cause of death in the EU and were responsible for almost 37% of all deaths in 2017 [28,29][23][24]. A biomarker may be a recording taken from an individual, an imaging test, or a biosample. Etymologically, the term “biomarker” comes from the Greek form βιο-, from βίος, meaning life, and the Old English word meaning a mark [34,35][25][26]. Bearing in mind this Greek root, using the word ‘life’ in the context of a post mortem may seem slightly incongruous. This was the case until recently, when the COVID-19 pandemic brought about a radical shift in routine post mortem practice [36][27].3. Options for Traditional Autopsy
Traditional autopsy may be criticized in the media, but it is an important tool for both criminal investigations and healthcare quality control. For this reason, minimally invasive alternatives to traditional autopsies are continuously emerging. Imaging and “verbal autopsy” (VA) were shown in a large series to be promising techniques compared with a full autopsy [37,38,39,40][28][29][30][31] (Figure 21). Various objective factors influence the autopsy rate, though it is less likely to be requested for deaths in the emergency department or on general surgery wards, and it is most likely to be requested for fetal, medicine-related, cardiothoracic surgery-related, and pediatric deaths [41][32]. Nevertheless, most countries globally do not report high autopsy rates (less than 70% of all-cause mortality) [42][33].
Figure 21. Schematic of a provisional post mortem protocol created by the author, with biomarkers included; TA—traditional autopsy; VA—verbal autopsy.
While the cost of electronic data systems and the long wait between data collection and analysis appear to be the main disadvantages of verbal autopsies, post mortem imaging is hampered by a lack of direct visualization of the soft tissue, as well as postmortem artifacts that obscure the natural causes of death and can be misinterpreted as antemortem pathologies [40,43][31][34]. However, VA has been preferred recently in the COVID-19-related pandemic context, with a satisfying effect [44][35].
For deaths that occur outside the health system, health information and a description of the events preceding death are included in the VA. It was first used in a public health project concerning the relationships between nutrition, infection, and child development in India [47][36]. Nowadays, this method has been improved and augmented so that it yields suitably complete death certificates and ultimately estimates cause-specific mortality. Specifically, VA means the collection of anamnestic data through an in-person interview with a close relative or caregiver of a deceased. The interview takes place within a short time of death; these data include symptoms, signs, and circumstances prior to death [48][37]. In settings where most deaths are otherwise undocumented, which typically means in low- and middle-income countries, VA attempts to establish causes of death, allowing scientists to analyze disease patterns and direct public health policy decisions. The body of relevant literature reports that the specificity of the VA is commonly found to be higher than sensitivity [49][38].
In agreement with contemporary attainments, even conducting an autopsy can be transferred to a computerized environment, and digital tools can be employed. Accordingly, another accessible and recently developed modality of postmortem healthcare is a radiographic examination of the body after death—postmortem radiology. As much as they provide a strong complementary tool to the TA, imaging techniques used in everyday clinical work are applied to post mortem processing [43][34].
The explicit potential economic benefits of the PMCT (magnetic resonance imaging—MRI) have not been assessed recently [38,57][29][39]; despite its numerous advantages, this method still exhibits the problem of a significant rate of diagnostic discrepancies [58,59][40][41]. Nevertheless, PMCT has 79% sensitivity and 92.1% specificity for the detection of the source of bleeding [60][42].
4. Post Mortem Biomarkers
Biomarkers provide plenty of information for enhancing all aspects of vascular homeostasis through vascular beds [1]. Biomarkers are characteristic indicators of disease, a disease state, or disease progression. They were at first described as a “measurable and quantifiable biological parameter that could serve as an index for health assessment” and were ultimately defined as “a characteristic that is objectively measured as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” [63,64][43][44].
The post mortem period involves events such as autolysis or decay, and biomarkers found in bodily fluids may represent the progression of the active disease or a reaction to the disease. Therefore, the value of post mortem biomarkers should be evaluated with this in mind, even if their efficacy is clinically confirmed [65][45]. This compounds the value of clinical post mortem studies as not only a method of control but also a means of improving teaching methods in hospitals [13]. The augmentation of post mortems with blood-based (circulating) biomarkers, in order to avoid invasive autopsies, would have cultural, religious, and potentially economic benefits [38,57,66][29][39][46].
