Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Peter Tang and Version 5 by Peter Tang.
Metformin (MF), a first-line drug to treat type 2 diabetes mellitus (T2DM), alone and in combination with other drugs, restores the ovarian function in women with polycystic ovary syndrome (PCOS) and improves fetal development, pregnancy outcomes and offspring health in gestational diabetes mellitus (GDM) and T2DM.
- metformin
- diabetes mellitus
- gestational diabetes mellitus
- polycystic ovary syndrome
- in vitro fertilization
- ovary
- insulin
- gonadotropin
- folliculogenesis
- steroidogenesis
1. Introduction
Metformin (1,1-dimethyl biguanide hydrochloride) (MF), an orally administered biguanide, is a first-line drug for the treatment of type 2 diabetes mellitus (T2DM). It reduces the adipose tissue mass and increases the tissue sensitivity to insulin, thereby reducing hyperglycemia, normalizing carbohydrate and lipid metabolism and preventing inflammation and oxidative stress in the tissues [1][2]. MF is also used to treat non-alcoholic fatty liver disease [3], coronary artery disease [4][5], acute kidney injury and chronic kidney disease [6], in patients with T2DM, metabolic syndrome (MetS) and obesity, and in patients without apparent symptoms of metabolic disorders [7]. There are numerous clinical and experimental studies indicating the effectiveness of MF as an anticancer drug, used to prevent the growth and metastasis in breast cancer [8][9], endometrial cancer [10][11][12], colorectal cancer [9][13], prostate cancer [14] and in a number of the other tumors [15][16].
Currently, there is a large body of evidence for the effectiveness of MF therapy in restoration of reproductive functions and fertility in women with polycystic ovary syndrome (PCOS), gestational diabetes mellitus (GDM) and T2DM, as well as to improve the effectiveness of the assisted reproductive technologies (ART), such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Important attributes of MF use in the treatment of pregnant women with PCOS and T2DM include its lack of teratogenic effect and established positive effect on fetal development, pregnancy outcomes and newborn health. Moreover, convincing evidence has been obtained for the restorative effects of MF on steroidogenic and spermatogenic functions in men with diabetes mellitus (DM) and MetS.
2. Summary of Cell Targets and Molecular Mechanisms of Action of Metformin
The signaling pathways of MF in cells of human and mammals are still not fully understood, they seem to be dependent on species and cell type, as well as doses and routes of administration, along with metabolic and hormonal status of subjects [17][18][19][20][21].
The molecule of MF, a small hydrophilic cation, is transported from the extracellular space to the cytoplasm of the target cell through organic cation transporters-1 and -2 (OCT1, OCT2), multidrug and toxin extrusion transporters (MATE), and ATM (ataxia telangiectasia mutated) transporter, and OCT1 and OCT2 are considered as the main functional units of MF transmembrane transport [22]. The transfer of MF across the placental barrier during pregnancy is largely dependent on the transporter OCT3 [23]. The ultimate intracellular target for MF is the 5′-adenosine monophosphate-activated protein kinase (AMPK), the key energy sensor of the cell, although MF does not interact directly with the enzyme [17][24][25][26]. In pathological conditions, like T2DM and MetS, the activity of AMPK is reduced. MF’s action increases the activity of AMPK, and consequently normalizes the energy metabolism of the target cell. The AMPK consists of a catalytic α-subunit and the regulatory β- and γ-subunits that form a functionally active αβγ-heterotrimeric complex, and is widely distributed in all subcellular compartments (cytoplasmic, lysosomal, mitochondrial, and nuclear). AMPK is activated by increasing levels of AMP, a positive allosteric regulator of the enzyme [25][27][28]. The interaction of AMP with the adenine nucleotides-binding sites located in the γ subunit leads to stabilization of the αβγ heterotrimeric complex and enables phosphorylation of the α-subunit by liver kinase B1 (LKB1), which leads to the increase in AMPK activity [25][26][29] (Figure 1). Activating phosphorylation of AMPK may be also mediated by Ca2+-calmodulin-dependent protein kinase kinase 2 (CaMKK2) [30][31] and transforming growth factor β activated kinase-1 (TAK1) [32][33][34], but LKB1 is most important for AMPK activation [25][28][35][36][37]. Allosteric binding of AMP and ADP to γ-subunit of AMPK increases the ability of LKB1 and CaMKK2 to phosphorylate AMPK α-subunit at the Thr172 [38][39][40]. In the lysosomes, the “non-canonical” pathway of LKB1-mediated AMPK activation is carried out through dissociation of fructose 1,6-bisphosphate from aldolase. At the lysosomal surface, free aldolase promotes the formation of a multiprotein complex, including the vacuolar H+-ATPase and the scaffold protein AXIN, and this complex ensures the effective binding between AMPK and LKB1, thereby activating AMPK [41][42]. A negative regulator of AMPK is the protein phosphatase 2C (PP2C), which dephosphorylates and inactivates the α-subunit of AMPK, causing the dissociation of the αβγ-heterotrimeric complex. Elevated levels of AMP lead to an inhibition of PP2C activity, which allows AMPK to remain stable in the active Thr172-phosphorylated state [43][44].
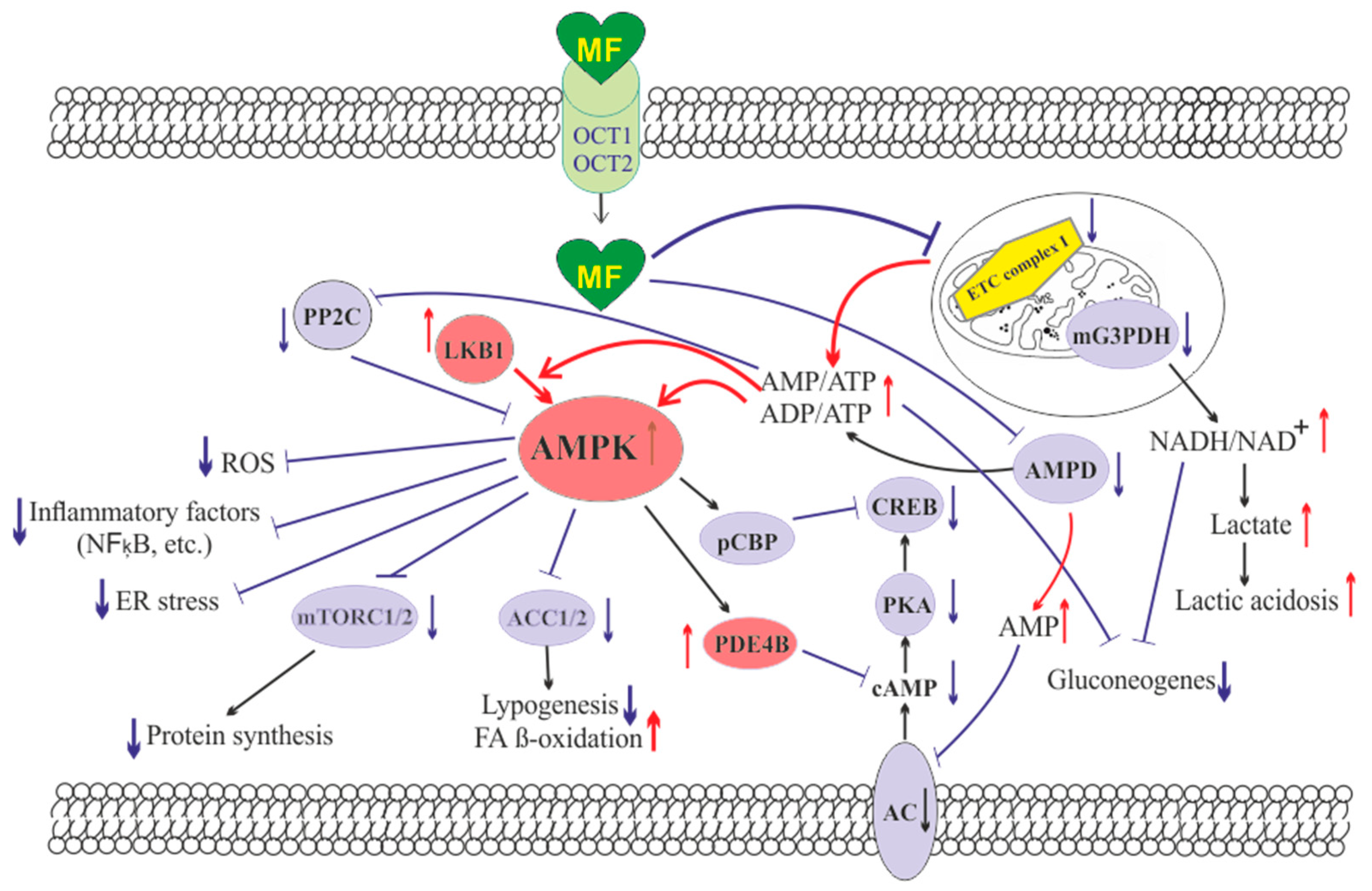
Figure 1. The cellular mechanisms of metformin action which are carried out by activation of the AMP-activated protein kinase and inhibition of the mitochondrial electron transport chain complex I. Abbreviations: AC, adenylyl cyclase; ACC1/2, acetyl-CoA carboxylases 1 and 2; AMPD, AMP deaminase; AMPK, the heterotrimeric AMP-activated protein kinase consisting of the α1/2 (the target for activation phosphorylation at the Thr172), β1/2 and γ1/2/3 subunits; CREB, cAMP-activated transcription factor (cAMP response element-binding protein); ETC complex I, the mitochondrial NADH-dehydrogenase complex, the first complex of the respiratory electron transport chain; FA, fatty acids; LKB1, liver kinase B1; mG3PDH, mitochondrial glycerol-3-phosphate dehydrogenase; mTORC2, the mTOR complex 2; NFκB, nuclear factor κB; OCT1/2, the organic cations transporters 1 and 2; pCBP, the Ser436-phosphorylated form of CREB-binding protein with acetyltransferase activity, a co-activator of the factor CREB; PDE4B, cAMP-specific 3′,5′-cyclic phosphodiesterase 4B; PKA, cAMP-dependent protein kinase; PP2C, protein phosphatase 2C; ROS, reactive oxygen species.
