You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Beatriz Oliveira.
Adapting our food production chain and increasing the flora and fauna’s livelihood in climate change-affected areas using Opuntia is not only theoretical but already exists in practice in many places. This cactus grows in unsuitable soil for most species as it is adapted to arid and semi-arid soils and hot weather. In these regions, Opuntia protects from erosion and contributes to soil health. Opuntia has a high potential as an invasive species, with caution always being recommended when dealing with this specie. The high content of specific compounds, such as proline, indicaxanthin, and betanin, found in Opuntia ficus-indica, influence the plant’s adaptation to unfavourable conditions.
- desertification
- Opuntia
- Opuntia ficus-indica
- drought
1. The Crassulacean Acid Metabolism (CAM) and Opuntia Productivity
Like many other arid-weather-adapted plants, the prickly pear follows a CAM metabolism, opening its stomata exclusively during the night period, thus avoiding loss of water during the day when temperatures and sun exposure are harsher and evaporation rates higher. At night, CO2 is fixed through the oxaloacetate and malate, accumulated in the cytosol and subsequently moved and stored in vacuoles [61][1]. During the day, CO2 is released from these molecules, and photosynthesis can proceed as a C3 plant (the most common of three metabolic pathways for carbon fixation, C3 carbon fixation occurs in all plants as the first step of the Calvin–Benson cycle), with the exception that the stomata remain closed as a way to prevent water vapour and CO2 escape.
Opuntia ficus-indica is considered an extremely productive CAM plant due to its ability to keep sequestrating CO2 even during prolonged drought periods, fixing large quantities of carbon in its shoot and root system [20][2]. An increase in the organic carbon sequestration of the soil is also observed where the prickly-pear is planted [17][3]. Furthermore, limiting soil and water erosion decreases the on-site loss of organic carbon [30][4]. Opuntia, being able to keep transforming CO2 even at high temperatures and thriving in places inhospitable for most plant species, could contribute to lowering CO2 in the earth’s atmosphere.
A peculiarity of C3 and CAM plants is that by increasing the partial CO2 pressure in the atmosphere to about 70 Pa, an increase in uptake and biomass accumulation on the plant from 30% to over 60% is observed [62][5]. Specifically, in Opuntia ficus-indica, Nobel and Israel [63][6] described that, in the first few months after planting in a high CO2 chamber, the daily CO2 uptake was increased by 70%, accompanied by an increase in biomass production, favouring growth. Doubling CO2 atmospheric concentration was also shown to enhance the activity of carbohydrate-metabolism enzymes and increase source carbohydrate production, photo assimilation transport, and sink strength [64][7]. After three months of exposure, glucose and starch in the chlorenchyma increased by 175% and 57%, respectively, compared to the initial value. Malate nocturnal production also increased by 75%, mirroring the changes found by Nobel and Israel [63][6], where an increase of 70% in CO2 uptake was registered.
The net assimilation rate reported by Acevedo et al. [65][8] was upbeat throughout the year, averaging 3.4 g per square meter per day for 1.0- and 2.0-year-old plants. This growth was shown to be seasonal, with most of the expansion occurring in the late spring to early summer months (October to January). It is also important to mention that the younger the cladodes, the higher the biomass growth, as the annual increase in the area averaged 30% for all cladodes on the youngest plants (0.4 years old) but only 3% for the oldest cladodes (4.9 years old).
2. Low Irrigation Need
Opuntia’s resistance to drought is in part due to its high capacity to store water in its succulent stems. As such, variations in cladode thickness can be a measure of plant dehydration and stress. When in optimal irrigation conditions, Opuntia sequesters water into their parenchymal tissue, becoming a recognizable mucous, thick, white layer (most of the inside of the cladode). When faced with hydric stress, this white mucous layer consistently shrinks to a measurable degree [61][1]. This remarkable ability of the parenchyma to store water provides the plant with an efficient buffer for many physiological responses to drought. When in severe drought, the shrinking of organs due to tissue dehydration was observed.
After two and a half months without irrigation, Opuntia’s acid accumulation and stomatal conductance were severely reduced, as shown in Scalisi et al. [61][1], although the parenchyma and chlorenchyma osmotic pressure is little affected. Moreover, plant turgor presence was also shown to be reduced by 86% compared to well-watered conditions.
