Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by laurent gallais and Version 2 by Rita Xu.
Since its inception in 1984, 3D printing has revolutionized manufacturing by leveraging the additivity principle and simple material–energy coupling. Stereolithography, as the pioneering technology, introduced the concept of photopolymerization with a single photon. This groundbreaking approach not only established the essential criteria for additive processes employing diverse localized energies and materials, including solid, pasty, powdery, organic, and mineral substances, but also underscored the significance of light–matter interactions in the spatial and temporal domains, impacting various critical aspects of stereolithography’s performance.
- 3D printing
- two-photon absorption
- printability
1. Introduction
The basic idea of additive manufacturing or 3D printing is presented in Figure 1. It involves an energetic stimulation to create an elementary volume called a “voxel” (different in terms of the nature of the initial material), which, when moved in space, leads to a series of voxels that only becomes a 3D object if there is sufficient cohesion between the voxels (notion of printability).
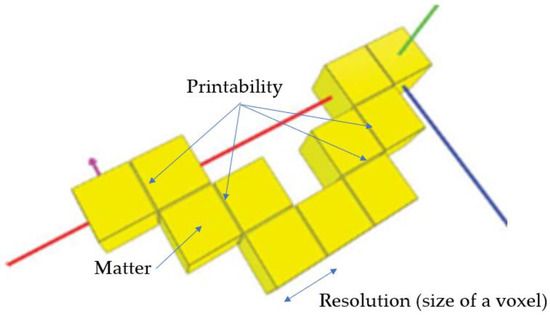
Figure 1. The fundamentals of additive manufacturing.
Because rwesearchers have known for a long time how to “play” with the energy of light, it was first possible to imagine a transformation of matter by the energy carried by light: polymerization by electronic excitation of a resin, thermally induced polymerization, etc. Under these conditions, researcherswe go from a fluid (the resin) to a solid voxel. In the first patent, in 1984 [1], the authors had not yet studied the evolution of the chemically-induced resolution on the final result. Thus, the global resolution is an association between two interdependent phenomena, one related to the light power present at a point in space, the other to the polymerization chemistry (and especially to its exothermicity).
If today there are other ways of energy stimulation (seven major families of additive manufacturing processes) [2], the processes using light retain their interest in many areas such as micro-fabrication, production of prototype parts, bio-printing, indirect realization of parts in their material of use (ceramics, glasses, etc.), etc. Obviously, the performances that are achieved depend on the methodologies that affect the spatial resolution and printability aspects presented above.
2. Resins and Additive Manufacturing
With acrylic type resins, it is possible to carry out chain radical polymerizations. However, these are protected by inhibitors, which, in continuous excitation with a photon lead to complex polymerization kinetics that must be taken into account in the realization of a voxel. This mechanistic aspect is the starting point of this part dedicated to the resins used in stereolithography. Before dealing with the consequences of the light–matter relationships, a singular point is underlined, that of the exothermicity of the polymerization reactions which can be taken advantage of, but with the risk of a loss in spatial resolution. In principle, it is possible to provide energy locally to carry out a chemical transformation (here, a radical chain polymerization). The chemical kinetics of radical polymerization reactions corresponds to a nonlinear process, qualitatively presented in Figure 2 [2][3][2,3]. Photochemical radical polymerization has a threshold behavior. Under these conditions, if one knows how to have a different light intensity from one point in space to another, or a sufficient exposure time, or finally an inhibitor concentration from one element in space to another, etc., the polymerization can be spatially controlled. The weakly irradiated regions do not contribute to the polymerization as long as one is zone I (presented in Figure 2; it corresponds to the total non-consumption of the inhibitors of radical polymerization, in particular of oxygen).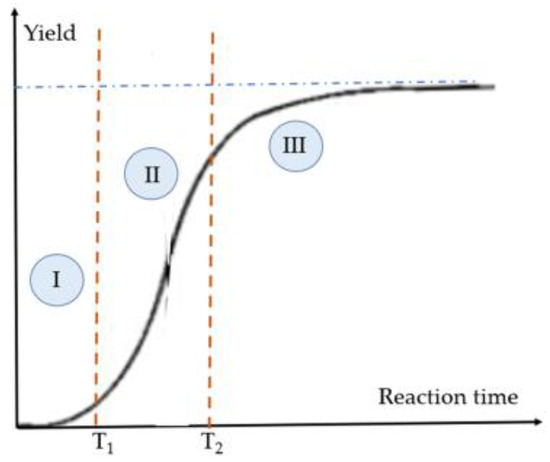
Figure 2. Typical polymerization kinetic curve under continuous irradiation. Phase I: very slow kinetics related to the consumption of the inhibitor(s); phase II: radical polymerization before reaching the gel point; phase III: cross-linking of the multifunctional monomer links.