MF penetrates into the mitochondria through intracellular space and accumulates in them. While in the mitochondria, MF inhibits the mitochondrial ETC complex I, which leads to decrease in ATP production and increase in the [AMP]i/[ATP]i and [ADP]i/[ATP]i ratios [45][46][47][48]. Moreover, MF decreases the activity of the enzyme AMP-deaminase (AMPD), which converts AMP to inosine monophosphate, inducing the accumulation of AMP within the cell [49]. The MF-induced increase in the intracellular AMP level leads to the activation of AMPK as described above [35][50]. The MF effect on AMPK activity is observed at drug concentrations below 80 μM, which are achieved with oral administration of therapeutic doses of MF [51]. The MF-induced activation of AMPK results in the stimulation of energy-producing catabolic pathways that mediate the increased glucose uptake by cells, the increased expression and activity of the membrane glucose transporters, the activated metabolic processes such as glycolysis and oxidative phosphorylation, and the normalization of mitochondrial biogenesis [18][52][53][54]. The MF-induced AMPK stimulation leads to phosphorylation of types 1 and 2 acetyl-CoA carboxylases (ACC1 and ACC2), inducing an inhibition of lipogenesis and stimulation of the β-oxidation of free fatty acids [55][56][57] (Figure 1). The ultimate results of this metabolic cascade is the decrease of T2DM- and MetS-produced dyslipidemia, and the normalization of lipid metabolism. In addition, the AMPK activation induces a plethora of cellular events, including regulation of autophagy and apoptotic processes, a decrease in the activity of inflammatory factors, including nuclear factor κB (NF-κB) and interleukin 1β, an inhibition of the ROS production, a decrease in the ER stress, as well as a decrease in insulin/IGF-1-induced activation of the mTORC1/2 complexes and a decrease in the protein synthesis [55][57][58][59][60][61].
The MF is a functional antagonist of cAMP-dependent signaling cascades, which are stimulated by hormones, glucagon in particular, through the Gs protein-coupled receptors and the membrane-bound forms of adenylyl cyclase (AC) [62][63]. The stimulation of AC results in an increase in the intracellular cAMP level and the activation of the protein kinase A (PKA) and the cAMP-activated transcription factor CREB (cAMP response element-binding protein). The MF-induced activation of AMPK promotes phosphorylation and activation of cAMP-specific 3′,5′-cyclic phosphodiesterase 4B (PDE4B), thereby reducing the intracellular level of cAMP [63]. Moreover, MF causes an increase in the intracellular level of AMP, a negative regulator of the catalytic site of AC, which leads to inhibition of AC activity and a decrease in cAMP production. An increase in the level of AMP can be the result of both inhibition of the mitochondrial ETC complex I, and suppression of the activity of AMP deaminase [49][64] (Figure 1). A decrease in the activity of cAMP-dependent pathways in the liver, like activation of AMPK, leads to the inhibition of glucose synthesis in hepatocytes. Furthermore, MF-induced AMPK activation induces the protein kinase ι/λ-mediated phosphorylation of cyclic AMP response element binding (CREB)-binding protein (CBP or CREBBP) at the Ser436, which leads to the inability of the phospho-CBP to form a functionally active complex with the factor CREB and thereby inhibits the cAMP-dependent gene transcription [65].
Along with AMPK-dependent, there are also AMPK-independent pathways of MF action on the intracellular effector systems and gene expression. High-dose MF inhibits the activity of the mitochondrial glycerol-3-phosphate dehydrogenase (mG3PDH) [66]. The inhibition of mG3PDH leads to an increase in NADH levels and decreases NAD+ levels, and this causes a deficiency in NAD+, which is involved in the conversion of lactate to pyruvate (Figure 1). Since a decrease in mG3PDH activity inhibits the conversion of lactate to glucose, the result of impaired gluconeogenesis in hepatocytes is an accumulation of lactate, which can cause lactic acidosis in the conditions of high-dose MF treatment [66][67]. Another target of MF is the enzyme H3K27me3-demethylase KDM6A/UTX, which is responsible for the transcriptional activity of a large number of genes [68].
The antidiabetic effects of MF may be due to the changes in the gut microbiota, due to stimulation of the growth of bacteria that produce short-chain fatty acids [69]. By modulating the composition of the microbiota in rodents with T2DM and MetS, MF reduces the levels of bacterial lipopolysaccharides in the blood [70], and activates AMPK-dependent pathways in the mucosal layer of the intestine, reducing glucose absorption [71].
The most important mechanism of action of MF on target cells is the enhancement of the insulin signaling pathways and the decrease in insulin resistance (IR). This may be due to inhibition of hyperactivated nuclear factor κB (NF-κB), a transcription factor that provokes the development of IR, as well as a decrease in the expression of the phosphatase and tensin homolog (PTEN), which dephosphorylates phosphatidylinositol-3,4,5-triphosphate and thereby prevents insulin-induced stimulation of Akt kinase, a key effector component in the 3-phosphoinositide signaling pathway. The inhibitory effect of MF on the activity of NF-κB-dependent signaling pathways is carried out mainly through the stimulation of AMPK [19][72][73]. Since NF-κB plays a key role in inflammatory reactions, its inhibition by MF promotes the weakening of inflammation and increases the cell survival, and these effects of MF are prevented by AMPK inhibitors [19][74][75].
