You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Paola Pontrelli and Version 2 by Catherine Yang.
Hypoxia activates hypoxia-related signaling pathways controlled by hypoxia-inducible factors (HIFs). HIFs represent a quick and effective detection system involved in the cellular response to insufficient oxygen concentration.
- hypoxia-inducible factors
- cellular aging
- cellular energy
- immunosenescence
1. Introduction
Aging is a physiological time-related process affecting the majority of living organisms and is characterized by cellular and functional deterioration. It results in an increased susceptibility to the development of many diseases, including cancer, as well as cardiovascular, metabolic, and neurodegenerative diseases [1]. Aging can be considered a mosaic of interactions between abnormal molecular signaling pathways, in which hypoxia plays an important role. Despite atmospheric oxygen levels being nearly 20%, its availability in human organisms is variable, and oxygen is distributed from the respiratory system to every tissue by blood flow; thus, not all cells are exposed to the same oxygen concentration [2]. Low oxygen concentration is associated with senescence, and this condition is characterized by the activation of signaling pathways and transcription factors, termed hypoxia-inducible transcription factors (HIFs), that regulate the expression of several genes involved in aging [3].
HIFs can also influence several aspects of immune cells, from viability to biological function and differentiation. Immune cells are susceptible to hypoxic conditions, and HIFs have a pivotal role in maintaining cellular homeostasis and in supporting high energy-dependent processes in the presence of low O2 concentrations. All immune cells express HIF-1, whereas neutrophils, activated T-cells, and NK cells selectively express HIF-2 [4].
2. HIFs and Immunosenescence
Immunosenescence is characterized by an extensive modification in the innate and adaptive response that leads to decreased immune response in the elderly, involving dysregulation in T-cell response [5][58], deprived B-lymphopoiesis [6][59], and impaired activity of antigen-presenting cells [7][60]. Aging is a significant risk factor for cancer, and the immune system plays a crucial immune surveillance role in the antitumor response but is also directly related to the development and progression of neoplasms. In this context, immunosenescence plays a key role in cancer risk, since the tumor microenvironment (TME) may affect various aspects of the immune system, accelerating its aging [8][61]. Programmed death-ligand 1 (PD-L1), an immunoinhibitory molecule, is encoded by the Pdcdl1 gene on chromosome 9 and is expressed by several immune cells within the TME. It binds programmed death-1 (PD-1), a T-cell co-inhibitory receptor, to promote immunosuppression, resulting in high tumor aggression and evasion of cancer cells from immune response [9][62]. Hypoxia is frequently present in the TME in solid tumors; it induces HIF-driven transcriptional responses in cancer cells, where a direct link between PDL-1 and HIF-1α has been reported [10][63]. Once the immune system is activated, ATP demand in immune cells increases and a metabolic shift is required to support cell proliferation and activity. Since immune cells are not able to use oxidative metabolism to support metabolic needs in hypoxic conditions, glycolysis, an oxygen-independent pathway, is required to supply ATP [11][64]. The first evidence of HIFs’ role in the control of the immune system was reported in 2003 by Cramer et al. [12][65]. Till then, several studies focused on the role of HIFs in the regulation of innate and adaptive immune cells, such as T and B cells, NK cells, dendritic cells, neutrophils, macrophages, and epithelial cells [13][14][15][66,67,68]. Innate immune system cells, such as neutrophils, dendritic cells, and macrophages, undergo phenotypic and metabolic changes in response to low oxygen environment, and activation of HIF precedes these adaptive processes. HIF-1 and HIF-2 affect several functions of macrophages, such as cytokine production, motility, and response to viruses and bacteria [16][17][69,70]. Depending on the stimuli, macrophages can polarize into the pro-inflammatory phenotype, which predominantly uses glycolysis as a source of ATP (M1-type macrophages), and the anti-inflammatory phenotype, which uses oxidative phosphorylation (M2-type macrophages) for ATP generation [18][71]. High expression of HIF-1α induces M1-type macrophages [19][72], which, through glycolysis, leads