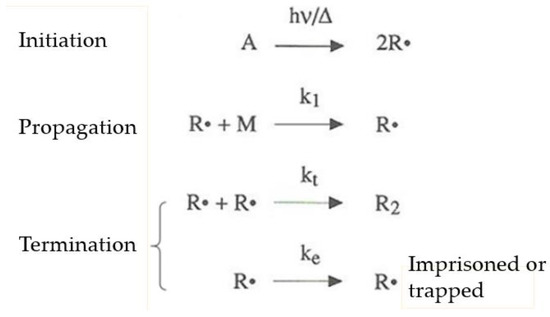
Figure 3. Radical mechanism of chain polymerization (A corresponds to the initiator producing free radicals R. reacting with the monomer M under light (hν) or thermal (Δ) initiation).
R· + Q → Consumption of Q
2.1. Materials
Several situations have been observed since 1984 [1]:-
The photopolymer must not dissolve in the resin that gave birth to it, which requires multifunctional monomers (see Figure 4);
-
The polymer is generally denser than the initial monomer, which leads to shrinkage (as in foundry); and if the object was not supported, it falls (Stokes’ law) to the bottom of the reactor by being deformed;
-
A complex deformation linked to the manufacturing process (anisotropy of local tensions and global deformation);
-
An obligation of post-treatment with the risk of an ageing likely to lead to the destruction in a few days of the realized object.
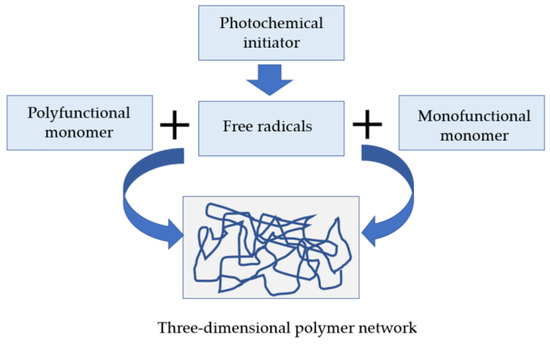
Figure 4. Radical photopolymerization of acrylic resins.
Table 1. Some resins applicable in stereolithography.
| Some Commercial Resins | References |
|---|---|
| Monomers, initiators: Acrylic resins and photochemical initiators (aromatic ketones). Examples: 2-hydroxyethyl methacrylate, 1,6-hexanediol diacrylate, pentaerythritol tetra-acrylate, etc. | Cf. Fluka; Sigma-Aldrich (St. Louis, MO, USA); Quick-Parts; Merck; Arkema; Norland; Yosra; Nanoscribe catalogs (2022) |
| Specific monomer: Ionic polymerization monomer | [5] |
| Commercial resins: Materialise, Acura AMX, Durable natural 3D printing resin (3D Systems), etc. | Materialise (2022); [6]; Additive 3D (2022); Evonik catalog; 3D Ceram (2022) |
2.2. Polymerization
In the presence of light, free radicals are produced; they consume the inhibitors and then react with the monomers to create macro-radicals which grow by reaction with “fresh” monomers with the risk of trapping free radicals in the polymer matrix. Given the concentration of monomers (quasi-pure monomer medium at the time of initiation), the polymerization takes place from close to close, on a molecular scale as shown in Figure 5. In addition, if multifunctional monomers are available, the polymer that is built up is normally insoluble in the monomer that gave rise to it [14].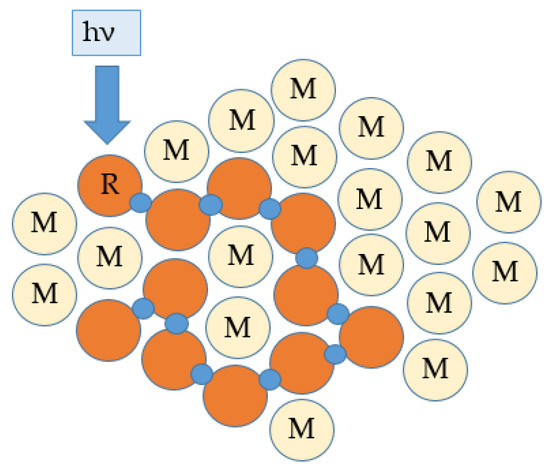
Figure 5. Spatial proximity polymerization (hν represents the energy of the photon, where ν is the frequency of the radiation and h is Planck’s constant, for a one-photon initiation). The red discs represent the elements that have reacted to form a polymer and M represents a monomer molecule.