3. Metformin and Polycystic Ovary Syndrome
3.1. Pathophysiology of Polycystic Ovary Syndrome
The PCOS occurs in average from 9% to 18% of women of reproductive age and includes a number of metabolic and endocrine dysfunctions [76]. Some of them are: (i) the ovarian dysfunction, characterized by irregular or no ovulation (oligo- or amenorrhea), the increased secretion of androgens (hyperandrogenism, HA) and estrogens, the endometrial hyperplasia and the increased size of the ovaries, (ii) the pancreatic dysfunction leading to insulin hypersecretion and, as a result, to insulin resistance (IR) development, (iii) the adrenal dysfunction, which leads to hyperproduction of androgens, and (iv) the functional changes in the hypothalamic and pituitary links of the female hypothalamic-pituitary-gonadal (HPG) axis [77][78][79][80]. Since these dysfunctions and changes are usually associated with obesity, MetS and T2DM, the PCOS is much more common in women with these metabolic disorders (on average in 30% of cases), with a significant proportion of PCOS patients having IR with accompanying compensatory hyperinsulinemia [81][82][83][84][85][86]. According to the Rotterdam criteria (2003), the main diagnostic criteria for PCOS are clinical or biochemical HA, oligo- or amenorrhea associated with chronic anovulation, and morphological features of PCOS, which include 12 or more follicles (2 to 9 mm) in each ovary and/or an increase in ovarian volume over 10 mL [87][88][89]. It should be noted that about 80% of women with anovulatory infertility have typical signs of PCOS [76].
The etiology and clinical manifestations of PCOS depend on many factors, as well as combinations and interactions between them. The genetic predisposition [90][91][92][93] and epigenetic factors, including an increased level of gene methylation, histone modification, and microRNA pattern variation [94][95][96], are important for the development of PCOS. Environmental and socioeconomic factors are also of great importance, including ethnic characteristics, nutrition, and adverse environmental factors (toxins, xenobiotics, chemical mutagens, and ionizing radiation) [97][98][99]. The development of PCOS in women largely depends on the effects of maternal hormones during the prenatal period, as well as on their metabolic and hormonal status in the early childhood [79][94][100][101][102].
3.2. The Use of Metformin in PCOS Women
In recent years, MF therapy has become widely used for correction of the metabolic and hormonal impairments in women with PCOS and for restoration of their reproductive functions [80][103][104][105], including the improvement of IVF/ICSI outcomes in PCOS [106][107][108][109]. MF is most effective in treating PCOS patients with the metabolic disorders such as T2DM, obesity, dyslipidemia, and severe IR [80][110][111]. This is majorly attributed to the alleviation of negative effects of these disorders on the female reproduction by MF, increased tissues sensitivity to insulin, improved lipid and glucose metabolism and cell metabolism, and reduced inflammation and oxidative stress in the ovaries as well as in other tissues. In cases where significant metabolic changes in PCOS patients are not observed during treatment, MF therapy can lead to energy and hormonal imbalance. The outcomes may be the opposite of improvement, but a further deterioration in reproductive functions. This possibility is supported by the data from clinical trials on metabolic changes, including an increase in fasting glucose clearance and endogenous glucose production [112][113], as well as changes in the microbiota in non-diabetic individuals [114], as well as data on metabolic and hormonal dysfunctions in normal rodents, for a long time receiving MF [115].
There is a lot of clinical evidence of the high efficacy of MF in PCOS, which makes it feasible to consider MF as a second-line drug for ovulation induction in women with PCOS [104][116][117][118][119][120][121][122][123]. MF is recommended for the induction of ovulation in PCOS women who are either resistant to clomiphene citrate (CC) or require antiandrogen therapy without the use of contraceptives [120], as well as in PCOS patients with severe obesity and impaired lipid metabolism [109]. One very important consideration during PCOS treatment with MF is that drug has no or little adverse effects on the outcomes of pregnancy as well as the health of fetus and newborn, which indicates the safety of MF therapy [121][122]. The gastrointestinal side effects of MF have been reported in a number of cases, but these effects did not significantly affect the health of PCOS women [103][107].
The MF treatment of PCOS women normalizes the frequency and regularity of ovulation, including when co-administered with exogenous gonadotropins [107][124][125]. This suggests that MF can also affect the sensitivity of ovarian cells to gonadotropins, which is important for the ART. As a result, during the ART, the most promising approach is the combined use of MF with gonadotropins [106][108]. In PCOS, MF improves clinical pregnancy rates and live birth rates [103][106][107][108][126][127][128][129][130][131], and also reduces the number of miscarriages and increases the rate of embryo implantation [132][133].
There is evidence of a positive effect of MF on the effectiveness of IVF and IVF/ICSI in PCOS women [109]. It is believed to be due to the normalization of metabolic and hormonal parameters and the androgen levels in PCOS, which leads to an improvement of embryo implantation, an increase in the ovarian response to gonadotropins and a decrease in the rates of miscarriage [107][130][133][134][135][136]. The increased gonadotropin sensitivity allows avoiding the use of high-dose gonadotropins and, thereby, preventing the ovarian hyperstimulation syndrome (OHSS), a severe complication of gonadotropin-induced ovulation induction. However, it should be noted that some data on the use of MF in the ART technology in PCOS women are not so unambiguous, and there are results that do not support the efficacy of MF in IVF/ICSI. The clinical studies carried out by Egyptian group of physicians showed no improvement in IVF rates in PCOS women who received MF [137]. However, in this study, overweight or obese PCOS women received short-term courses of low-dose MF (1000 mg/day), from the start of ovarian stimulation with gonadotropins until proof of clinical pregnancy. As a result, in this case, the period of time for the manifestation of the restorative effects of MF on the ovaries and folliculogenesis in PCOS patients may not have been long enough. Potentially, for an adequate estimation of MF effectiveness in PCOS patients it is necessary to separate them in the groups, based on the severity and duration of the disease and in the body mass index [103][109], as well as the severity of IR, dyslipidemia and hyperglycemia.
3.3. Combined Use of Metformin with Clomiphene Citrate, Letrozole, Liraglutide, Saxagliptin, or Oral Contraceptives
A promising approach to treat PCOS is the use of combination of MF with the other drugs that improve the ovarian function and metabolic parameters in PCOS, with the best candidates for co-administration are CC, a mild nonsteroidal estrogen antagonist belonging to the family of selective estrogen receptor modulators, and letrozole, a non-steroidal aromatase inhibitor that prevents the conversion of androgens to estrogens [111][126][138][139][140][141][142][143].
The CC is the main drug of choice for treatment of PCOS, yet a significant proportion of PCOS women have weak or no response to CC therapy. Therefore, a search is underway for drugs that can potentiate the therapeutic effects of CC in PCOS, and MF is one of the most promising candidates [103][126][129][138][139][141]. Combined use of MF plus CC in PCOS showed significant improvement in clinical indices of pregnancy and the combination therapy is more effective than the use of CC alone. However, a number of studies reported no effect [144] or relatively weak potentiating effect of MF for CC therapy [129]. One of the possible reasons for these contradictory results may be the difference in the sensitivity to CC and MF in PCOS patients. The most profound potentiating effect of MF on the induction of ovulation and pregnancy rates is found in patients with a pronounced resistance to CC [139][141][145]. However, some PCOS patients may be also insensitive to MF, which is due to many factors, including the polymorphisms and inactivating mutations in the transmembrane proteins facilitating intracellular transport MF [146]. As a result, the combined therapy is expected to benefit mainly PCOS patients with reduced sensitivity to CC, pronounced obesity, IR and dyslipidemia, and high sensitivity to MF.
In recent years, the data have been obtained for the effectiveness of the combined use of MF and letrozole, an aromatase inhibitor that is widely used to restore the ovarian cycle and induction of ovulation and improves oocyte implantation and pregnancy rates in women with PCOS, primarily those with reduced sensitivity to CC [139][143][145]. The combined therapy with MF demonstrated enhancement for the improving effects of letrozole on the pregnancy and live birth rates. Moreover, there are clinical results showing that the combined use of letrozole and MF is more effective than the combined use of CC and MF [140][142].
