You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Filipp Lavrentev and Version 2 by Catherine Yang.
Diffusion is one of the key nature processes which plays an important role in respiration, digestion, and nutrient transport in cells. In this regard, the presearchnt article aims to review various diffusion approaches used to fabricate different functional materials based on hydrogels, unique examples of materials that control diffusion. They have found applications in fields such as drug encapsulation and delivery, nutrient delivery in agriculture, developing materials for regenerative medicine, and creating stimuli-responsive materials in soft robotics and microrobotics.
- diffusion
- hydrogel
- drug delivery
- encapsulation
1. Introduction
Nature has had millions of years of evolution to obtain materials with the necessary properties. In this regard, using approaches implemented in nature is promising for developing new materials [1][2][3][1,2,3]. One of the key processes in nature is diffusion, which ensures the movement of molecules of a substance from an area of higher concentration to an area of lower concentration. Diffusion plays an important role in many processes, such as respiration, digestion, and nutrient transport [4][5][6][4,5,6]. When breathing, oxygen and carbon dioxide move through the membranes of the lungs, and the process itself is possible only due to a concentration gradient. In the process of digestion, the absorption of nutrients occurs through the intestinal membranes due to the difference in their concentration [7][8][9][7,8,9]. It is diffusion that provides our cells with nutrients and removes the products of their vital activity [10][11][10,11].
Thus, diffusion plays an important role in the life of organisms, ensuring the movement of various substances through membranes and maintaining the necessary concentrations of substances to sustain life. Thus, the study of diffusion features under multiple conditions can be used to simulate processes in living matter. Understanding diffusion is crucial for such diverse approaches as the design of new materials that can be used for prolonged controlled release [12][13][14][15][12,13,14,15], self-absorbable hydrogels for regenerative medicine [16][17][16,17], the creation of artificial muscles [18][19][18,19] and robots [20][21][20,21], and many others.
Hydrogels are a unique example of materials that allow control of diffusion and have found their application in wide fields such as encapsulation of drugs [22][23][24][22,23,24] and cells [25][26][25,26], analytical chemistry [27][28][27,28], fabrication of biosensors [29][30][31][32][33][29,30,31,32,33], and biomaterials for the bioactive agents’ delivery [34][35][34,35] as well as stimuli-responsive materials [36][37][38][36,37,38] and soft robotics [39][40][39,40].
All these applications mainly capitalize upon the hydrogel’s ability to restrict a solute’s diffusive movement [41]. Thus, understanding the parameters governing solute diffusion within hydrogels and how they affect diffusion is very important.
2. Diffusion-Controlled Drug Delivery by Hydrogel Systems
Diffusion plays a crucial role in hydrogel drug delivery, as it allows the controlled release of therapeutic drugs to targeted sites. In general, the majority of mechanisms of release are mediated by diffusion kinetics or hybrid processes within the polymeric drug carriers [42][43][47,91]. Usually, the carrier systems are presented by either reservoir or matrix systems, through which the encapsulated drug diffuses by a nonequilibrium concentration gradient. The compartmentalization approach allows shielding of sensitive drug molecules with hydrogel capping shells and ideally provides the payload release directly at the specific site within a certain time and with an accurate amount of loaded drug. Hydrogel-based colloidal networks, or micro- and nanogels, made by crosslinking polymers, are biocompatible and capable of encapsulating small drugs and molecules into the polymer network, making them almost ideal platforms for delivering pharmaceuticals. However, the transportation and targeting processes, fulfilled by different stimuli and environmental conditions, remain critical. The efforts to overcome these challenges and improve the diffusivity of drugs using hydrogel-based drug delivery platforms are discussed in this section. Functionalization and the introduction of compartments can bring about a diverse range of compositions with the possibility of spatial control and regulation of the diffused drug levels. Compartmentalization opens up new ways for variation in the inner structure of a complex hydrogel material, and in general, the compartmentalized compositions for drug delivery can be divided into three groups: (1) micellar/vesicular compartments [44][92], (2) droplet compartments [45][93], (3) multilayer compartments [46][47][94,95]. The presence of charged functionalities in the network is essential for achieving stimuli-responsiveness in hydrogels, a critical attribute for encapsulating and retaining theranostic cargo. Drug-releasing