In another study, Aguilar Becerril and Peña Valdivia [66][9] determined the physiological response of Opuntia to extreme and intermediate drought. It was found that, under intermediate drought, the plant’s photosynthetic machinery is stable to intermediate drought (two irrigations in a 180-day period).
Acevedo et al. [65][8] reported that severe water stress suppressed the nocturnal stomatal openings on mature cladodes but not on young cladodes. The characteristic acidification of the plant during the nocturnal period (due to rising concentrations of malate and oxaloacetate) was considered not sensitive to water stress.
In Madagascar, livestock keepers have long incorporated Opuntia into their rural economy. Assisting the cattle during the dry season is the key to successful production, and rather than looking for water through the land, pastoralists have turned to the cactus as a water source [67][10].
In May 2021, Madagascar was facing its worst drought in the past 40 years. The Observer Research Foundation reports that the rainfall deficit has led to massive crop failures and adversely affected livestock [68][11]. Attempts are being made to “climate-proof” the island’s food production, the prickly pear having a central role. The population already relies heavily on prickly-pear consumption, as most households produce or forage what they consume [69][12].
As climate change is a global phenomenon, climate-proofing the food supply chain is highly important, assuring food security.
The presence of this cactus in the European Mediterranean region is nothing new, as it was brought to the continent by the Spanish [70][13] between the end of the 15th century and the beginning of the 16th century. It quickly spread to Southern Italy and Greece, where the climate accommodated the plant’s growth. Its primary use, however, was the production of the Cochineal red dye and not human consumption. The observed increase in the market value of this fruit in Europe in recent years has been driven by two factors: the shortages of water observed due to climate change and the consumers’ habits. Consumers are becoming more climate- and health-conscious and are looking for local produce and a varied diet. There is also awareness of how Opuntia ficus-indica requires less water and maintenance to grow, having a lower environmental impact when compared to other fruit. The plant also appeals to farmers as it is considered a “hands-off” culture, decreasing the workload and investment. More recently, the Alentejo region of Portugal has been experiencing a severe yearly drought during the Summer, making it hard for farmers to maintain their conventional crops, such as olive trees, due to the increasing watering expenses. As a result, many of these farmers have turned to prickly-pear cultivars as a far more profitable solution.
Nevertheless, higher market growth for the fruit is necessary for its more widespread plantation. Consumers in Europe are not entirely familiar with the fruit. The number of seeds, presence of spikes, and unfamiliarity with how to consume it are probably its main limiting factors in the European market.
3. High Proline Content in the Fruit and Resistance to Arid Environments
Proline is the primary amino acid found in Opuntia’s fruit. Other reported primary amino acids include taurine and glutamine [71,72][14][15]. Differences between samples are usually observed, which may be attributed to the maturity of the fruit, its variety, and the growth environment. According to Hernández-Urbiola et al. [73][16], physical conditions such as water availability, temperature, and light–dark periods are primarily implicated in protein synthesis. Furthermore, several studies have demonstrated that protein synthesis increases as cellular protection when the soil is too acidic or saline [63,66,74][6][9][17].
Proline hyperaccumulation is reported to accompany the extremophile characteristics of some plants, and it is believed to contribute to stress tolerance capacity [75,76][18][19]. Proline content is reported to increase when the plant is under drought conditions, high salinity, high light and UV radiation, or heavy metal or oxidative stress [77][20]. In addition, proline accumulating mutants of some plants demonstrate that the accumulation of this amino acid has a complex effect on the plant’s response to adverse environmental conditions [78,79][21][22].
Proline is not only able to work as an osmoregulatory molecule, but it is also able to influence stress tolerance in several ways. For example, proline has been shown to work as a molecular chaperone that can protect protein integrity and enhance the activity of enzymes [77][20].
Moreover, some antioxidant features have been attributed to proline. Proline has been shown to protect human cells against carcinogenic oxidative stress and to alleviate mercury toxicity in rice (Orzya sativa), and free radical levels were shown to be reduced in transgenic algae and tobacco plants with hyper-accumulated proline. The damaging effects of singlet oxygen and hydroxyl radicals in thylakoid membranes in plants were also significantly reduced by proline [77][20].