In PCOS patients, the efficiency of MF therapy is increased when MF is used with oral estrogen-progestin contraceptives, both acting similar, by suppressing ovarian androgen overproduction and normalizing menstrual cycle, most noticeably in obese PCOS women [147][148]. On a contrary, when the same combined treatment (MF and oral contraceptives) is used for PCOS women with normal or reduced body weight, it results in a decrease in their muscles mass, leads to the water retention and the formation of an “osteosarcopenic” phenotype [149]. Two main reasons are behind the decrease in the muscles mass during combined therapy. First, MF and oral contraceptives reduce the blood androgen levels. It is known that in PCOS there is a significant positive correlation between the blood level of androgens and the muscles mass [150]. Second, MF-induced activation of AMPK and changes in mitochondrial energy status stimulate catabolic processes in the muscles tissue, which leads to muscles atrophy, as shown in patients with T2DM [151]. In this regard, it should be noted that MF treatment of T2DM patients leads to an increase in the blood level of fibroblast growth factor 21 (FGF21), which is one of the specific markers of muscles damage and degeneration [152]. Thus, it is highly recommended to take into account the proportion of the muscle tissue and body mass index in PCOS women, as well as the severity of HA when considering the option of using the combined therapy of MF and oral contraceptives [149].
The agonists of glucagon-like peptide-1 (GLP-1) receptor and the inhibitors of dipeptidyl peptidase-4 are widely used to treat T2DM and MetS [153][154][155][156], but they can also be used to correct the metabolic alterations and IR in PCOS women, as well as in pregnant women with GDM and T2DM [157][158]. It is shown that MF enhances the beneficial effect of liraglutide, a selective GLP-1 receptor agonist, on insulin sensitivity and glucose homeostasis. The 12-week treatment of 30 obese PCOS women with a combination of MF (1000 mg twice a day) and liraglutide (1.2 mg/day) causes a decrease in IR and normalizes the sensitivity of patients to glucose, and the combined therapy was more effective than monotherapy [159]. The treatment of premenopausal PCOS women with MF (2000 mg/day), saxagliptin (5 mg/day), an inhibitor of dipeptidyl peptidase-4, or a combination of MF and saxagliptin leads to normalization of glucose tolerance on average of 56% of patients [160]. Moreover, in the group treated with MF alone or saxagliptin alone, the improvement of glycemic control is demonstrated only in 25 and 55% of patients, respectively, while the combined therapy restores glucose tolerance in 91% of women with PCOS [160]. A high efficacy of the combined therapy was shown by other group of authors who monitored the 16-week treatment of 38 women with pre-diabetes and PCOS using the MF plus saxagliptin [161]. Weight loss and decrease in hyperglycemia and IR, which are induced by treatment of obese PCOS patients with GLP-1 receptor agonists, lead to a decrease in HA [162][163][164] and an improvement in menstrual frequency [162][164]. Liraglutide, an analogue of GLP-1, normalizes the menstrual cycle and fertility in women with HAIR-AN syndrome, which is due to a decrease in the levels of androgens and insulin [165]. Consequently, in PCOS patients, MF-induced potentiation of the metabolic-improving effects of GLP-1 agonists may also increase their restorative effects on the menstrual cycle and fertility.
3.4. The Mechanisms of Metformin Effects on Reproductive Functions in PCOS
3.4.1. Metformin-Induced Inhibition of Hyperandrogenism and Normalization of the Steroid Hormones Balance
One of the main mechanisms of the restorative effect of MF on ovarian function, ovulation and pregnancy in PCOS women mediates through the pronounced antiandrogenic effect of MF, both in monotherapy and in combination with other drugs [136][147][166][167][168][169][170][171][172][173][174]. One-year treatment of overweight PCOS women with MF (1700 mg/day) reduced the levels of free testosterone, dehydroepiandrosterone (DHEA) and androstenedione, and significantly weakened the signs of hirsutism. This effect of MF was strongly associated with a decrease in the homeostasis model assessment of insulin resistance index (HOMA-IR) and an improvement in glucose tolerance, but was weakly associated with a decrease in the body weight, which indicates a main contribution of a decrease in IR and hyperinsulinemia to the antiandrogenic effect of MF [175]. An antiandrogenic effect was demonstrated in the treatment of overweight and obese adolescents with MF (1000–2000 mg/day), and was accompanied by a significant decrease in IR [147][168][169][171][172]. MF reduced both the basal and gonadotropin-stimulated testosterone levels, and these effects were observed even with short-term MF treatment. The administration of MF for two days to PCOS women caused a decrease in their testosterone levels stimulated by luteinizing hormone (LH). This effect was not due to a decrease in the body weight and the changes in metabolic indices, pointing to potential direct influence of MF on steroidogenic activity in ovarian cells [176].
In PCOS, the severity of IR is positively correlated with the severity of HA and dysregulations of the ovulatory cycle. The PCOS women with oligomenorrhea and without HA usually do not have IR, while the PCOS women with oligomenorrhea and HA often show significant signs of IR [177]. In turn, in PCOS women with regular ovulatory cycle, IR was less pronounced than in women with PCOS and irregular or no ovulation [178].
The inhibitory effect of MF on the production of steroid hormones by ovarian cells was demonstrated in the in situ experiments using different cell lines [89][179][180][181]. Cultured human ovarian cells grown in the presence of MF, showed a decrease in production of basal and gonadotropin- or insulin-stimulated steroid hormones. Similar effects were shown for progesterone and estradiol in granulosa cells and androstenedione in theca cells. Inhibitory effect of MF was dose-dependent and most pronounced in the measure of suppression of hormone-stimulated steroidogenesis [180]. MF (10 mM) treatment of bovine granulosa cells isolated from small follicles led to a decrease in both the basal and follicle-stimulating hormone (FSH)- and IGF-1-stimulated production of progesterone and estradiol [181].
When deciphering the mechanisms of the inhibitory effect of MF on steroidogenic activity in the ovaries, a key role is assigned to stimulation of AMPK, and the triggering of AMPK-dependent pathways in ovarian cells [89][181][182] (Figure 2). The mechanisms of AMPK activation in ovarian cells are the same, and are triggered by MF-induced inhibition of electron transport chain in mitochondrial respiratory complex I [183]. It is worth noticing that in humans, other mammals and birds (cows, goats, sheep, pigs, rats, mice, chicken), AMPK is widely expressed in different types of the ovarian cells (oocyte-cumulus complexes, granulosa cells, and theca cells) and in the corpus luteum [181][182][184].