platforms rely on the diffusion of therapeutics from macro-, micro-, and nanogels. Macroscopic drug-encapsulated hydrogels reach several centimeters, with the mesh diameter ranging from 5 to 100 nm [48][96]. The mesh diameter of the polymer network (or the mesh size, as the spacing between polymer chains is usually called) determines the amount and velocity of the encapsulated species diffused through the polymer matrix. Therefore, the mesh dimensions concerning the drug molecule size determine the diffusion limitation. More rapid diffusion occurs when the mesh size is larger than the encapsulated drug (rmesh/rdrug > 1) unless specific bonding forms between the molecule and the polymer chain [49][97]. When the mesh size value is close to the drug size (rmesh/rdrug ≈ 1), steric hindrance significantly impacts drug diffusion. For giant drug molecules compared to the mesh size (rmesh/rdrug < 1), steric hindrance leads to diffusion limitation, and the drug stay trapped within the network. Therefore, the molecule can only be released with the degradation of the network. This immobility of the drug until further net destruction is widely applied in stimuli-responsive hydrogels, which are discussed further in this section. Currently, existing models are already able to predict the diffusivity of solutes through poly(ethylene glycol) (PEG) hydrogels by combining three distinct diffusion mechanisms: (a) hydrodynamic, (b) free volume, and (c) obstruction theory [50][58]. At the same time, the previously employed Stokes–Einstein equation for predicting the diffusivity of solutes in hydrogels did not prove its accuracy, even when the mesh size was significantly larger than the drug size. It was shown that a more accurate diffusivity estimation is obtained when two diffusion modes contribute to the total probability: (i) via accessible volume voids and/or (ii) alongside water through the polymer mesh. The provided model explains the diffusivity more precisely and can be applied to explain the release within the drug carriers, even though the calculations include the solutes as hard spheres and the solutes and the polymer’s intermolecular forces are not considered. In general, the majority of mechanisms of release are mediated by diffusion kinetics or hybrid processes within the polymeric drug carriers [43][91]. Usually, carrier systems are presented by either reservoir or matrix systems, through which the encapsulated drug diffuses by a nonequilibrium concentration gradient. The diffusion rate may be increased by incorporating drugs into the polymer above the saturation point when the drugs remain undissolved within the matrix. For example, in-droplet precipitation of drugs before the polymer solidification or the lower critical solution temperature effect of thermoresponsive polymers and drug crystallization over the saturation level [51][52][98,99], amplifying the amount of the released drug at the disease site, may be applied. These diffusion mechanisms typically contribute to the passive transportation of drug carriers discussed below. In terms of operational mechanisms which determine diffusion modes, there are two primary modes of transporting micro- and nanogels to the intended destination, which are passive and selective transportation [53][100]. Passive transport of hydrogels is based on blood circulation, diffusion, and accumulation at the disease site. Passive transport is a process that does not require cellular energy expenditure and involves the movement of a substance across a membrane, following its concentration gradient. Typically, a phenomenon of the enhanced permeability and retention (EPR) effect [54][55][56][101,102,103] of tumor tissue increases the accumulation effect of passive transport systems (liposomes, nanogels, and macromolecules). These nanoparticles mostly rely on the degradation of the matrix and passive diffusion of entities through the cell membrane down the diffusion gradient, as well as on the enhanced leakage of newly formed blood vessels of the tumor tissues, allowing more accessible accumulation of carrier systems due to the EPR effect. The release of drugs through diffusions is determined by the mesh size, which typically falls within the range of hundreds of nanometers as an optimal size, approximately 100–200 nm [57][104]. The matrix’s degradation and swelling, whether time-dependent or triggered, can modify the retention effect of the drug carriers and, therefore, the efficiency of delivery systems. Although many drug carriers’ issues, such as activation signals, control of the trigger in vivo, and materials’ complexity, have already been presented in various laboratory situations, clinical treatments still rely on passive diffusion systems [58][105]. Hydrogel-based platforms are often decorated with PEG hydrophilic blocks [59][60][61][106,107,108] to multiply the nano- and microgels’ blood circulation cycles due to better phagocytosis resistance and increased retention by the EPR effect [62][109]. However, although the FDA has approved other PEG-modified drug nanocarriers like liposomes