The high amount of proline found in OFI fruit when compared to other common fruits (see Table 1) may have an osmoregulatory function, protecting from the environmental stresses that the plant is exposed to. Proline could be the key to this plant’s survival in arid, sunny environments where it thrives.
4. Salt Tolerance
Recent water scarcity has restricted the availability of good-quality water for irrigation. This has driven the seeking of salt-tolerant plants that can be watered with salty water without further treatment. The search for plants with increased salt tolerance has included Opuntia, with studies showing that this plant has a higher-than-average salt tolerance; however, the tolerance level is very variable between varieties.
The sodium (Na) accumulation in the soil or inside the plant makes the water and nutrients required unavailable. A stress response to salinity seen in Opuntia is the proline accumulation, used as an osmolyte for intracellular osmotic adjustment [83][26]. Silva Ortega et al. [83][26] noted that this proline accumulation was correlated with the expression of the gene Osp5cs. Radi et al. [84][27] recorded that after the first week of saline stress, there is a notable decline in the levels of glycine betaine and proline. However, increasing the duration of the stress promoted the accumulation of these substances. This could be due to an adaptation period to the stress. The prickly pear already shows a high amount of proline in normal conditions, believed to help the plant survive in the semi-arid areas it inhabits. Proline accumulation is, therefore, an adaptation to protect the plant from hydric stress and saline stress.
Despite these adaptations, Opuntia is not entirely resistant to saline stress. Salinity irrigation of 20 dS m−1 (desisiemens per meter) for 90 days decreased plant height by 36.5% compared to the control and reduced cladode thickness [85][28]. This thickness reduction is synonymous with a decrease in water storage brought upon by the high salinity. Radi et al. [84][27] showed that salt stress at 15.6 dS m−1 reduced shoot proliferation, growth, and fresh and dry weights. Additionally, after a stress duration of 3 weeks, the survival rate was significantly decreased, being the lowest at 62.25% for remote shoots. However, explants were not affected. Opuntia seeds have been shown