-
a zone (I) with very slow kinetics where the free radicals formed in the initiation step consume the inhibitors (oxygen);
-
the zone (II) of the polymerization itself;
-
the terminal step, linked to the imprisonment of the free macro-radicals which can no longer reach the reactive bonds of the remaining monomers because of the passage from a fluid material to a highly polymerized entity in which the diffusion of the reactive species is strongly hindered (cf. Figure 5).
2.3. Exothermicity
Polymerization reactions are generally exothermic and there can be a local (and temporary) rise in temperature which can induce a change in the refractive index of the polymerizable liquid and then of the solid state polymer. Depending on the transparency of the resin, it is practically no longer possible to consider that the path of the light follows a straight line beyond several centimeters (loss in resolution or transformation). Under these conditions, it is necessary to build forms of object adapted to avoid the problems mentioned above or to work under spatial and/or chemical conditions where these phenomena are not preponderant or likely to be masked. This goal can be obviously achieved if one wants to create, for example, small objects, which is well suited for commercial picosecond lasers and microscopes. There exists a critical distance for thermal polymerization. To take into account the phenomena involved, it is possible to consider a spherical structure consisting of a polymer at a temperature T1, bathed in a liquid medium containing the monomer and its thermal initiator at a temperature T2, lower than T1. Initiation by AIBN or benzoyl peroxide starts at a temperature between T1 and T2. If the radius of the sphere is small, with a high surface to volume ratio, the heat from the sphere must dissipate before the thermal initiator has time to produce free radicals. On the other hand, for high values of this radius, the heat transfer has time to generate free radicals and provide additional heat energy that drives the polymerization from the edges of the sphere into the space accessible for the reaction. ResearchWers can therefore imagine the existence of a critical radius that corresponds to the escape of the reaction from the space that was intended for it. Moreover, from one voxel to another, the reaction medium heats up, which can lead to minor or major losses of resolution. Then, choosing a space of spherical symmetry and an initial temperature θ0 = 50 °C, Figure 6 plots the spatial variations in temperature as a function of dimensionless time τ (equal to the product of time times the thermal diffusion coefficient divided by the spot of the squared radius R of an electromagnetic wave). With a thermal initiator such as benzoyl peroxide or AIBN [18], after a dimensionless time on the order of unity, the heat, initially carried uniformly in the sphere of radius R, can be considered dissipated throughout the reactant fluid. The polymerization reaction could have started, but stops by quenching (hence no loss in spatial resolution).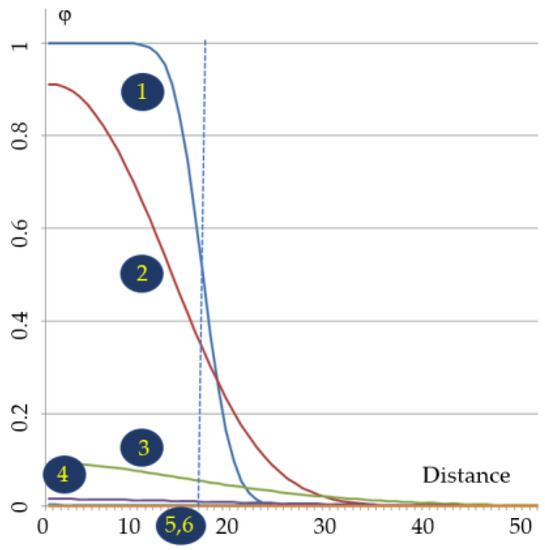
Figure 6. Temperature variations during the dimensionless polymerization time for θ0 = 50 °C. Relative temperature (φ) beyond θ0 is represented on the ordinate; on the abscissa, 100 units represent 5 × R (i.e., in theiour conditions, 0.5 mm). 1: τ = 0.01; 2: τ = 0.1; 3: τ = 1; 4: τ = 2; 5: τ = 3; 6: τ = 5.