In fact, no contemporary studies compare the costs of the various post mortem optional modalities.
5. Biomarkers of Vascular Quiescence
5.1. Circulating Markers of the Extracellular Matrix: Biomarkers Related to the Vascular Wall
Collagen fragmentation is typically found in abdominal aortic aneurysm (AAA) biopsies as an indicator of new types I and III collagen synthesis [69][47]. AAA is interesting in the context of post mortems since it bears the risk of a rupture or a dissection—life-threatening conditions with high mortality rates [70,71][48][49]. This mortality is about 25% at 6 h and rises to 50% by 24 h; this can be compared to the rates of 40–70% in cases of sepsis [72,73][50][51]. Therefore, the search for highly sensitive and specific biomarkers for AAA should be equally focused. Both the carboxy-terminal and amino-terminal ends of the precursor molecule are released during collagen synthesis, and fragments represent candidate biomarkers. A larger study and confirmation of clinical validity in a larger cohort is needed to link these molecules to AAA. In that regard, another candidate biomarker that has been suggested is tenascin-X, due to its involvement in Ehlers–Danlos syndrome. AAA patients showed elevated serum levels compared to controls [74,75][52][53]. Considering that serum elastin peptide (SEP) is a degradation product of elastin, its role as a biomarker has been shifted from sepsis to the extracellular matrix in vascular quiescence [76,77][54][55]. The fragmentation of the extracellular matrix implies the involvement of elastases and matrix metalloproteinases (MMPs) in the pathophysiology of AAAs. As AAAs are a setting for the abundant expression of the MMP-9, it is considered to play a pivotal role in their formation. Therefore, this enzyme was explored as a possible biomarker for the presence of AAA in case–control studies. Patients with AAA demonstrated elevated concentrations of circulating MMP-9 [79][56]. The possible use of elastases as serum biomarkers of extracellular matrix remodeling is the basis of some studies involving alpha-1 antitrypsin or p-elastase [80,81,82][57][58][59].5.2. Proteins Associated with Vascular Lumen: Inflammation and Thrombosis Biomarkers
Whether as the final product or an outgrowth of the signaling pathway of degradation, markers of inflammation in vascular disease include cell adhesion molecules, cytokines, pro-atherogenic enzymes, and CRP [82,92][59][60]. Biomarkers used to identify thrombosis are unlikely to translate into a universal clinical tool; conversely, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT) are often used [93][61]. Moreover, hyperhomocysteinemia has been identified as an indicator of oxidant stress and a significant cardiovascular risk factor [94,95][62][63], although this association is weak. The principal markers that have been evaluated are fibrinogen, D-dimer, homocysteine, and CRP, the elevation of which is intimately linked to other inflammatory cytokines, including interleukins (ILs; e.g., IL-6) and macrophage activation [96,97][64][65]. Assessing protein complexes embedded in the coagulation cascade and CRP levels, which are elevated in large aneurysms, covers both processes [98][66]. The molecular basis of blood coagulation first attracted attention in the search for blood-based biomarkers due to a plasma fibrinogen concentration that was positively correlated with the AAA diameter [106][67]. Nonetheless, its elevated plasma concentrations are induced by smoking, so the association can only be linked to the “black box” of smoking [107][68]. Due to various functional interactions, fibrinogen plays a crucial role in hemostasis. Specifically, it is a substrate for three major enzymes: thrombin, plasmin, and factor XIIIa [106][67]. The currently available D-dimer assays are not standardized and it is unclear whether these differences have an impact. On the other hand, these tests are rapid, simple, and inexpensive [113][69]. Therefore, to explore the differences between D-dimer assays and their impact on the diagnostic outcome, a prospective multicenter cohort outcome study evaluating 3462 patients with suspected PE (the YEARS study) was conducted. Four different D-dimer assays were used, and the median D-dimer concentrations differed significantly between the assays. The sensitivity, specificity, positive predictive value (PPV), and NPV for the detection of PE of all four assays were determined, using a cutoff level of 1000 ng/mL [31][70]. CRP and D-dimer are of significant interest, as they are widely used in clinical work [116][71]. While the role of both of these molecules as candidate biomarkers in clinical work has been explored, their use in post mortem processing is more a matter of the pathologist’s discretion.6. Vascular Cognitive Impairment: Room for Biomarkers at Post Mortem