to germinate up to 45.65 dS m−1 but showed a low ability to recover after salt exposure, some even entering a secondary dormancy state [86][29]. However, a saline concentration of 3.6 dS m−1 was reported by Fonseca et al. [87][30] to not have stressed the plant. On the contrary, a 3-day interval treatment with saline water was reported to have increased plant height, the number of cladodes, cladode area index, green mass, and dry matter yield. These results directly contradict those acquired by de Lira Freire et al. [88][31], who applied the same 3.6 dS m−1 saline water treatment at a 14-day interval until the plants showed extreme chlorosis and dehydration (considered 100% damage). From the 24 genotypes studied, the least resistant was the F-8 variety, showing 100% damage at 130 days, and the most resistant variety was Liso forageiro, surviving 419 days.
5. Proline, Indicaxanthin, and Proline Betaine
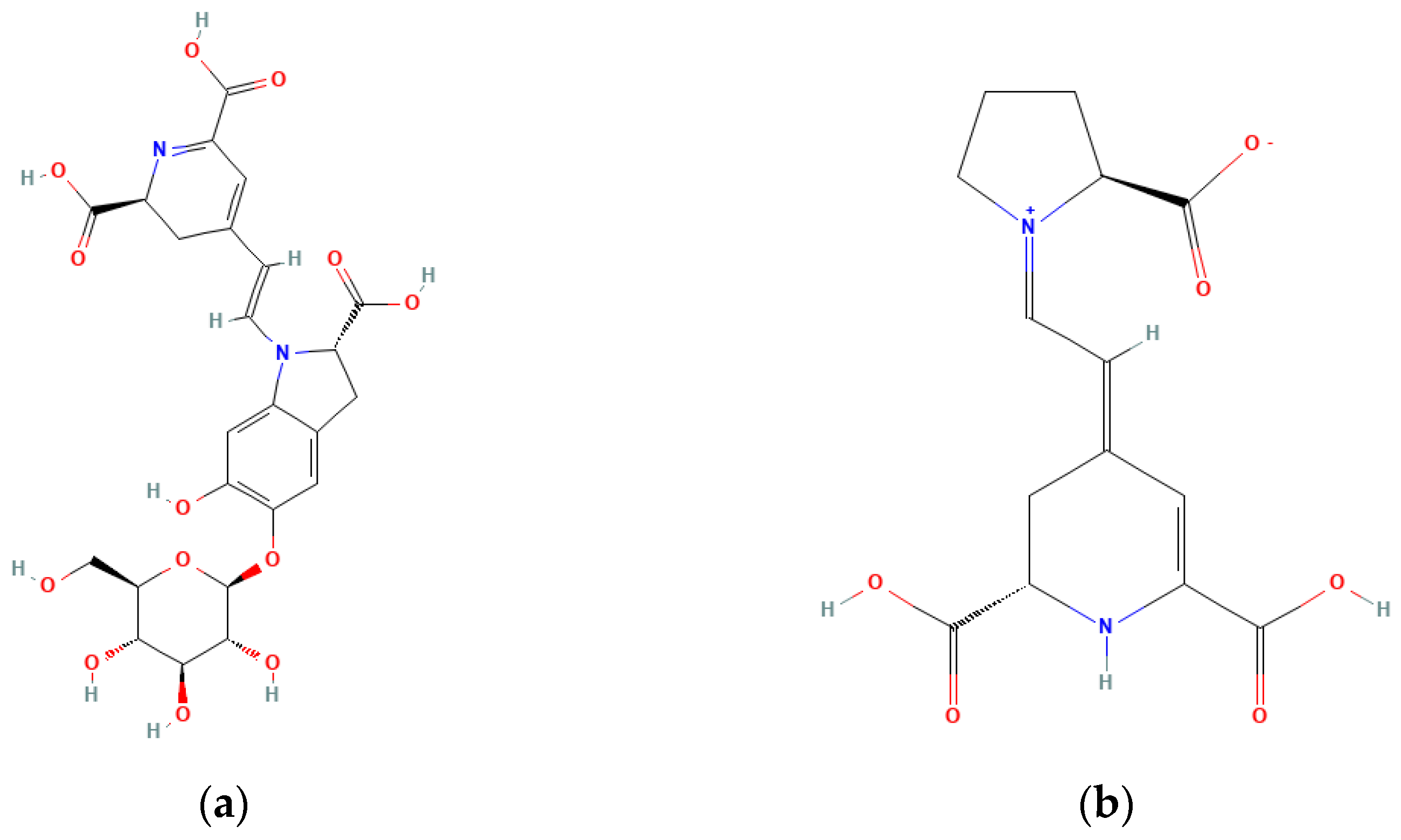
Betalains such as betacyanins and betaxanthins are rare in the human diet, being only significantly present in beets and prickly pear [89][32]. Different betalains (Figure 1) are detected in Opuntia, with indicaxanthin being usually the dominant one [89,90[32][33][34][35],91,92], although it mostly depends on fruit variety.
Indicaxanthin has a yellow colouration, while betanin has a red colouration. As such, depending on the fruit variety (which have different colourations), the relative amounts of these two pigments differ. In red varieties, betanin is prevalent, while in yellow varieties, indicaxanthin predominates (see Table 2).
Table 2. Amount of betanin and indicaxanthin in the prickly pear pulp (mg/100 g of edible pulp) [95].
| Fruit Variety | Indicaxanthin | Betanin |
|---|---|---|
| Yellow | 8.42 ± 0.51 | 1.04 ± 0.12 |
| Red | 2.61 ± 0.30 | 5.12 ± 0.51 |
| White | 5.86 ± 0.49 | 0.10 ± 0.02 |
Indicaxanthin is derived from L-proline instead of tyrosine. Unlike anthocyanin, commonly found in plants, betalains contain nitrogen, as they are aromatic indole derivatives synthesized from tyrosine. Opuntia shows much higher levels of proline compared to tyrosine.
Physiologically, indicaxanthin is a very stable molecule that is considered a significant antioxidant. In vitro, tests point to its ability as a radical scavenger and protector against cytotoxicity by various agents [96][39].