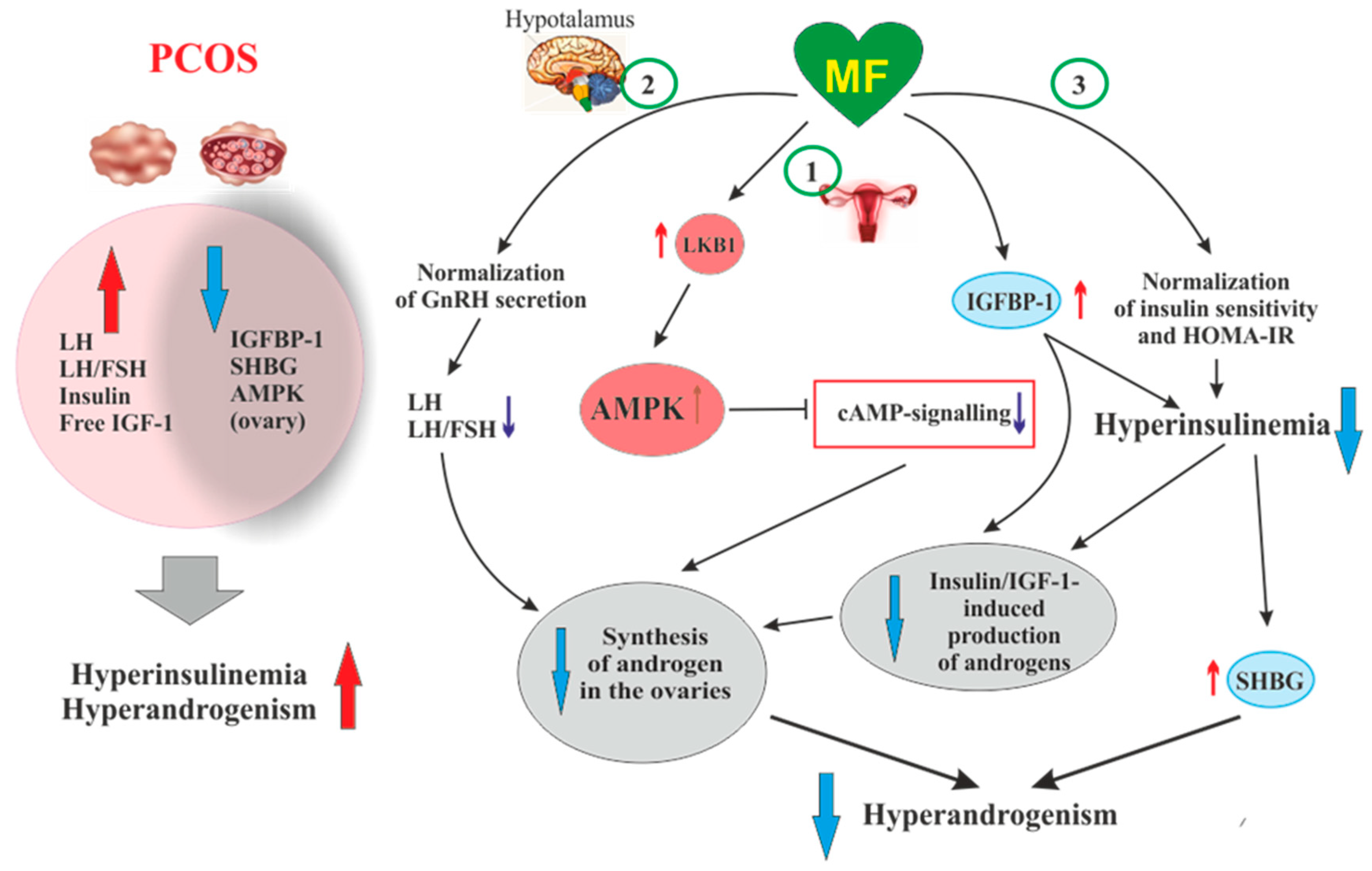
Figure 2. The pathways involved in the inhibitory effect of metformin on hyperandrogenism in PCOS. Hyperinsulinemia and HA are among the key pathogenetic factors in the development of PCOS, which is why, their attenuation by MF is the most important mechanism for improving effect of this drug on ovarian function in PCOS women. In PCOS, MF-induced increase in insulin sensitivity leads to a decrease in the HOMA-IR and a weakening of compensatory hyperinsulinemia. Another mechanism for lowering insulin levels may be an increase in the level of IGFBP-1, which specifically binds insulin and IGF-1. In PCOS, the expression of IGFBP-1 is generally reduced, and MF treatment may be one way to normalize it. A reduced hyperinsulinemia and an increase in IGFB-1 levels lead to a decrease in the stimulating effect of insulin and IGF-1 on the ovarian steroidogenesis and a weakening of HA. Hyperinsulinemia leads to a decrease in the production of SHBG, which provokes HA in PCOS. MF-induced reduction of hyperinsulinemia leads to the normalization of the SHBG levels, thereby preventing excess androgen levels in the blood. By improving the functionality of the hypothalamic signaling network responsible for the pulsatile secretion of GnRH, treatment with MF leads to the normalization of blood LH levels and the LH/FSH ratio, both of which are increased in PCOS. A decrease in blood LH levels results in a weakening of gonadotropin-induced androgen production by the ovaries. A direct regulatory effect of MF on ovarian steroidogenesis was also established. By inhibiting the mitochondrial ETC complex I, stimulating the LKB1 activity and, as a result, increasing the AMPK activity, MF reduces the synthesis of androstenedione in the ovarian cells and prevents HA. It can be assumed that the prevalence of some mechanisms of the inhibitory effect of MF on HA is due to the characteristic features of PCOS pathogenesis and the metabolic and hormonal status of the ovaries. Abbreviations: AMPK, AMP-activated protein kinase; FSH, follicle-stimulating hormone; HA, hyperandrogenism; HOMA-IR, homeostasis model assessment of insulin resistance; IGF-1, insulin-like growth factor-1; IGFBP-1, insulin-like growth factor-binding protein-1; LH, luteinizing hormone; LKB1, liver kinase B1; SHBG, androgen and sex hormone-binding globulin.
There is a large body of experimental data that AMPK is essential for the regulation of folliculogenesis and meiotic activity, both control the maturation of oocytes [181][185][186][187][188][189], and that AMPK is involved in the regulation of steroidogenesis in ovarian granulosa cells [182][190]. Deletion of the AMPK α1-subunit in mouse oocytes leads to a 27% decrease in litter size, and after IVF, the number of embryos in these mutant mice decreases by 68% [191]. In the ovaries of mutant mice, the levels of transmembrane connexin-37 and N-cadherin, which mediate the intercellular communication and are involved in the formation of the oocyte-cumulus complexes, were significantly reduced. The activity level within cAMP-dependent cascade, which includes PKA and factor CREB, and the activity of mitogen-activated protein kinase (MAPK) cascade are reduced, indicating weakening of cAMP- and MAPK-mediated signal transduction [190][191]. The components of these signaling pathways are involved in the junctional communication between the oocyte and the cumulus/granulosa cells. The MII oocytes in mice lacking the α1-AMPK have a significantly reduced intracellular ATP level and decreased levels of cytochrome c and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α), which indicates the impaired mitochondrial biogenesis and the activation of apoptotic processes [191].
In ovarian cells, through the activation of AMPK, MF inhibits the cAMP signaling pathways, decreases the expression and activity of the steroidogenic enzymes and the production of androstenedione, a precursor of testosterone (Figure 2). When exposed to cultured human theca cells, both in the basal and forskolin-stimulated state, application of MF (50 and 200 μM) caused the AMPK stimulation and reduced androstenedione synthesis in dose-dependent manner. In theca cells stimulated by forskolin, a non-hormonal AC activator, MF suppressed the expression of StAR and Cyp171a genes encoding StAR protein, which carries out cholesterol transport into mitochondria (the first, rate-limiting stage of steroidogenesis), and cytochrome P450c17α, which catalyzes the synthesis of androstenedione [179]. The inhibitory effect of MF on steroidogenesis in the rat and bovine granulosa cells was also due to AMPK activation, as indicated by an increase of Thr172 phosphorylation of AMPK α-subunit, as well as an increase of Ser79 phosphorylation and inhibition of the main target of AMPK, the enzyme acetyl-CoA-carboxylase [181][192]. The involvement of AMPK in the antiandrogenic action of MF is supported by the data on a similar effect of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), the pharmacological activator of AMPK [181]. The MF-induced stimulation of AMPK in granulosa cells results in a decrease in the activity of MAPK cascade, primarily the kinases ERK1/2, and inhibition of phosphorylation of MAPK-activated protein kinase-1, also referred to as ribosomal s6 kinase (p90RSK) [181][192][193]. Therefore, through the AMPK-dependent mechanisms, MF not only reduces the excessive steroidogenic activity in ovarian cells, but also suppresses and/or modulates the cell growth and intracellular protein synthesis.
An important role of AMPK-dependent mechanisms in the antiandrogenic action of MF in PCOS is supported by the data on the relationship between the androgen production and the activity of LKB1 in the ovaries of mice with experimental HA [194]. The LKB1 expression in the ovaries of hyperandrogenic mice is inhibited by high concentrations of androgens through activation of intracellular androgen receptors. In opposite, LKB1 activation leads to a decrease in the androgens production by theca cells, but increases the estrogen production by granulosa cells. Transgenic mice overexpressing LKB1 are characterized by the increased resistance to the development of HA [194]. Mice with a functionally inactive ovarian gene Lkb1 have significantly enlarged ovaries and activated entire pool of primordial follicles, but without further maturation and ovulation, which results in the premature ovarian failure and severely reduced fertility [195]. The data indicates that MF-induced activation of LKB1/AMPK pathway in PCOS ovaries normalizes ovarian steroidogenesis and counteracts HA (Figure 2).
Another, very important mechanism of antiandrogenic action of MF is largely due to an MF-induced increase in insulin sensitivity and consequent weakening of compensatory hyperinsulinemia, the main pathogenic factor in PCOS, also closely associated with HA [196][197][198] (Figure 2).