Vascular cognitive impairment (VCI) is a term used to encompass the entire spectrum of cognitive disorders related to the mental abilities of awareness, thinking, and feeling. It is associated with a variety of cerebral vascular brain injuries. VCI symptoms can range from forgetfulness to more serious problems with attention, memory, language, and executive functions such as problem solving. Cerebrovascular disease (CeVD) and neurodegenerative forms of dementia, such as Alzheimer’s disease (AD), are frequently associated comorbidities in the elderly, with similar risk factors and pathophysiological mechanisms, including neuroinflammation [117][72]. As an inflammatory marker that is upregulated in vascular diseases, as well as in AD, protein secreted to plasma (i.e., osteopontin (OPN)) has been tested as a biomarker of AD and VCI [118,119][73][74]. OPN’s involvement in lipid metabolism likely explains its role in conditions that fall under the spectrum of VCI.7. Applying Clinical Biomarkers in a Post Mortem Setting
Applying clinical biomarkers in a post mortem setting does not violate the medicolegal requirements for death investigations. Nevertheless, instead of limiting the contents of the death investigation toolbox, biomarkers could be used to decrease the rate of clinical–autopsy discrepancies and to reduce post mortem healthcare inequalities [12,123,124][12][75][76]. Predominately as a consequence of the decline in rates of clinical (hospital) autopsies, overall autopsy rates have declined in recent decades in many high-income countries [127][77]. This negative trend has been attributed to various factors such as costs, a lack of medical education, the development of new clinical diagnostic tools, medical malpractice implications, and difficulties in obtaining permission from relatives [128][78]. Even if performed, autopsies tend to be negative, failing to produce findings that reveal the cause of death. On the other hand, studies show substantial discrepancies between autopsy results and pre-mortal clinical diagnoses [21,129][16][79]. Healthcare practices have come a long way in reducing mortality, but the decreasing number of TAs demonstrates the need for a feasible alternative. Nonetheless, any form of post mortem investigative tool can provide additional information or a change in diagnosis regarding the cause of death in a great number of cases, either because of discrepancies between the clinical and autopsy diagnoses or through inconclusive autopsies.References
- Nordon, I.M.; Hinchliffe, R.J. Biomarkers in Vascular Disease. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists ; University of Adelaide Press: Adelaide, Australia, 2011.
- Cui, Z.; Zhao, G.; Liu, X. Blood fibrinogen level as a biomarker of adverse outcomes in patients with coronary artery disease: A systematic review and meta-analysis. Medicine 2022, 101, e30117.
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400.
- Kutlu, E.; Cil, N.; Avci, E.; Bir, F.; Kilic, I.D.; Dereli, A.K.; Acar, K. Significance of postmortem biomarkers and multimarker strategy in sudden cardiac death. Leg. Med. 2023, 61, 102212.
- Cao, Z.; Zhao, M.; Xu, C.; Zhang, T.; Jia, Y.; Wang, T.; Zhu, B. Evaluation of Agonal Cardiac Function for Sudden Cardiac Death in Forensic Medicine with Postmortem Brain Natriuretic Peptide (BNP) and NT-proBNP: A Meta-analysis. J. Forensic. Sci. 2020, 65, 686–691.
- Puchenkova, O.A.; Soldatov, V.O.; Belykh, A.E.; Bushueva, O.; Piavchenko, G.A.; Venediktov, A.A.; Shakhpazyan, N.K.; Deykin, A.V.; Korokin, M.V.; Pokrovskiy, M.V. Cytokines in Abdominal Aortic Aneurysm: Master Regulators With Clinical Application. Biomark. Insights 2022, 17, 11772719221095676.
- Medeiros, F.A. Biomarkers and Surrogate Endpoints: Lessons Learned From Glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO20–BIO26.
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cifkova, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532.
- Basso, C.; Stone, J.R. Autopsy in the era of advanced cardiovascular imaging. Eur. Heart J. 2022, 43, 2461–2468.
- (WHO), W.H.O. Autopsy Rate (%) for All Deaths. Available online: https://gateway.euro.who.int/en/indicators/hfa_545-6410-autopsy-rate-for-all-deaths/ (accessed on 3 May 2023).