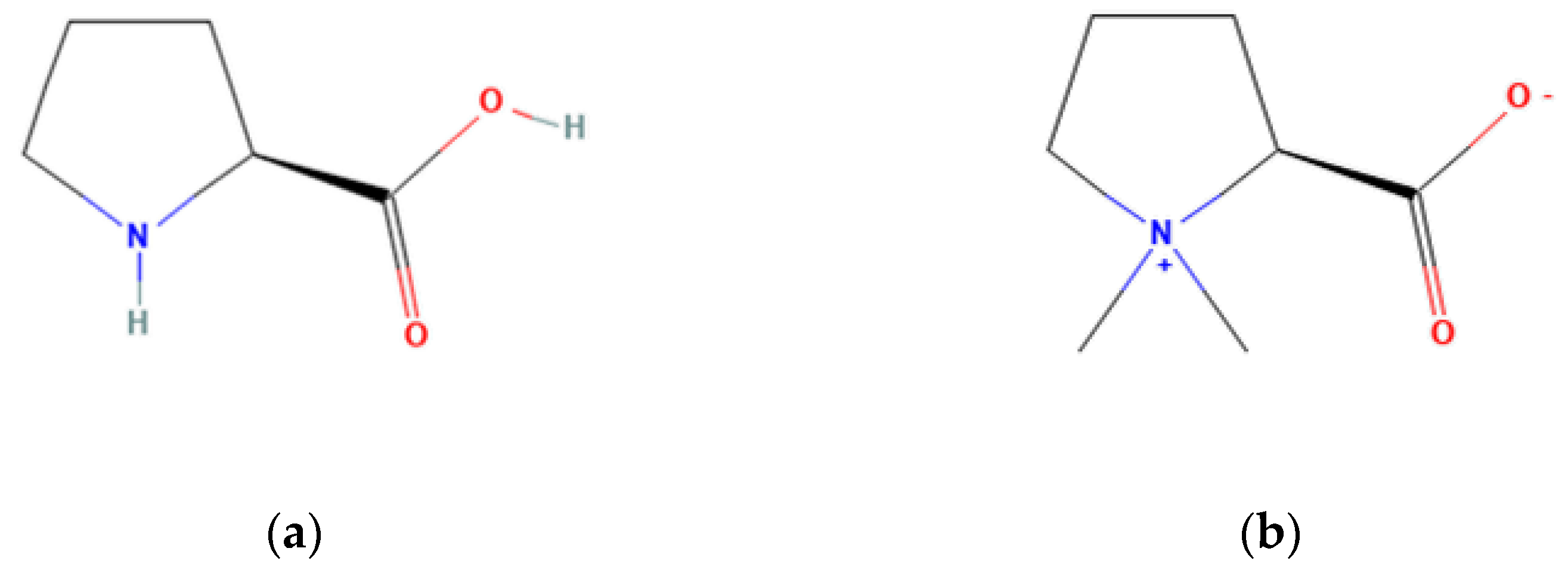
Another compound, proline betaine, is produced by the methylation of proline in plants (See Figure 2). This molecule has not been assessed in the Opuntia plant. In alfalfa plants (Medicago sativa), it was shown that high salinity originated a 10-fold increase of proline betaine in the shoots [97][40], suggesting an osmotic adjustment role. This protective role of proline betaine, however, remains controversial, as not all plant species produce this compound and introducing it to a plant does not always lead to an increased environmental stress tolerance [98][41] Genetic engineering has been used to produce a higher amount of Proline Betaine in Citrus, resulting in an increased stress resistance as described in Nolte et al. [99][42]. Since a high amount of proline is naturally found in Opuntia and betaine, it can be hypothesized that proline betaine could also be present and have a role in the adaptations to unfavourable conditions of this plant.
References
- Scalisi, A.; Morandi, B.; Inglese, P.; Lo Bianco, R. Cladode growth dynamics in Opuntia ficus-indica under drought. Environ. Exp. Bot. 2016, 122, 158–167.
- Nefzaoui, A.; Salem, H. Opuntia spp. A strategic fodder and efficient tool to combat desertification in the WANA region. In Cactus (Opuntia spp.) as Forage; Mondragon-Jacobo, C., Perez-Gonzalez, S., Eds.; FAO Plant Production and Protection Paper 169; FAO: Rome, Italy, 2001; pp. 73–90.
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. Agroecol. Sustain. Food Syst. 2022, 46, 815–841.