3.4.2. Protective effect of Metformin against Excess Androgens in PCOS
In addition to reducing HA in PCOS, MF is able to prevent the negative effect of excess androgens on the ovarian cells [199][200]. It was shown in mice model of PCOS, that treatment using MF (500 mg/kg, 20 days) after DHEA induction improved the quality of oocytes and normalized the early stages of embryonic development. In the ovaries of MF-treated mice, the restoration of the number of metaphase II oocytes, mitochondrial membrane potential and ATP levels were shown. Along with this, MF attenuated oxidative stress, as indicated by a decrease in reactive oxygen species levels and an increase in the reduced form of glutathione [199].
The inhibition of endoplasmic reticulum (ER) stress and the prevention of MAPK cascade hyperactivation make a significant contribution to protective effects of MF in PCOS-associated HA. The activation of ER stress in the ovaries and the triggering of signaling pathways induced by the unfolded protein response lead to impaired synthesis and post-translational modification of proteins and the mitochondrial dysfunction, all of which negatively affects folliculogenesis and meiotic maturation of oocytes [201][202][203]. The effector components of MAPK cascade p38-MAPK is important for the activation of the unfolded protein response signaling and apoptosis in ovarian cells [204][205]. More recently, it was shown that an excess of androgens led to activation of ER stress and apoptosis in human and mouse cumulus cells [200][206]. The MF treatment reduces ER stress and inhibits p38-MAPK phosphorylation, which is significantly increased in cumulus cells of PCOS women and in the granulosa cells and the oocyte-cumulus complexes in mice with DHEA-induced PCOS [200]. There is every reason to believe that this effect of MF is based on its ability to inhibit HA.
3.4.3. Effects of Metformin on FSH-Activated Signaling in the PCOS Ovaries
Another mechanism of the MF restoring effect on ovarian function in PCOS is the inhibition of expression of the Cyp19a1 gene encoding aromatase. Reduced levels of aromatase result in a decrease in estrogen response to FSH, insulin and IGF-1 in the ovaries [180][207][208][209]. A large number of PCOS patients have increased sensitivity of granulosa cells to stimulation with FSH, insulin, or IGF-1. This is due to the fact that in granulosa cells of PCOS women, the expression of the FSH and IGF-1 receptors and the IRS1 and IRS2 proteins are significantly increased [210][211][212][213][214][215]. In addition, the expression of PTEN, a negative regulator of signaling pathway involving insulin/IGF-1 receptors, IRS proteins, phosphatidylinositol 3-kinase (PI 3-K) and Akt-kinase, is reduced, which leads to hyperactivation of Akt-kinase by insulin and IGF-1 [214]. In the PCOS ovaries, the important mechanism of suppression of PTEN expression and hyperactivation of the insulin and IGF-1 signaling pathways is an increase in the expression of two microRNAs of miR-200 family, miR-200b and miR-200c, which negatively affect the expression of the PTEN gene [215]. In addition, a decrease in the expression of miR-99a, a negative regulator of IGF-1 receptor expression, leads to an increase in the sensitivity of granulosa cells to IGF-1 [216]. An increase in the expression and activity of the receptor and postreceptor components of the FSH-, insulin- and IGF-1-regulated signaling systems in PCOS results in the accelerated growth and proliferation of the ovarian cells, primarily granulosa cells, in the response to the stimulating effect of these hormones. Moreover, this potentiates already pre-existing increased ovarian reactivity and premature luteinization [217][218][219].
MF reduces the expression of FSH receptors thereby weakening the stimulating effects of FSH on steroidogenesis and proliferation of granulosa cells, increased in PCOS, which leads to the normalization of folliculogenesis and ovulation. Under the conditions of ovarian dysfunctions in PCOS, MF treatment postpones the triggering of processes that ensure the normal growth of antral follicles, thus providing more appropriate window of time required for their differentiation and development (on average about three months) [208]. By reducing the ovarian sensitivity to FSH, MF prevents the OHSS, the most common complication of gonadotropin-stimulated induction of ovulation [130][131][133][220][221].
The inhibitory and modulating effects of MF on the effector components of gonadotropin-stimulated cascades in ovarian cells can be realized through both AMPK-dependent and AMPK-independent pathways, including the MAPK cascade [207][208]. Through AMPK-independent pathways, MF reduces FSH-induced increases in aromatase activity and estradiol synthesis in granulosa cells, and this effect is not reproduced when using AICAR [208]. The inhibitory effect of MF on the expression and activity of aromatase can be elicited through at least three well understood mechanisms.
3.4.4. The Effect of Metformin on the Production of Anti-Müllerian Hormone in PCOS
In PCOS, one of the targets of MF therapy is AMH, a dimeric glycoprotein that is produced by the granulosa cells of the primary, preantral and small antral follicles [222][223]. AMH concentration in the blood of women positively correlates with the follicular reserve and, as a result, in PCOS, the blood levels of AMH are usually increased by two or more fold [214][223][224][225][226]. Excess levels of AMH lead to an impaired folliculogenesis, preventing the recruitment of primordial follicles into the pool of growing follicles and reducing the responsiveness of growing follicles to FSH [212][227][228][229]. The increased levels of AMH may be due to HA and hyperinsulinemia, which are characteristic features of PCOS and are closely interrelated [212][225][230][231][232], as well as to an increase in blood LH levels or the sensitivity of granulosa cells to LH, typical for PCOS patients [232][233][234][235]. In in vitro experiments using lutein granulosa cells obtained from oligo/anovulatory PCOS women, the LH increases the AMH production, while the expression of type II AMH receptors in these cells does not change significantly. In the case of lutein granulosa cells obtained from healthy women and normo-ovulatory PCOS women, the stimulating effect of LH on AMH production is almost completely inhibited, but the inhibiting effect of LH on the expression of type II AMH receptors is preserved. This effect was reproduced with the use of cAMP analogs, which indicates the participation of cAMP-dependent mechanisms in it [233]. All this indicates that PCOS women with an impaired ovulatory cycle have an increase in both the LH-induced AMH production and the responsiveness of lutein granulosa cells to this factor.
By lowering insulin and androgen levels and normalizing gonadotropin levels, MF attenuates ovarian AMH secretion, which leads to a decrease of its inhibitory effect on folliculogenesis and a weakening of the signs of PCOS [222][223][236][237][238][239][240]. Eight-week treatment of PCOS women with MF (1500 mg/day) reduced the blood AMH levels from 10 ± 3.75 to 7.8 ± 3.7 ng/mL [223]. Six-month treatment with MF at the same dose led to a decrease in AMH, ovarian volume and antral follicle number in PCOS women [222]. The treatment of PCOS women with MF at the doses of 850 mg/day (first week), 850 mg/12 h (second week) and 850 mg/8 h (next six weeks), along with the restoration of ovulation and the normalization of LH and testosterone levels, caused a decrease in the blood AMH levels, from 8.99 ± 0.99 to 6.28 ± 0.46 ng/mL [238]. The combined therapy with MF and resveratrol of rats with DHEA-induced PCOS reduced ovarian size, improved ovarian follicular cell architecture, and decreased AMH production [241]. It is assumed that in PCOS, a decrease in the blood AMH level to control values can be considered as one of the prognostic factors of the effectiveness of MF therapy [237][238][242].
3.4.5. Effect of Metformin on Metalloproteinases in PCOS
Women with PCOS usually have the increased serum levels of type 9 matrix metalloproteinase (MMP-9) and MMP-2 [243][244], and the altered concentrations of the types 1 and 2 tissue inhibitor of MMP (TIMP-1 and TIMP-2) [243]. The changes in the concentrations and balance of MMPs and TIMPs lead to the remodeling in the ovarian stroma, increased ovarian angiogenesis and impaired folliculogenesis [243][244]. The activation of AMPK causes a decrease in the activity of the pathway involving mTOR (mammalian target of rapamycin) and ribosomal protein kinase p70S6K, and suppresses the expression and functional activity of MMPs. Consistently, stimulation of Akt kinase and mTOR enhances the expression of MMPs and, thereby, stimulates angiogenesis, cell migration, cell proliferation and protein synthesis [245][246].
