You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Virgínia Cruz Fernandes and Version 3 by Fanny Huang.
Plastic is an indispensable material in modern society; however, high production rates combined with inadequate waste management and disposal have resulted in enormous stress on ecosystems. In addition, plastics can become smaller particles known as microplastics (MPs) due to physical, chemical, and biological drivers. MP pollution has become a significant environmental problem affecting terrestrial and aquatic ecosystems worldwide.
- microplastics
- plastics
- pollution
1. Introduction
Plastic debris and microplastics (MPs) (defined as plastic particles <5 mm) have been widely found in a variety of environmental media, such as air, water, and soil, and their persistence and complexity are considered major global concerns [1][2][1,2]. MPs are classified as primary if they are industrially produced, or secondary if they are derived from the fragmentation of larger plastic items [3][4][3,4]. Owing to the widespread use and disposal of plastics, as well as the heterogeneity of polymers, the sources of MPs in the environment and the routes of contamination can be difficult to determine. Land-based activities are considered the primary source of MPs, but ocean-related activities such as fishing and shipping can also contribute [5][6][5,6]. Typically, MPs tend to be more prevalent in urbanized areas and regions with ineffective waste management, although they can also be dispersed by the airstream and deposited across water and land [7][8][9][7,8,9]. Information on the presence, fate, and long-term effects of MPs on ecosystems (particularly in freshwater and terrestrial environments) is scarce. Indeed, there is a lack of standardized sampling, pretreatment, extraction, and analysis methods for MPs, making comparisons between studies quite challenging [2][4][2,4]. There is growing evidence suggesting that MPs can have negative effects on individual organisms [10][11][12][13][14][15][16][17][10,11,12,13,14,15,16,17]; for instance, MPs ingested by zebrafish (Danio rerio) can accumulate in the gut, causing inflammation and oxidative stress in tissues [13].
Research and development of effective methods to detect and monitor MPs in various environmental media is essential because of the significant environmental impact and potential health risks associated with them. The standardization of analytical methods would provide more reliable and robust data, which in turn would facilitate the development of effective regulations to mitigate the impact of MPs, promote the use of alternative materials to plastics, and establish defensible waste management practices.
2. Emerging Contamination: From Plastic to Microplastics
Plastics comprise a wide variety of materials that are produced from different sources, namely fossil origins (i.e., crude oil, gas, etc.), renewable (e.g., sugar cane, vegetable oils, etc.), or mineral base (i.e., salt) [18]. Large-scale plastic production began in the early 1950s and products made from these materials are all around playing a fundamental role in a myriad of sectors, such as packaging, construction, electronics, agriculture, textile, cosmetics, among others [19][20][21][19,20,21]. The use of plastics deeply shapes the development of modern society as they bring safety, hygiene, comfort, and well-being [18][20][18,20]. Many types of chemical additives are used in plastic production to improve its properties, including plasticizers, stabilizers, flame retardants, and colorants [22][23][24][22,23,24]. It is important to note that the characteristics that make plastic materials desirable (hydrophobicity, durability, etc.) are those that make them persistent and prevalent in terrestrial, freshwater, estuarine, coastal, and marine environments [2][25][2,25].
In 2021, global plastic production reached 390.7 million tonnes, with Asia being responsible for half of the production followed by North America and Europe. Packaging, building and construction, and the automotive sector were the three biggest end-use markets, with 44%, 18%, and 8% of the consumption, respectively [26]. Fossil-based resins represented 90.2%, and the most produced polymers were polypropylene (PP) (19.3%), low-density polyethylene (PE-LD) (14.4%), polyvinyl-chloride (PVC) (12.9%), high-density polyethylene (PE-HD) (12.5%), polyethylene terephthalate (PET) (6.2%), polyurethane (PUR) (5.5%), and polystyrene (PS) (5.3%). Circular plastics (i.e., recycled and biobased/bioattributed plastics) represent approximately 9.8% of the global production [26]. It was estimated that in 2015, around 8.3 billion tonnes of plastics were produced worldwide, of which 6.3 billion tonnes ended up as waste with 9% being recycled, 12% energetically recovered, and 79% deposited in landfills or leaked out of formal waste collection systems accumulating in terrestrial or marine environments (i.e., single-use packaging) [21][27][28][21,27,28]. According to Plastics Europe, the separate waste collection enables recycling rates 13 times higher than mixed waste collection systems [26].