- (WHO), W.H.O. Autopsy Rate (%) for Hospital Deaths. Available online: https://gateway.euro.who.int/en/indicators/hfa_544-6400-autopsy-rate-for-hospital-deaths/ (accessed on 3 May 2023).
- Waidhauser, J.; Martin, B.; Trepel, M.; Markl, B. Can low autopsy rates be increased? Yes, we can! Should postmortem examinations in oncology be performed? Yes, we should! A postmortem analysis of oncological cases. Virchows Arch. 2021, 478, 301–308.
- Bunei, M.; Muturi, P.; Otiato, F.; Njuguna, H.N.; Emukule, G.O.; Otieno, N.A.; Dawa, J.; Chaves, S.S. Factors Influencing Acceptance of Post-Mortem Examination of Children at a Tertiary Care Hospital in Nairobi, Kenya. Ann. Glob. Health 2019, 85, 95.
- Rosendahl, A.; Mjörnheim, B.; Eriksson, L.C. Autopsies and quality of cause of death diagnoses. SAGE Open Med. 2021, 9, 20503121211037169.
- Lawrence, S.; Namusanya, D.; Hamuza, A.; Huwa, C.; Chasweka, D.; Kelley, M.; Molyneux, S.; Voskuijl, W.; Denno, D.M.; Desmond, N. Hypothetical acceptability of hospital-based post-mortem pediatric minimally invasive tissue sampling in Malawi: The role of complex social relationships. PLoS ONE 2021, 16, e0246369.
- Kurz, S.D.; Sido, V.; Herbst, H.; Ulm, B.; Salkic, E.; Ruschinski, T.M.; Buschmann, C.T.; Tsokos, M. Discrepancies between clinical diagnosis and hospital autopsy: A comparative retrospective analysis of 1112 cases. PLoS ONE 2021, 16, e0255490.
- Buja, L.M.; Barth, R.F.; Krueger, G.R.; Brodsky, S.V.; Hunter, R.L. The Importance of the Autopsy in Medicine: Perspectives of Pathology Colleagues. Acad. Pathol. 2019, 6, 2374289519834041.
- van den Tweel, J.G.; Wittekind, C. The medical autopsy as quality assurance tool in clinical medicine: Dreams and realities. Virchows Arch. 2016, 468, 75–81.
- Rodrigues, F.S.; Oliveira, I.C.; Cat, M.N.L.; Mattos, M.C.L.; Silva, G.A. Agreement between Clinical and Anatomopathological Diagnoses in Pediatric Intensive Care. Rev. Paul. Pediatr. 2021, 39, e2019263.
- Thiene, G.; Saffitz, J.E. Autopsy as a Source of Discovery in Cardiovascular Medicine: Then and Now. Circulation 2018, 137, 2683–2685.
- Bombi, J.A.; Llebaria, C.; Rives, A. Analysis of a series of 500 clinical post mortem studies. II. Basic diagnosis (author’s transl). Med. Clin. 1981, 77, 185–189.
- Smith, A.M.; Lingard, L.; Heslop, P.; Gray, J.; Walker, D.J. Vascular disease as a cause of death in patients with severe disability due to osteoarthritis and rheumatoid arthritis. Springerplus 2015, 4, 328.
- Petersen, S.; Rayner, M.; Leal, J.; Luengo-Fernandez, R.; Gray, A. European Cardiovascular Disease Statistics; British Heart Foundation: Glasgow, UK, 2000.
- EUROSTAT. Causes of Death Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Causes_of_death_statistics (accessed on 3 May 2023).
- Aronson, J.K. When I use a word.... Too much healthcare—Biomarkers. BMJ 2022, 379, o2533.
- Aronson, J.K.; Ferner, R.E. Biomarkers-A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9–23.
- Solarino, B.; Ferorelli, D.; Dell’Erba, A. Post-mortem routine practice in the era of the COVID-19 pandemic. J. Forensic. Leg. Med. 2020, 74, 102010.
- Roberts, I.S.; Benamore, R.E.; Benbow, E.W.; Lee, S.H.; Harris, J.N.; Jackson, A.; Mallett, S.; Patankar, T.; Peebles, C.; Roobottom, C.; et al. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: A validation study. Lancet 2012, 379, 136–142.