- Girmay, A. Contributions of prickly pear cactus towards achieving household food security in Tigray: The case of Tabia Kihen in Kilte Awlaelo Wereda of Eastern Tigray, Northern Ethiopia Meaza Taddele, Workneh Negatu, Abbadi Girmay. In Proceedings of the Improved Utilization of Cactus Pear for Food, Feed, Soil and Water Conservation and Other Products in Africa Proceedings of International Workshop Conference, Mekelle, Ethiopia, 19–21 October 2009.
- Drake, B.G. The impact of rising CO2 on ecosystem production. Water Air Soil Pollut. 1992, 64, 25–44.
- Nobel, P.S.; Israel, A.A. Cladode development, environmental responses of CO2 uptake, and productivity for Opuntia ficus-indica under elevated CO2. J. Exp. Bot. 1994, 45, 295–303.
- Wang, N.; Nobel, P.S. Doubling the CO2 Concentration Enhanced the Activity of Carbohydrate-Metabolism Enzymes, Source Carbohydrate Production, Photoassimilate Transport, and Sink Strength for Opuntia ficus-indica. Plant Physiol. 1996, 110, 893–902.
- Acevedo, E.; Badilla, I.; Nobel, P.S. Water Relations, Diurnal Acidity Changes, and Productivity of a Cultivated Cactus, Opuntia ficus-indica. Plant Physiol. 1983, 72, 775–780.
- Aguilar Becerril, G.; Peña Valdivia, C.B. Alteraciones fisiológicas provocadas por sequía en nopal (Opuntia ficus-indica). Rev. Fitotec. Mex. 2006, 29, 231–237.
- Kaufmann, J.C. Prickly Pear Cactus and Pastoralism in Southwest Madagascar. Ethnology 2004, 43, 345–361.
- Chakrabarty, M. Plight of Madagascar: Severe Drought, Raging Famine and Lack of Adaptation Funds. Available online: https://www.orfonline.org/expert-speak/plight-of-madgascar-raging-famine-causes-rise-in-food-insecurity/ (accessed on 3 May 2023).
- Kaamil Ahmed, R.R. At Least 1 m People Facing Starvation as Madagascar’s Drought Worsens. Available online: https://www.theguardian.com/global-development/2021/may/10/at-least-1m-people-facing-starvation-madagascar-droughtworsens#:~:text=At%20least%201m%20people%20facing%20starvation%20as%20Madagascar%27s%20drought%20worsens,This%20article%20is&text=Madagascar%27s%20worst%20drought%20in%2040,year%20of%20desperate%20food%20shortages (accessed on 3 May 2023).
- Barbera, G.; Carimi, F.; Inglese, P. Past and Present Role of the Indian-Fig Prickly-Pear (Opuntia ficus-indica (L.) Miller, Cactaceae) in the Agriculture of Sicily. Econ. Bot. 1992, 46, 10–20.
- Stintzing, F.C.; Schieber, A.; Carle, R. Amino acid composition and betaxanthin formation in fruits from Opuntia ficus-indica. Planta Medica 1999, 65, 632–635.
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901.
- Hernández-Urbiola, M.; Contreras-Padilla, M.; Perez-Torrero, E.; Hernández-Quevedo, G.; Rojas-Molina, I.; Cortes, M. Study of Nutritional Composition of Nopal (Opuntia ficus indica cv. Redonda) at Different Maturity Stages. Open Nutr. J. 2010, 4, 11–16.
- Drennan, P.M.; Nobel, P.S. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant Cell Environ. 2000, 23, 767–781.
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759.
- Kishor, P.; Sangam, S.; Amrutha, R.; Laxmi, P.; Naidu, K.; Sambasiva Rao, K.R.S.; Rao, S.; Reddy, K.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 429–438.
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97.
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Verma, D.P. Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000, 122, 1129–1136.
- Mattioli, R.; Marchese, D.; D’Angeli, S.; Altamura, M.; Costantino, P.; Trovato, M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008, 66, 277–288.
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Amino Acid Determination in Grape Juices and Wines by HPLC Using a Modification of the 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate (AQC) Method. Chromatographia 2003, 58, 29–35.
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93.