The different physical properties of each polymer may affect its behavior in the environment. For instance, in the aquatic environment, the tendency of particles to float or settle in sediments is usually related to the density of the polymer [2][9][2,9]. However, buoyant particles of PP and PE can sink and be retained within sediments, which means that even when physical properties are well known, predicting the fate of polymers in the environment is very challenging [2][29][2,29]. From sediments in deep seas to underwater canyon, encapsulated in Arctic sea ice, or massive accumulations at sea surface waters, such as the Great Pacific Garbage Patch, plastic materials have been identified all across the globe [29][30][31][32][29,30,31,32]. Moreover, the ability of plastics to fuse with volcanic rocks, sediments, and organic materials may lead to the formation of solid rocklike structures, the plastiglomerates (Figure 1a) [33][34][33,34]. Furthermore, it has been shown that chemical additives can leach out during the life cycle of the product [22][35][36][22,35,36]. At the same time, due to the hydrophobic surface of plastic materials, they can adsorb other chemical contaminants (e.g., metals, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, and/or pesticides) [22][35][36][37][22,35,36,37]. Synergistic interactions may occur between them, resulting in an enhancement of the toxicity in living organisms [22][35][36][37][38][22,35,36,37,38]. It is possible that if plastic particles were taken up by biota, they could act as carriers for other contaminants along the food chain—a Trojan horse effect [3][37][39][40][41][3,37,39,40,41].
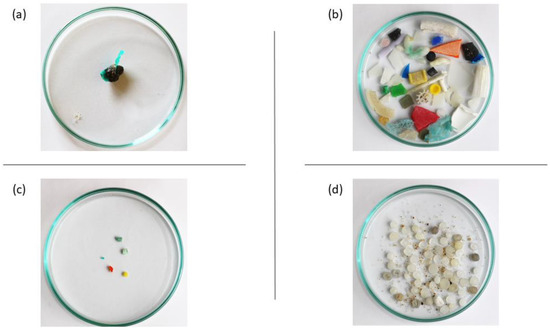
Figure 1. Types of plastic debris found on a beach in Northern Portugal. The plastic materials include: (a) a plastiglomerate, (b) mesoplastic fragments, (c,d) microplastics.
Plastic litter can be degraded by physical, chemical, and biological drivers (i.e., ultraviolet radiation, wind or water erosion, etc.) and become smaller pieces, usually classified by size, namely, megaplastics (>100 mm), macroplastics (>20 mm), mesoplastics (20–5 mm, Figure 1b)), MPs (<5 mm, Figure 1c,d), and nanoplastics (NPs) (1 to 1000 nm), although the adopted terminology can vary [7][39][42][43][44][7,39,42,43,44]. Plastic debris was pointed out as a serious environmental issue in the 1970s, though only in 2004 was the term microplastic applied for the first time [9][45][9,45]. Currently, environmental contamination caused by MPs is in the public domain, being considered an emerging issue of global interest [16][46][47][16,46,47]. MPs fall into two groups: primary MPs if they are produced for industrial purposes, such as PE microbeads used as exfoliating agents in personal care products; or secondary MPs if they are derived from the fragmentation of larger items [6][39][40][6,39,40]. Secondary MPs correspond to the majority of MPs that can be found in the natural environment [3][48][3,48].
Owing to the massive production of plastic materials and the ineffectiveness of waste management and disposal, there is no question about the challenge that plastic pollution represents in our society [36]. The wide variability of plastic types and sizes has hindered the development of standardized extraction and detection methods for MPs in environmental samples [35][49][35,49]. There has been an increase in the development of programs and guidelines for assessing marine litter and in the number of studies on the fate and effects of marine compartments [8][45][8,45]. However, sources, in situ formation, distribution, transport pathways, interactions, and ecotoxicological effects of MPs and other chemical contaminants on the environment, particularly in terrestrial and freshwater compartments, have not received sufficient attention, and the data are scattered [39][45][39,45]. Both have been seen mainly as sinks and transport routes for MPs to reach the seas and oceans [2][6][2,6]. Monitoring programs are generally based on the measurement of chemical concentrations in different compartments. They do not provide useful information on real impacts on organisms and ecosystems, making it difficult to prepare protection and mitigation plans [36][39][36,39]. In Europe, approximately 26 million tonnes of plastic waste are produced annually [50]. To tackle plastic pollution and marine litter and to accelerate the transition to a resource-efficient circular plastic economy, the European Commission has established the European Plastics Strategy as part of the Circular Economy Action Plan and the Framework on Biobased, Biodegradable, and Compostable Plastics [50][51][50,51]. It brings clarity to consumers and the industry on single-use plastics, plastic packaging, MPs, and circular plastics. It is estimated that between 75,000 and 300,000 tonnes of MPs are released into the environment every year in the EU [50]. While there is still no law in place for MPs, the European Chemical Agency has put forward a proposal for a restriction on MPs intentionally added to mixtures, which is currently under discussion with member state authorities and voting. If adopted, this restriction would reduce the quantity of MPs released to approximately 500,000 tonnes over 20 years [52].