to accumulation of Krebs cycle intermediate. In particular, high levels of succinate can inhibit PDHs and therefore lead to the stabilization and accumulation of HIF-1α [20][73]. Moreover, upregulation of MTORC1 is associated with M1 polarization [21][74], and a reduction in pro-inflammatory macrophages expression has been observed through the inhibition of HIF-1α and mTOR [22][75]. Hypoxia can also induce gene expression in human and murine macrophages in a HIF-independent way by upregulating NF-kβ (nuclear factor kappa-light-chain-enhancer of activated B cells), activating transcription factor 4 (ATF4) and early growth response-1 [23][76]. Fangradt et al., in fact, demonstrated the presence of HIF-1 in the cytoplasm of primary human monocytes and macrophages, whereas NF-kB1 (p50) was found in monocyte nuclei under hypoxic conditions [24][77]. Dendritic cells (DC) can be considered a key link between innate and adaptive immune systems; thus, it is important to evaluate the role played by hypoxia on DC and its influence on the quality and intensity of immunoreaction. Jantsch et al., demonstrated that hypoxia alone cannot activate murine dendritic cells, but hypoxia combined with lipopolysaccharides (LPS) can induce HIF-dependent changes affecting cell survival, migration, and T-cell activation [25][78]. Nandini et al., demonstrated that hypoxia exposure in human monocyte-derived dendritic cells is associated with upregulation of HIF-1α and downregulation of anti-apoptotic molecule Bcl-2, thus resulting in cellular death. Cell death was not observed in LPS-induced mature DC, despite high expression of HIF-1α via PI3K/Akt pathway; however, the inhibition of this signaling pathway was correlated with the death of hypoxic mature DC [26][79]. Upregulation of glycolysis is associated with activation of DCs via toll-like receptors (TLRs). The glycolytic process in DC can be divided in two phases: acute induction, which occurs within minutes, lasts for hours, and is necessary for early DC maturation; and long-term induction, which is mandatory for metabolic adaptation. HIF-1α activity depends on mTOR and promotes inducible nitric oxide synthase (iNOS) expression during long-term induction, leading to a NO-mediated suppression of DC mitochondrial activity. It has been demonstrated that mTOR inhibition is ineffective in controlling DCs’ acute induction of glycolysis, but it is required for the reduction of NO production, which leads to increased DC mitochondrial activity [27][80]. Although the role of HIF-1α in DCs needs to be more precisely defined, there is evidence supporting its involvement in the activation, maturation, and migration of dendritic cells with a pro-inflammatory profile [28][81]. Hypoxic conditions induce a metabolic switch towards glycolysis and in polymorphonuclear neutrophils (PMNs), as described in macrophages and dendritic cells. PMNs are cells of the innate cellular system. After migration to sites of infection and inflammation, they produce ROS and proinflammatory cytokines like TNF-α, IL-1β, and interferons [29][82]. Moreover, they can entrap and kill pathogens through neutrophil extracellular traps (NETs) [30][83]. During hypoxia, the PMN metabolic switch towards glycolysis is required for mitochondrial ROS production, and this is essential for HIF-1α stabilization [31][84] and the release of NETs. In human neutrophils, a correlation between mTOR-dependent upregulation of HIF-1α and NETs formation has been demonstrated after LPS stimulation [32][85], but excess NET formation and therefore excessive inflammation can damage the host. For example, during sepsis, neutrophil dysfunction has been reported, and the inhibition of glycolysis via downregulation of the PI3K/Akt-HIF-1α pathway seems to contribute to neutrophil immunosuppression [33][86]. Under hypoxic conditions, HIF-1 triggers NF-κB in PMNs to promote the survival of these cells. Increased levels of NF-κB encourage neutrophil activation, which increases the generation of nitric oxide and pro-inflammatory cytokines while decreasing cellular apoptosis [34][87]. Besides HIF-1α, HIF-2 seems to be involved in the regulation of PMNs, and, in particular, its expression is increased in the context of inflammatory diseases [35][88]. In terms of cytotoxicity, natural killer (NK) lymphocytes are the major innate effector cells, especially against tumor [36][89], and the tumor microenvironment is often characterized by hypoxia, which promotes cell proliferation and angiogenesis [37][90]. NK cells are characterized by the presence on their surface of