- Blokker, B.M.; Wagensveld, I.M.; Weustink, A.C.; Oosterhuis, J.W.; Hunink, M.G. Non-invasive or minimally invasive autopsy compared to conventional autopsy of suspected natural deaths in adults: A systematic review. Eur. Radiol. 2016, 26, 1159–1179.
- Wichmann, D.; Obbelode, F.; Vogel, H.; Hoepker, W.W.; Nierhaus, A.; Braune, S.; Sauter, G.; Pueschel, K.; Kluge, S. Virtual autopsy as an alternative to traditional medical autopsy in the intensive care unit: A prospective cohort study. Ann. Intern. Med. 2012, 156, 123–130.
- Flaxman, A.D.; Stewart, A.; Joseph, J.C.; Alam, N.; Alam, S.S.; Chowdhury, H.; Mooney, M.D.; Rampatige, R.; Remolador, H.; Sanvictores, D.; et al. Collecting verbal autopsies: Improving and streamlining data collection processes using electronic tablets. Popul. Health Metr. 2018, 16, 3.
- Sinard, J.H. Factors affecting autopsy rates, autopsy request rates, and autopsy findings at a large academic medical center. Exp. Mol. Pathol. 2001, 70, 333–343.
- Paratz, E.D.; Rowe, S.J.; Stub, D.; Pflaumer, A.; La Gerche, A. A systematic review of global autopsy rates in all-cause mortality and young sudden death. Heart Rhythm. 2023, 20, 607–613.
- Michaud, K.; Jacobsen, C.; Basso, C.; Banner, J.; Blokker, B.M.; de Boer, H.H.; Dedouit, F.; O’Donnell, C.; Giordano, C.; Magnin, V. Application of postmortem imaging modalities in cases of sudden death due to cardiovascular diseases–current achievements and limitations from a pathology perspective. Virchows Archiv. 2023, 482, 385–406.
- De Souza, P.M.M.; Gerson, G.; Dias, J.S.; De Melo, D.N.; De Souza, S.G.; Ruiz, E.M.; Fernandes Tavora, F.R.; Cavalcanti, L.P.D.G. Validation of verbal autopsy and nasopharyngeal swab collection for the investigation of deaths at home during the COVID-19 pandemics in Brazil. PLoS Neglected Trop. Dis. 2020, 14, e0008830.
- Singh, A. Childhood Malnutrition in India. In Perspective of Recent Advances in Acute Diarrhea; IntechOpen: London, UK, 2020.
- Caleo, G.; Sy, A.; Balandine, S.; Polonsky, J.; Palma, P.; Grais, R. The 2012 WHO verbal autopsy instrument. Lancet 2018, 12, 1–11.
- Thomas, L.M.; D’Ambruoso, L.; Balabanova, D. Verbal autopsy in health policy and systems: A literature review. BMJ Glob. Health 2018, 3, e000639.
- Hyde, G.; Rummery, R.; Whitby, E.H.; Bloor, J.; Raghavan, A.; Cohen, M.C. Benefits and Limitations of the Minimally Invasive Postmortem: A Review of an Innovative Service Development. Pediatr. Dev. Pathol. 2020, 23, 431–437.
- Zech, W.D.; Jackowski, C.; Schwendener, N.; Brencicova, E.; Schuster, F.; Lombardo, P. Postmortem CT versus forensic autopsy: Frequent discrepancies of tracheobronchial content findings. Int. J. Legal. Med. 2016, 130, 191–198.
- Mondello, C.; Baldino, G.; Bottari, A.; Sapienza, D.; Perri, F.; Argo, A.; Asmundo, A.; Ventura Spagnolo, E. The role of PMCT for the assessment of the cause of death in natural disaster (landslide and flood): A Sicilian experience. Int. J. Legal. Med. 2022, 136, 237–244.
- Chatzaraki, V.; Thali, M.J.; Ampanozi, G. Diagnostic accuracy of postmortem computed tomography for bleeding source determination in cases with hemoperitoneum. Int. J. Legal. Med. 2021, 135, 593–603.
- Puntmann, V.O. How-to guide on biomarkers: Biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad. Med. J. 2009, 85, 538–545.