3. The Ubiquity of Microplastics in the Environment: Input Pathways and Transport
The sources of MPs in the environment can be numerous, and the routes of contamination can be difficult to define because of the massive plastic production and consumption (especially single-use packaging), the leakage of plastic from waste streams, and the heterogeneity of polymers [2][6][53][2,6,53]. Land-based activities are pointed out as the main sources of MPs, and as environmental compartments are linked, it is expected that contaminants migrate between them (Figure 2) [5][6][5,6]. Moreover, ocean-based activities, such as fishing, aquaculture, and merchant ships, might also release MPs directly into the environment, either by the accidental loss and fragmentation of plastic materials or by illegal dumping of plastic waste at sea [6][41][53][6,41,53]. MPs are likely to be more abundant in urbanized areas as well as in areas where waste management programs are ineffective [2][22][54][2,22,54]. Additionally, lighter plastic items from construction materials, artificial turf, and household dust can be transported by wind action and deposited by atmospheric fallout across water bodies and/or land [8][9][30][8,9,30].

Figure 2. Sources and routes of microplastics and plastic-related chemical pollution in soil environment.
Industrial complexes might contribute to the input of primary MPs into the environment, mostly due to improper handling during production, packaging, and transportation [25][37][25,37]. Some examples are plastic resin pellets (commonly known as nurdles, Figure 1d), flakes, or plastic powder, used as raw materials in the production of larger items, waste from plastic production, regranulate from plastic recycling, and products containing abrasives [37]. Furthermore, wastewater treatment plants (WWTPs) are thought to be an important land-based source of primary MPs, namely plastic particles from personal care products (i.e., hand cleaners or toothpaste, to name a few) and medical products [3][22][25][55][3,22,25,55]. In addition, wastewater may contain secondary synthetic microfibers from laundry (i.e., PA), and other plastic debris that, due to inadequate disposition, end up in wastewater streams [2]. The resulting sewage sludge is often disposed of in soil or converted into compost or biosolids (pasteurized sewage sludge) and applied as fertilizer (Figure 2) [25][28][55][25,28,55]. Regarding the effluents of WWTPs, they can be discharged into the aquatic environment or be used as reclaimed water and are frequently seen as a major source of MPs [3][8][25][39][56][3,8,25,39,56].
Approximately 95% of MPs captured by WWTPs are retained in the sludge phase [8][55][57][8,55,57]. Every day, large amounts of sewage sludge are produced and widely applied to the soil for agricultural purposes [57][58][57,58]. Considering the properties of polymers, it is expected that MPs will remain barely unchanged over time [2][25][59][2,25,59]. For example, the original properties of microfibers, which are considered the most abundant microparticles in natural environments, have been preserved for 15 years after biosolid application [22]. The remaining 5% of MPs that pass through WWTPs are directly and continuously released into aquatic systems [8][25][8,25]. As MPs can be retained within sediments and buried close to the outlet of treated wastewater, not all plastic particles discharged into freshwater are transported to marine environments [22][60][22,60]. For instance, in industrialized areas, rivers may have higher concentrations of MPs than marine environments [22]. Furthermore, when modern WWTP facilities are not available or in the case of an overflow, wastewater is directly input into aquatic systems without any treatment [2][9][2,9]. Nonetheless, it is undeniable that rivers and freshwater bodies are the major carriers of MPs from land to oceans [6][8][6,8]. According to Zhang and Liu, only 1% of global plastic waste is directly input into the marine environment, which means that the largest amount is transferred from freshwater and soil [28].
A direct source of secondary MPs is the fragmentation of meso- and macroplastic litter already present in the environment [1][2][1,2]. Physical abrasion and ultraviolet radiation are considered to be the driving forces that trigger the fragmentation of plastic items by altering their chemical, physical, and mechanical properties [9][61][62][9,61,62]. Another source of secondary MPs is living organisms that mistake plastic for food [63][64][63,64]. For example, caddisfly larvae use external feeding appendages to actively fragment and physically alter plastics [63]. Similarly, earthworms contribute to the biofragmentation of plastic litter, as reported by Kwak and An [64]. In addition, landfills can facilitate the entry of MPs into the environment, either through the loss of plastic materials during waste collection or mismanagement [6][25][54][6,25,54]. MPs can also accumulate in the environment through the fragmentation of tire wear particles, road-marking paints, and particles derived from vehicle components. Tire abrasion particles may be introduced into roadside environments via dust or wash-off, decreasing plant growth even at low concentrations, altering bulk density and soil aeration, and ultimately the biogeochemical cycling [39][54][65][66][39,54,65,66].
The plastic mulching used in agriculture promotes the incorporation of MPs into the environment [25][39][54][25,39,54]. This widespread agricultural technique contributes to improve production, but plastic-related chemicals such as phthalates (plastic plasticizers) can be released into crops along with MPs [39][54][39,54]. Moreover, it is expected that dense polymers remain in the soil and are transported into deeper soil layers by the action of biota, harvesting, ploughing, soil cracking, and wet–dry cycles [64][67][68][64,67,68]. On the other hand, lighter polymers are more likely to be transported across water bodies or land, by the action of wind and water (Figure 2) [8][9][8,9]. It is important to note that plastic debris is not only transported by rivers from land to the ocean; plastic litter in the aquatic environment can return to land due to high tides or floods, reinforcing the idea that pollutants drift between environmental compartments [2][30][39][2,30,39].