- Tezcan, F.; Uzaşçı, S.; Uyar, G.; Öztekin, N.; Erim, F.B. Determination of amino acids in pomegranate juices and fingerprint for adulteration with apple juices. Food Chem. 2013, 141, 1187–1191.
- Silva-Ortega, C.O.; Ochoa-Alfaro, A.E.; Reyes-Agüero, J.A.; Aguado-Santacruz, G.A.; Jiménez-Bremont, J.F. Salt stress increases the expression of p5cs gene and induces proline accumulation in cactus pear. Plant Physiol. Biochem. 2008, 46, 82–92.
- Radi, H.; Bouchiha, F.; El Maataoui, S.; Oubassou, E.-Z.; Rham, I.; Alfeddy, M.N.; Aissam, S.; Mazri, M.A. Morphological and physio-biochemical responses of cactus pear (Opuntia ficus indica (L.) Mill.) organogenic cultures to salt and drought stresses induced in vitro. Plant Cell Tissue Organ Cult. 2023, 154, 1–14.
- Nerd, A.; Karadi, A.; Mizrahi, Y. Salt tolerance of prickly pear cactus (Opuntia ficus-indica). Plant Soil 1991, 137, 201–207.
- Podda, L.; Santo, A.; Leone, C.; Mayoral, O.; Bacchetta, G. Seed germination, salt stress tolerance and seedling growth of Opuntia ficus-indica (Cactaceae), invasive species in the Mediterranean Basin. Flora 2017, 229, 50–57.
- Fonseca, V.A.; Dos Santos, M.R.; da Silva, J.A.; Donato, S.L.R.; Rodrigues, C.S.; Brito, C.F.B. Morpho-physiology, yield, and water-use efficiency of Opuntia ficus-indica irrigated with saline water. Acta Sci. Agron. 2019, 41, 42631.
- De Lira Freire, J.; Dos Santos, M.V.F.; Dubeux, J.C.B.; Neto, E.B.; De Andrade Lira, T.L.M.; Da Cunha, M.V.; Dos Santos, D.C.; De Mello, A.C.L.; Da Silva Oliveira, C.G. Evaluation of cactus pear clones subjected to salt stress. Trop. Grassl. 2021, 9, 235–242.
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307.
- Stintzing, F.C.; Schieber, A.; Carle, R.J.E.F.R. Phytochemical and nutritional significance of cactus pear. Eur. Food Res. Technol. 2001, 212, 396–407.
- Piattelli, M.; Minale, L.; Prota, G.J.T. Isolation, structure and absolute configuration of indicaxanthin. Tetrahedron 1964, 20, 2325–2329.
- Fernández-López, J.A.; Almela, L. Application of high-performance liquid chromatography to the characterization of the betalain pigments in prickly pear fruits. J. Chromatogr. A 2001, 913, 415–420.
- National Institutes of Health (NIH), PubChem, Compound Summary for CID 6540685, Betanine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Betanine (accessed on 14 April 2023).
- National Institutes of Health (NIH), PubChem, Compound Summary for CID 6096870, Indicaxanthin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Indicaxanthin (accessed on 14 April 2023).
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901.
- Habtemariam, S. The chemical and pharmacological basis of prickly pear cactus (Opuntia species) as potential therapy for type 2 diabetes and obesity. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases, 1st ed.; Habtemariam, S., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2019; pp. 435–472.
- Trinchant, J.-C.; Boscari, A.; Spennato, G.; Van de Sype, G.; Le Rudulier, D. Proline betaine accumulation and metabolism in alfalfa plants under sodium chloride stress. Exploring its compartmentalization in nodules. Plant Physiol. 2004, 135, 1583–1594.
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216.
- Nolte, K.D.; Hanson, A.D.; Gage, D.A. Proline Accumulation and Methylation to Proline Betaine in Citrus: Implications for Genetic Engineering of Stress Resistance. J. Am. Soc. Hortic. Sci. 1997, 122, 8–13.
- National Institutes of Health (NIH), PubChem, Compound Summary for CID 145742, Proline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Proline (accessed on 14 April 2023).
- National Institutes of Health (NIH), PubChem, Compound Summary for CID 115244, Stachydrine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Stachydrine (accessed on 14 April 2023).
More