- Bondareva, O.; Sheikh, B.N. Vascular Homeostasis and Inflammation in Health and Disease-Lessons from Single Cell Technologies. Int. J. Mol. Sci. 2020, 21, 4688.
- Almulhim, A.M.; Menezes, R.G. Evaluation of Postmortem Changes; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- O’Keefe, H.; Shenfine, R.; Brown, M.; Beyer, F.; Rankin, J. Are non-invasive or minimally invasive autopsy techniques for detecting cause of death in prenates, neonates and infants accurate? A systematic review of diagnostic test accuracy. BMJ Open 2023, 13, e064774.
- Qian, G.; Adeyanju, O.; Olajuyin, A.; Guo, X. Abdominal Aortic Aneurysm Formation with a Focus on Vascular Smooth Muscle Cells. Life 2022, 12, 191.
- Pal, D.; Szilagyi, B.; Berczeli, M.; Szalay, C.I.; Sardy, B.; Olah, Z.; Szekely, T.; Racz, G.; Banga, P.; Czinege, Z.; et al. Ruptured Aortic Aneurysm and Dissection Related Death: An Autopsy Database Analysis. Pathol. Oncol. Res. 2020, 26, 2391–2399.
- Takada, M.; Yamagishi, K.; Tamakoshi, A.; Iso, H. Height and Mortality from Aortic Aneurysm and Dissection. J. Atheroscler. Thromb. 2022, 29, 1166–1175.
- Levy, D.; Goyal, A.; Grigorova, Y.; Farci, F.; Le, J.K. Aortic Dissection. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023.
- La Russa, R.; Maiese, A.; Viola, R.V.; De Matteis, A.; Pinchi, E.; Frati, P.; Fineschi, V. Searching for highly sensitive and specific biomarkers for sepsis: State-of-the-art in post-mortem diagnosis of sepsis through immunohistochemical analysis. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419855226.
- Imanaka-Yoshida, K.; Matsumoto, K.I. Multiple Roles of Tenascins in Homeostasis and Pathophysiology of Aorta. Ann. Vasc. Dis. 2018, 11, 169–180.
- Brady, A.R.; Thompson, S.G.; Fowkes, F.G.; Greenhalgh, R.M.; Powell, J.T.; Participants, U.K.S.A.T. Abdominal aortic aneurysm expansion: Risk factors and time intervals for surveillance. Circulation 2004, 110, 16–21.
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287.
- Bown, M.J.; Sutton, A.J.; Bell, P.R.; Sayers, R.D. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br. J. Surg. 2002, 89, 714–730.
- Li, T.; Jiang, B.; Li, X.; Sun, H.Y.; Li, X.T.; Jing, J.J.; Yang, J. Serum matrix metalloproteinase-9 is a valuable biomarker for identification of abdominal and thoracic aortic aneurysm: A case-control study. BMC Cardiovasc. Disord. 2018, 18, 202.
- Bihlet, A.R.; Karsdal, M.A.; Sand, J.M.; Leeming, D.J.; Roberts, M.; White, W.; Bowler, R. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir. Res. 2017, 18, 22.
- Kristensen, J.H.; Karsdal, M.A.; Sand, J.M.; Willumsen, N.; Diefenbach, C.; Svensson, B.; Hagglund, P.; Oersnes-Leeming, D.J. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm. Med. 2015, 15, 53.
- Aragon-Vela, J.; Alcala-Bejarano Carrillo, J.; Moreno-Racero, A.; Plaza-Diaz, J. The Role of Molecular and Hormonal Factors in Obesity and the Effects of Physical Activity in Children. Int. J. Mol. Sci. 2022, 23, 15413.
- Hong, L.Z.; Xue, Q.; Shao, H. Inflammatory Markers Related to Innate and Adaptive Immunity in Atherosclerosis: Implications for Disease Prediction and Prospective Therapeutics. J. Inflamm. Res. 2021, 14, 379–392.
- Soleimani, Z.; Amighi, F.; Vakili, Z.; Momen-Heravi, M.; Moravveji, S.A. Diagnostic value of procalcitonin, erythrocyte sedimentation rate (ESR), quantitative C-reactive protein (CRP) and clinical findings associated with osteomyelitis in patients with diabetic foot. Hum. Antibodies 2021, 29, 115–121.