activating and inhibitory receptors, and a balance between the two is essential for the recognition of target cells [38][91]. When exposed to low-oxygen environments, NK cells show impaired cytolytic function [39][92], increased expression and stabilization of HIF-1α, and major responsiveness to IL-2, which mediates NK cell proliferation, in order to execute their functions [40][93]. Upregulation of HIF-1α in NK cells requires both hypoxia and IL-2 stimulation, since hypoxia is implicated in HIF-1α stabilization, whereas IL-2 leads to the activation of PI3K/mTOR signaling, which is vital for HIF-1α protein synthesis [41][94]. The balance between activating and inhibitory receptors might also be negatively affected by hypoxia. This condition leads to an impaired transcription of some of the receptors implicated in NK cell activation, such as NKp46, NKp30, NKp44, and NKG2D, resulting in impaired capacity to kill infected cells or tumor cells [42][95]. NK cells also undergo metabolic reprogramming when exposed to hypoxia in the tumor microenvironment. In patients with liver cancer, NK cells were found with fragmented mitochondria, which causes less oxidative phosphorylation and therefore low ATP production; moreover, they exhibit lower expression of granzyme B. Hypoxic conditions upregulate Drp1, a central player in mitochondrial fission. Zheng et al., highlighted how a low oxygen environment results in constant activation of mTOR-Drp1 GTPase in NK cells in tumor tissue, causing excessive mitochondrial fission into fragments. All of these features are related to reduced cytotoxicity and NK cell loss, which promote tumor evasion of NK cell-mediated surveillance [43][96]. A decline in adaptive immune responses occurs with the aging process, leading to increased susceptibility to infection due to thymic atrophy, a reduction in the number of peripheral blood naïve cells, and an increase in memory cells [44][97]. In older people, T-cell mitochondria are characterized by impaired oxidative phosphorylation even though they contain a larger quantity of protein than younger subjects. This impaired function causes a dysregulation of aged T-cell signaling pathways and leads to the activation of the inflammasome together with an aged T-cell proinflammatory phenotype through the activation of PI3K-AKT-mTOR and MAPK signaling [45][98]. Upregulation of glycolysis is mandatory for effector T-cell differentiation, and the activation of HIF-1α through metabolic reprogramming towards glycolysis can improve effector cell functions. Inhibition of glycolysis results in T-cell anergy and in a shift of effector T cells towards Tregs, whose metabolism is based on lipid oxidation and oxidative phosphorylation [46][99]. The development and differentiation of T lymphocytes is significantly influenced by the transcription factor NF-κB. It participates in the development of T cells from the early stage of thymocyte differentiation to post-selection maturation. It has been proven that there is a cross-talk between HIF signals and NF-κB [47][100], and according to Bruzzese et al., NF-κB activation increases the sensitivity of T cells to hypoxia. Moreover, both signals are important in the differentiation of regulatory T cells [48][101]. HIF-1α has also a key role in B-cell metabolism and functions. B-cell development and differentiation is affected by the different levels of oxygen to which they are exposed, especially in the light zones of the germinal centers that are known to be hypoxic. HIF-1α plays a vital role in hypoxia-induced B-cell cycle arrest and causes an increase in glycolytic metabolism while reducing B-cell proliferation and increasing B-cell death [49][102]. In germinal center B cells, HIF-1 is highly expressed. High-affinity plasma cells were produced as a result of defective class-switch recombination caused by impaired germinal center in response to HIF-1α knockout B cells [50][103]. A cross-talk between HIF, aging, and immune cells is undoubtedly present, and an implication of HIF dysregulation is expected in many different immune-mediated diseases, including sepsis, cancer, and inflammatory bowel disease [51][104]. Therefore, more studies on this topic can lead us to understand the underlying mechanisms of aging in immune cells when exposed to a hypoxic environment. (Figure 13).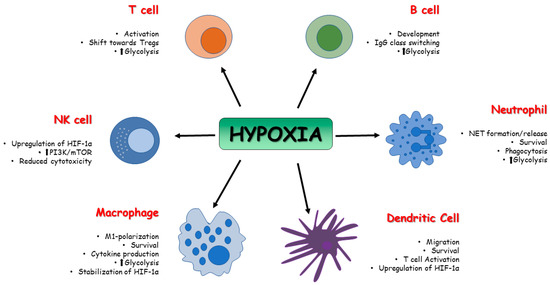
Figure 13. Effect of HIFs on immune cell metabolism. The activity of HIFs affects the function and the metabolism of innate and adaptive immune cells in terms of survival, activation, development, and polarization.