By activating AMPK, MF reduces the expression and activity of MMPs, and this is largely responsible for the well-described antitumor effect of MF [247]. MF treatment (10 mM) of cultured human ovarian granulosa (HTOG) cells, leads to an increase in AMPK activity, an inhibition of mTOR-dependent cascade, and a decrease in the expression of MMP-2 and MMP-9 [248]. Another MF-mediated mechanism for down regulating the MMP expression involves triggering the H19/miR-29b-3p signaling pathway. MF increases the expression of miR-29b-3p, a negative regulator of the MMP-2 and MMP-9 expression via decreasing the expression of histone H19 through methylation of the promoter in the gene encoding this histone, which leads to a decrease in the production of these enzymes and contributes to normalization of ovarian morphology in PCOS [248]. MF-induced activation of the H19/miR-29b-3p pathway is attenuated by the increased expression of histone H19, which decreases the expression of miR-29b-3p. The miR-29b-3p-mediated effect of MF on MMP expression is independent of AMPK, in contrast to MF regulation of mTOR signaling cascade [248].
3.4.6. Influence of Metformin on Inflammation and Lipid Status in PCOS
Dyslipidemia, oxidative stress and inflammation, along with IR, are essential for the pathogenesis of PCOS. These factors are similar to those in MetS and T2DM [249][250]. As a result, the ability of MF to improve energy metabolism, prevent lipotoxicity and restore the redox balance in the ovaries is another mechanism of its beneficial effect on folliculogenesis and the ovulatory cycle in PCOS. In human granulosa cells, through AMPK-dependent mechanisms, MF suppresses the tumor necrosis factor-α (TNF-α)-induced production of pro-inflammatory cytokines, interleukin-8 and chemokine CXCL1/GROα [251]. The other AMPK activators, such as AICAR and Baicalin, also reduce TNF-α- and chemokine-mediated inflammatory responses in ovarian cells [251][252]. In PCOS ovarian cells, the mechanism of MF suppressive effect on the TNF-α-induced cascades includes a decrease in the gene expression of TNF-α [241].
The severity of metabolic disorders positively correlates with the therapeutic effect of MF, which is due not only to its effect on the ovaries, but also to the effect on the metabolic processes in the other tissues. A prominent feature of PCOS is decreased level of high-density lipoprotein cholesterol (HDL-C), and MF is most effective in PCOS patients with blood HDL-C levels below 50 mg/dL [253]. HDL-C modulates glucose homeostasis through AMPK-dependent mechanisms, whereby in PCOS, MF-induced AMPK activation may significantly contribute to the insulin sensitivity-restoring effect of MF [253].
3.5. The Sensitivity of PCOS Women to Metformin Therapy
The responsiveness of PCOS women to MF may be due to the efficiency of MF transport into the target cell and the distribution and pharmacokinetics of this drug in the tissues [22] (Figure 3). Despite the ability to freely penetrate the blood-tissue barriers and redistribute in the tissues, MF transport into the cell is carried out through the specialized transporters, including OCT1, OCT2, ATM and MATE. A decrease in their functional activity due to inactivating mutations in their genes, primarily in the OCT1 gene, has a negative effect on the therapeutic effect of MF, in some cases making PCOS patients completely resistant to MF [146][254][255]. The incidence of OCT1, OCT2 and ATM polymorphisms in PCOS is much higher than in women without PCOS. During genotyping of PCOS patients, non-functional alleles of the OCT1, OCT2, and ATM genes were detected in 29.8%, while low-functional alleles in 57.9% of cases. Non-functional alleles in the OCT1 and OCT2 genes were associated with poor response to MF, as well as with glucose intolerance and significantly elevated levels of proinsulin C-peptide after glucose loading [255]. In the recent work of Taiwanese scientists, the polymorphisms rs683369 (allele G) and rs628031 (allele A) were identified in the OCT1 gene of PCOS women, and they were associated with a decrease in sensitivity of patients to both the MF and insulin therapy [146]. It should be noted that of the 38 currently identified OCT1 gene polymorphisms, the Met408Val (rs628031) variant exhibits the most suppressed MF therapeutic effect [256]. Quite unexpected finding was that single-nucleotide polymorphism in the OCT2 gene did not significantly affect the affinity to MF, although there is evidence of the involvement of the OCT2 in MF transfer into the target cells, including the ovarian cells [257].
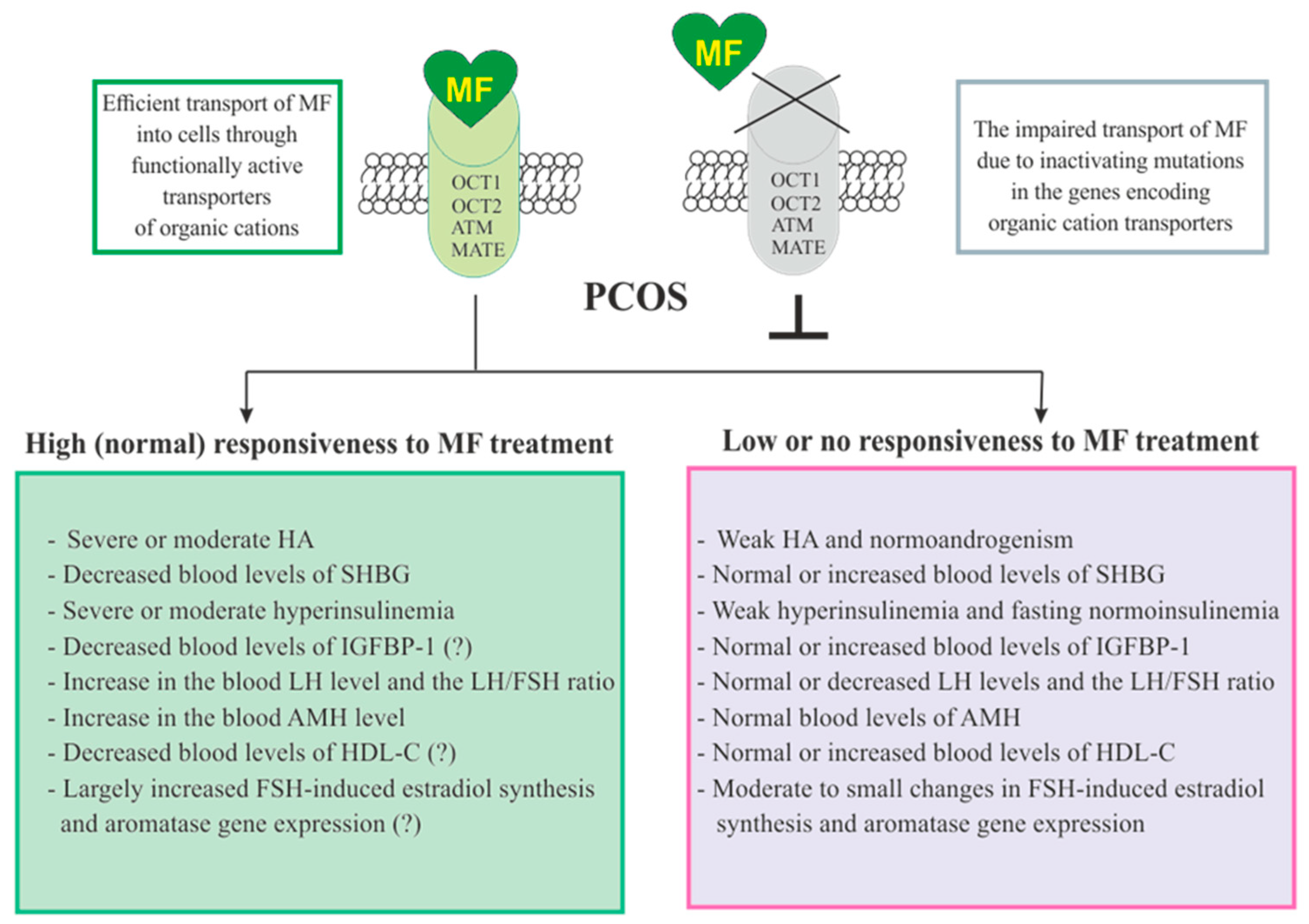
Figure 3. Factors determining responsiveness to metformin and the effectiveness of metformin therapy in women with PCOS. Women with PCOS, as well as the patients with other pathologies, must have functionally active transporters of organic cations (OCT1, OCT2, and others) in order to respond to MF, since inactivating mutations and polymorphisms in the genes encoding these transporters lead to impairment of MF transport into the cell and make MF therapy ineffective. Since MF improves metabolic parameters and insulin sensitivity, its effectiveness in PCOS women with overweight or obesity, as well as with severe dyslipidemia and impaired glucose tolerance, is usually higher. There is evidence that MF therapy is most effective in PCOS women who have pronounced signs of hyperinsulinemia and hyperandrogenism, the increased LH levels and the LH/FSH ratio, the decreased levels of SHBG, IGFBP-1 and HDL-C, and the increased levels of AMH. It can also be assumed that MF will be more effective in patients with increased aromatase expression and ovarian hypersensitivity to FSH, since one of the mechanisms of MF action is normalization of the expression of genes encoding the FSH receptor and aromatase, as well as normalization in the response of ovarian cells to stimulation of FSH. Abbreviations: AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein cholesterol; IGFBP-1, insulin-like growth factor-binding protein-1; LH, luteinizing hormone; OCT1 and OCT2, organic cation transporters-1 and 2; SHBG, androgen and sex hormone-binding globulin.