that have been on the market since 1995 [63][110], only a few nanogel systems have been taken for clinical studies [64][111]. Currently, the limitations for their widespread application include the fast elimination of nanogels from the body by organs like the kidney, liver, and spleen. Therefore, further designing of the nanogel platforms relies on shape and size optimization and focusing on stimuli-responsiveness as a crucial attribute for more precise and rapid release of the theranostic cargo. Selective transport for drug delivery is developed to control the diffusion and drug release profile. It refers to the ability of a drug delivery system to selectively transport drugs to a specific target site while avoiding nontarget sites. Several ways exist to improve drug delivery processes by amplifying mass transfer between drug-carrying vehicles and cells and reducing systemic toxicity by localized and controllable “on-demand” release [65][112]. Smart hydrogels may sense their microenvironment and react dynamically, mimicking the responsiveness of living organisms. Stimuli-responsiveness may be caused by two large groups of triggers [66][113]: (a) exogenous stimuli (temperature [67][68][114,115], light [69][70][116,117], ultrasound treatment [71][72][118,119], magnetic [73][74][120,121] or electric field [75][76][122,123]) and (b) endogenous stimuli (pH [77][78][124,125], enzyme concentration [79][80][126,127], or redox gradients [81][82][128,129]). Stimuli-activated release affects the properties, shape, structure, or integrity of the polymer matrix, which leads to hydrogel degradation by breakage of the polymer bonds or swelling of the hydrogel and drug release by solute adsorption. In the first case, the network serves as an actuator and degrades, and the mesh size increases, allowing the drugs to diffuse through the hydrogel. Typically, this type of release is determined by a complex mechanism of diffusion–erosion, when the polymer degrades, leaving large pores in the matrix that allow water to penetrate the polymer and intensify the release process. The balance between the diffusion rate and the degradation rate defines the nature of this type of release. When the diffusion rate exceeds the degradation rate, it leads to diffusion-controlled kinetics. When the degradation occurs before the drug release, it results in erosion-controlled kinetics [83][130]. Functional groups, transducing entities, and other environmental sensors play an important role in drug diffusion by matrix degradation. For example, pH-triggered release often includes carboxyl or amino groups, such as polydopamine (MPDA) nanoparticles, explicitly designed for the controllable release of tetracycline [84][131]. pH-dependent carriers are mainly chosen due to the pH variation within the human body from strong acidic to basic. This property may be applied to target specific tissues or organs, which is advantageous as it can provide a sustained release of the drug, leading to better therapeutic outcomes. Medical drugs are frequently released by enclosing them in a polymer that may expand upon fluid absorption and impact the diffusivity of the therapeutics. The drug is discharged from the domain’s boundaries, which may also increase in size as they absorb water or fluids. Swelling and deswelling of the hydrogel enable another diffusion-driven release mechanism by the increased porosity, allowing the encapsulated drugs to diffuse out of the drug carrier freely. Swelling and degradation may be actuated by various stimuli, including pH, temperature, magnetic field, etc. [85][132]. Specific polymer blocks may control the degree of swelling. For example, temperature-responsive polymers (poly(N-isopropyl acrylamide) (PNIPAM) [86][133], poly(N,N-dimethylacrylamide) (PDEAAm) [87][134], poly[2-(dimethylamino) ethyl methacrylate] (PDMAEMA) [88][135]) are designed in a unique way to stretch or shrink. These polymers usually include thermoactuating blocks such as hydrophobic (e.g., isopropyl) and hydrophilic (e.g., amide) groups, which allow the hydrogel to undergo a reversible phase transition, or swelling–deswelling, at a close to physiological temperature. An illustrative example is the thermoresponsive gelatin-based copolymer, designed to perform temperature-controlled prolonged release of T-lymphocyte-associated protein-4 for efficient and durable antitumor immunotherapy, or gingipain-responsive hydrogel (PEGPD@SDF-1) [89][90][136,137]. This effect allows the controlled release of drugs over a prolonged period, increasing their efficacy and reducing side effects. Figure 12 summarizes hydrogels’ described drug release mechanisms and compares their kinetics.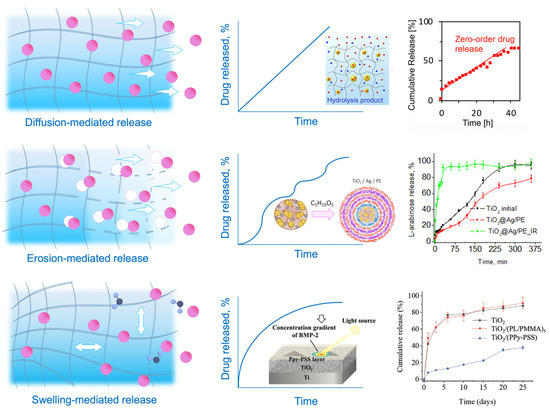
Figure 12. Mechanisms of release and drug diffusion kinetics. Drugs can diffuse with the liquid within the hydrogel [91][138] (diffusion-mediated release), through the increased mesh size by the matrix degradation [92][139] (erosion-mediated release), and through the increased mesh size by matrix expansion and water absorption [93][140] (swelling-mediated release).