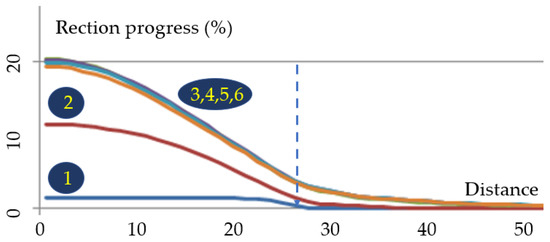
Figure 7. Variations in monomer concentration for different dimensionless times related to polymerization for θ0 = 50 °C and θ1 = 150 °C (by heating the irradiated area). Polymerization advancement (%) is shown on the ordinate; on the abscissa, 100 units represent 5*R (i.e., in theiour conditions, 0.5 mm). 1: τ = 0.01; 2: τ = 0.1; 3: τ = 1; 4: τ = 2; 5: τ = 3; 6: τ = 5.

Figure 8. Thermally cured part in a conductive sleeve. All the material accessible to the exothermic reaction is transformed into the polymer (see photograph on the right).
2.4. Resolution
Provided that a reaction is not degenerate (see Figure 8), i.e., limited to initiation, with chain lengths of about 100 and monomer size of 5 nm, it is under the best possible conditions conceivable to occupy an approximate volume of 100 × 125 nm3, corresponding to a cubic voxel size of the order of 20–25 nm on a side. On this basis, it is not the chemistry that limits the spatial resolution.2.5. Initiation Time
Assuming a time-independent rate of chain-bearing radical production (R0), the Q inhibitors are in a situation where their concentration decreases before cancelling out, corresponding to:R0 = 2kt·(R·)2,
T1 = ((2kt/R0)1/2)/kq
2.6. Composites
The additive manufacturing discussed here results from a transformation of a liquid into a solid under the action of electromagnetic radiation. The fillers that can be included in the monomer must be small [25] for various reasons, at least one of which is related to Stokes’ law, associated with the size of particles subject to the gravitational effect. With large particles, with passive and long-term storage of reagents, all charges can be at the bottom of the fabrication device. RWesearchers also suppress shadow effects that can be detrimental to the mechanical quality of 3D objects thus realized. If the effect associated with Stokes’ law is present in the layers (initial process of stereolithography), the 3D object will have strongly anisotropic mechanical properties. Indeed, additive manufacturing (AM) refers to “a process in which materials are assembled, usually layer by layer, to make an object that conforms to the 3D data that model it” [2][17][2,17]. A structural composite material is generally composed of a reinforcement and a matrix. The reinforcement, most often in fibrous or filamentary form, provides the essential mechanical properties [26]. However, in another context, when it comes to “green” parts, for example in ceramic production, it is not the mechanical strength that is strictly sought but the possibility of a part with little deformation that, by various operations (de-binding, sintering), will lead to the final usable part. The matrix plays the role of binder to protect the reinforcement from the environment, to maintain it in its initial position, and to ensure the transmission of forces. Composite materials can be classified according to the nature of their matrix: organic matrix, ceramic matrix, or metal matrix composite material. In fact, as indicated by [27], all powdered materials can be considered, even lunar dust. This context, as defined above, is based on the principle of printability (deep association of voxels as shown in Figure 1 by adherence), and thus raises the question of the quantity of filler that will allow the desired performance to be obtained in the composite. What happens when the fillers are no longer fully associated with their carrier polymer? With mono-dispersed spheres (simplifying assumption), the interparticle porosity ε represents the fraction of the volumes between spheres relative to the volume of the considered set. If ρapp and ρsol represent the bulk and bulk solid densities, then:ε = 1 − ρapp/ρsol
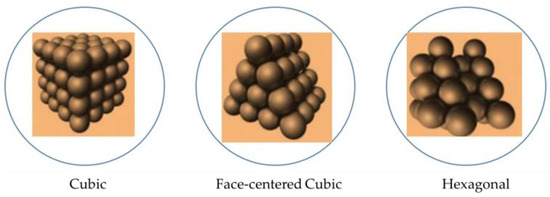
Figure 9. Density linked to regular stacks of spheres (according to http://villemin.gerard.free.fr/Wwwgvmm/Geometri/SpheEmpi.htm, accessed on 22 March 2023)—with the possibility of linking the spheres representing the charges by capillarity and polymerization.
Figure 10. Apollonian stacking.