- Albu, E.; Filip, C.; Zamosteanu, N.; Jaba, I.M.; Linic, I.S.; Sosa, I. Hyperhomocysteinemia is an indicator of oxidant stress. Med. Hypotheses 2012, 78, 554–555.
- Atre, A.S.; CR, W.D.S.; Suresh, V.; Nagaraja, M.; Madhuvan, H. Evaluation of Plasma Total Antioxidant Capacity Levels and Osteocalcin in Prediabetes and Healthy Subjects. RGUHS J. Med. Sci. 2020, 10, 20–26.
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148.
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Bradwin, G.; Hasan, A.A.; Rifai, N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: Secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur. Heart J. 2020, 41, 2952–2961.
- Holcomb, D.; Alexaki, A.; Hernandez, N.; Hunt, R.; Laurie, K.; Kames, J.; Hamasaki-Katagiri, N.; Komar, A.A.; DiCuccio, M.; Kimchi-Sarfaty, C. Gene variants of coagulation related proteins that interact with SARS-CoV-2. PLoS Comput. Biol. 2021, 17, e1008805.
- Al-Barjas, H.S.; Ariens, R.; Grant, P.; Scott, J.A. Raised plasma fibrinogen concentration in patients with abdominal aortic aneurysm. Angiology 2006, 57, 607–614.
- Menekşe, E.; Düz, M.E. Changes in D-dimer, Ferritin, and Fibrinogen in Healthy Smokers and Nonsmokers during the COVID-19 Outbreak. J. Surg. Res. 2023, 6, 94–99.
- Crawford, F.; Andras, A.; Welch, K.; Sheares, K.; Keeling, D.; Chappell, F.M. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst. Rev. 2016, 2016, CD010864.
- Hamer, H.M.; Stroobants, A.K.; Bavalia, R.; Ponjee, G.A.E.; Klok, F.A.; van der Hulle, T.; Huisman, M.V.; Hendriks, H.A.; Middeldorp, S. Diagnostic accuracy of four different D-dimer assays: A post-hoc analysis of the YEARS study. Thromb. Res. 2021, 201, 18–22.
- Goncalves, F.A.R.; Besen, B.; Lima, C.A.; Cora, A.P.; Pereira, A.J.R.; Perazzio, S.F.; Gouvea, C.P.; Fonseca, L.A.M.; Trindade, E.M.; Sumita, N.M.; et al. Use and misuse of biomarkers and the role of D-dimer and C-reactive protein in the management of COVID-19: A post-hoc analysis of a prospective cohort study. Clinics 2021, 76, e3547.
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713.
- Han, X.; Wang, W.; He, J.; Jiang, L.; Li, X. Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncol. Lett. 2019, 17, 2592–2598.
- Chai, Y.L.; Chong, J.R.; Raquib, A.R.; Xu, X.; Hilal, S.; Venketasubramanian, N.; Tan, B.Y.; Kumar, A.P.; Sethi, G.; Chen, C.P.; et al. Plasma osteopontin as a biomarker of Alzheimer’s disease and vascular cognitive impairment. Sci. Rep. 2021, 11, 4010.
- Bhatt, M.; MovaseghiGargari, M.; Chand, M.T. The importance of autopsies despite the declining number amidst the COVID-19 pandemic. Autops. Case Rep. 2022, 12, e2021371.
- Elmsjo, A.; Vikingsson, S.; Soderberg, C.; Kugelberg, F.C.; Green, H. Post-Mortem Metabolomics: A Novel Approach in Clinical Biomarker Discovery and a Potential Tool in Death Investigations. Chem. Res. Toxicol. 2021, 34, 1496–1502.
- Goldman, L. Autopsy 2018: Still necessary, even if occasionally not sufficient. Circulation 2018, 137, 2686–2688.
- Lunetta, P.; Lounamaa, A.; Sihvonen, S. Surveillance of injury-related deaths: Medicolegal autopsy rates and trends in Finland. Inj. Prev. 2007, 13, 282–284.
- Perkins, G.D.; McAuley, D.F.; Davies, S.; Gao, F. Discrepancies between clinical and postmortem diagnoses in critically ill patients: An observational study. Crit. Care 2003, 7, R129–R132.
More
