You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Ivan Šoša and Version 3 by Lindsay Dong.
Autopsies are still needed for the determination and correction of causes of death, even in “clear-cut” cases. Moreover, post mortem sample handling and analysis are challenges that need to be addressed, as they can produce variability in the findings; for this reason, validation with biomarkers is of key importance.
- blood-based biomarkers
- clotting
- inflammation
- post mortem
1. Introduction
Biomarkers found in bodily fluids may represent the active disease process or the patient’s reaction to that disease [1]. Moreover, they can act as an alternative measure of outcomes to assess the efficacy of therapy. According to common wisdom, a biomarker is a protein, enzyme, or cytokine with discriminatory value in clinical care [2][3][2,3]. A variety of molecules have been evaluated, and although post mortem biomarkers and a multimarker strategy are best investigated in the light of sudden cardiac death and agonal cardiac function [4][5][4,5], their significant potential in relation to peripheral vasculature is yet to be addressed [1][6][1,6]. All biomarkers must meet certain criteria to constitute a surrogate endpoint, or to be able to predict a clinically relevant endpoint, such as the loss of vision or a decrease in quality of life. In addition, the effect of a proposed treatment on the surrogate must capture the effect of the treatment on the clinically relevant endpoint [7][8][7,8].
This information should be considered in the context of the fact that autopsies face a number of challenges; for example, the lack of regulation for governmental funding for hospital-based autopsies, or hospitals rejecting autopsies requested by families [9]. In any case, autopsy numbers have fallen significantly worldwide [10][11][12][13][14][10,11,12,13,14], and the accessibility of post mortem healthcare is uneven [15]. It is necessary to improve these statistics and also to address the major problem of discrepancies between clinical diagnosis and initial autopsy findings regarding the panel of clinical biomarkers.
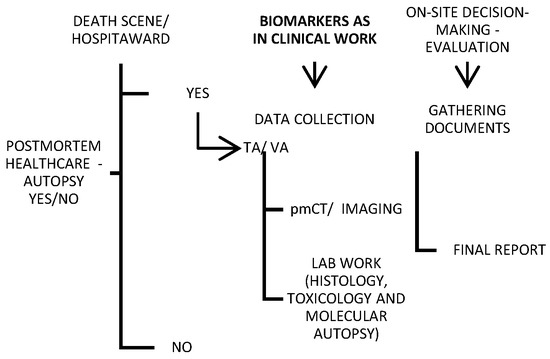
2. Traditional Post Mortem Healthcare
Despite its discrepancies with clinical records, autopsy remains the gold standard as the ultimate diagnostic procedure [16][17][21,22]. Although these discrepancies have decreased significantly over time, in 2010 their rate remained high [18][23]; in the “post-COVID” era, the rate has reached an unprecedented 42% [19][24]. This renders between one in two and one in three autopsies superfluous. Our knowledge about normal circulation stems entirely from thorough post mortem dissection [20][25]. More than 40 years ago, in a series of 500 clinical autopsies, vascular disorders were found to account for 25.2% of anatomopathological diagnoses [21][26]. These figures were more or less the same in osteoarthritis/rheumatoid arthritis research from 2015 [22][27]. Data from the Eurostat indicate the same phenomenon: diseases of the circulatory system are the main cause of death in the EU and were responsible for almost 37% of all deaths in 2017 [23][24][28,29]. A biomarker may be a recording taken from an individual, an imaging test, or a biosample. Etymologically, the term “biomarker” comes from the Greek form βιο-, from βίος, meaning life, and the Old English word meaning a mark [25][26][34,35]. Bearing in mind this Greek root, using the word ‘life’ in the context of a post mortem may seem slightly incongruous. This was the case until recently, when the COVID-19 pandemic brought about a radical shift in routine post mortem practice [27][36].3. Options for Traditional Autopsy
Traditional autopsy may be criticized in the media, but it is an important tool for both criminal investigations and healthcare quality control. For this reason, minimally invasive alternatives to traditional autopsies are continuously emerging. Imaging and “verbal autopsy” (VA) were shown in a large series to be promising techniques compared with a full autopsy [28][29][30][31][37,38,39,40] (Figure 12). Various objective factors influence the autopsy rate, though it is less likely to be requested for deaths in the emergency department or on general surgery wards, and it is most likely to be requested for fetal, medicine-related, cardiothoracic surgery-related, and pediatric deaths [32][41]. Nevertheless, most countries globally do not report high autopsy rates (less than 70% of all-cause mortality) [33][42].
Figure 12. Schematic of a provisional post mortem protocol created by the author, with biomarkers included; TA—traditional autopsy; VA—verbal autopsy.
While the cost of electronic data systems and the long wait between data collection and analysis appear to be the main disadvantages of verbal autopsies, post mortem imaging is hampered by a lack of direct visualization of the soft tissue, as well as postmortem artifacts that obscure the natural causes of death and can be misinterpreted as antemortem pathologies [31][34][40,43]. However, VA has been preferred recently in the COVID-19-related pandemic context, with a satisfying effect [35][44].
For deaths that occur outside the health system, health information and a description of the events preceding death are included in the VA. It was first used in a public health project concerning the relationships between nutrition, infection, and child development in India [36][47]. Nowadays, this method has been improved and augmented so that it yields suitably complete death certificates and ultimately estimates cause-specific mortality. Specifically, VA means the collection of anamnestic data through an in-person interview with a close relative or caregiver of a deceased. The interview takes place within a short time of death; these data include symptoms, signs, and circumstances prior to death [37][48]. In settings where most deaths are otherwise undocumented, which typically means in low- and middle-income countries, VA attempts to establish causes of death, allowing scientists to analyze disease patterns and direct public health policy decisions. The body of relevant literature reports that the specificity of the VA is commonly found to be higher than sensitivity [38][49].
In agreement with contemporary attainments, even conducting an autopsy can be transferred to a computerized environment, and digital tools can be employed. Accordingly, another accessible and recently developed modality of postmortem healthcare is a radiographic examination of the body after death—postmortem radiology. As much as they provide a strong complementary tool to the TA, imaging techniques used in everyday clinical work are applied to post mortem processing [34][43].
The explicit potential economic benefits of the PMCT (magnetic resonance imaging—MRI) have not been assessed recently [29][39][38,57]; despite its numerous advantages, this method still exhibits the problem of a significant rate of diagnostic discrepancies [40][41][58,59]. Nevertheless, PMCT has 79% sensitivity and 92.1% specificity for the detection of the source of bleeding [42][60].
4. Post Mortem Biomarkers
Biomarkers provide plenty of information for enhancing all aspects of vascular homeostasis through vascular beds [1]. Biomarkers are characteristic indicators of disease, a disease state, or disease progression. They were at first described as a “measurable and quantifiable biological parameter that could serve as an index for health assessment” and were ultimately defined as “a characteristic that is objectively measured as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” [43][44][63,64].
The post mortem period involves events such as autolysis or decay, and biomarkers found in bodily fluids may represent the progression of the active disease or a reaction to the disease. Therefore, the value of post mortem biomarkers should be evaluated with this in mind, even if their efficacy is clinically confirmed [45][65]. This compounds the value of clinical post mortem studies as not only a method of control but also a means of improving teaching methods in hospitals [13]. The augmentation of post mortems with blood-based (circulating) biomarkers, in order to avoid invasive autopsies, would have cultural, religious, and potentially economic benefits [29][39][46][38,57,66].
In fact, no contemporary studies compare the costs of the various post mortem optional modalities.