In addition to negative effects for MF transport into the cell, a number of other factors contribute the sensitivity of PCOS patients to MF therapy. Among these factors are the severity of hyperinsulinemia and HA, the blood levels of the binding proteins for insulin, IGF-1 (IGFBP-1) and androgens (SHBG), and the blood levels of AMH and HDL-C (Figure 3).
References
- Madsen, K.S.; Chi, Y.; Metzendorf, M.I.; Richter, B.; Hemmingsen, B. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2019, 12, CD008558.
- Gnesin, F.; Thuesen, A.C.B.; Kähler, L.K.A.; Madsbad, S.; Hemmingsen, B. Metformin monotherapy for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 6, CD012906.
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668.
- Dziubak, A.; Wójcicka, G.; Wojtak, A.; Bełtowski, J. Metabolic Effects of Metformin in the Failing Heart. Int. J. Mol. Sci. 2018, 19, 2869.
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417.
- Bell, S.; Farran, B.; McGurnaghan, S.; McCrimmon, R.J.; Leese, G.P.; Petrie, J.R.; McKeigue, P.; Sattar, N.; Wild, S.; McKnight, J.; et al. Risk of acute kidney injury and survival in patients treated with Metformin: An observational cohort study. BMC Nephrol. 2017, 18, 163.
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191.
- Lee, J.; Yesilkanal, A.E.; Wynne, J.P.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.C.; Rustandy, F.D.; Tiwari, P.; et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019, 568, 254–258.
- Meyerhardt, J.A.; Irwin, M.L.; Jones, L.W.; Zhang, S.; Campbell, N.; Brown, J.C.; Pollak, M.; Sorrentino, A.; Cartmel, B.; Harrigan, M.; et al. Randomized Phase II Trial of Exercise, Metformin, or Both on Metabolic Biomarkers in Colorectal and Breast Cancer Survivors. JNCI Cancer Spectr. 2019, 4, pkz096.
- Ko, E.M.; Walter, P.; Jackson, A.; Clark, L.; Franasiak, J.; Bolac, C.; Havrilesky, L.J.; Secord, A.A.; Moore, D.T.; Gehrig, P.A.; et al. Metformin is associated with improved survival in endometrial cancer. Gynecol. Oncol. 2014, 132, 438–442.
- Tang, Y.L.; Zhu, L.Y.; Li, Y.; Yu, J.; Wang, J.; Zeng, X.X.; Hu, K.X.; Liu, J.Y.; Xu, J.X. Metformin Use Is Associated with Reduced Incidence and Improved Survival of Endometrial Cancer: A Meta-Analysis. Biomed. Res. Int. 2017, 2017, 5905384.
- Mu, N.; Xu, T.; Gao, M.; Dong, M.; Tang, Q.; Hao, L.; Wang, G.; Li, Z.; Wang, W.; Yang, Y.; et al. Therapeutic effect of metformin in the treatment of endometrial cancer. Oncol. Lett. 2020, 20, 156.
- Zhang, Z.J.; Zheng, Z.J.; Kan, H.; Song, Y.; Cui, W.; Zhao, G.; Kip, K.E. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: A meta-analysis. Diabetes Care 2011, 34, 2323–2328.
- Pircher, A.; Zieher, M.; Eigentler, A.; Pichler, R.; Schäfer, G.; Fritz, J.; Puhr, M.; Steiner, E.; Horninger, W.; Klocker, H.; et al. Antidiabetic drugs influence molecular mechanisms in prostate cancer. Cancer Biol. Ther. 2018, 19, 1153–1161.
- Vallianou, N.G.; Evangelopoulos, A.; Kazazis, C. Metformin and cancer. Rev. Diabet. Stud. 2013, 10, 228–235.
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Postepy Hig. Med. Dosw. 2017, 71, 170–175.
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585.
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020, 21, 3240.
- An, H.; He, L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J. Endocrinol. 2016, 228, R97–R106.
- He, L. Metformin and Systemic Metabolism. Trends Pharm. Sci. 2020, 41, 868–881.
- Cioce, M.; Pulito, C.; Strano, S.; Blandino, G.; Fazio, V.M. Metformin: Metabolic Rewiring Faces Tumor Heterogeneity. Cells 2020, 9, 2439.
- Chan, P.; Shao, L.; Tomlinson, B.; Zhang, Y.; Liu, Z.M. Metformin transporter pharmacogenomics: Insights into drug disposition-where are we now? Expert Opin. Drug Metab. Toxicol. 2018, 14, 1149–1159.
- Lee, N.; Hebert, M.F.; Wagner, D.J.; Easterling, T.R.; Liang, C.J.; Rice, K.; Wang, J. Organic Cation Transporter 3 Facilitates Fetal Exposure to Metformin during Pregnancy. Mol. Pharmacol. 2018, 94, 1125–1131.
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262.
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 27, 190–201.
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313.
- Hardie, D.G. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32, S7–S12.
- Hardie, D.G. Keeping the home fires burning: AMP-activated protein kinase. J. R. Soc. Interface 2018, 15, 20170774.
- Lizcano, J.M.; Göransson, O.; Toth, R.; Deak, M.; Morrice, N.A.; Boudeau, J.; Hawley, S.A.; Udd, L.; Mäkelä, T.P.; Hardie, D.G.; et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004, 23, 833–843.
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19.
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33.
- Momcilovic, M.; Hong, S.P.; Carlson, M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 2006, 281, 25336–25343.
- Jia, J.; Abudu, Y.P.; Claude-Taupin, A.; Gu, Y.; Kumar, S.; Choi, S.W.; Peters, R.; Mudd, M.H.; Allers, L.; Salemi, M.; et al. Galectins Control mTOR in Response to Endomembrane Damage. Mol. Cell 2018, 70, 120–135.
- Jia, J.; Bissa, B.; Brecht, L.; Allers, L.; Choi, S.W.; Gu, Y.; Zbinden, M.; Burge, M.R.; Timmins, G.; Hallows, K.; et al. AMPK, a Regulator of Metabolism and Autophagy, Is Activated by Lysosomal Damage via a Novel Galectin-Directed Ubiquitin Signal Transduction System. Mol. Cell. 2020, 77, 951–969.
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174.
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008.
- Cao, J.; Meng, S.; Chang, E.; Beckwith-Fickas, K.; Xiong, L.; Cole, R.N.; Radovick, S.; Wondisford, F.E.; He, L. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J. Biol. Chem. 2014, 289, 20435–20446.
- Oakhill, J.S.; Steel, R.; Chen, Z.P.; Scott, J.W.; Ling, N.; Tam, S.; Kemp, B.E. AMPK is a direct adenylate charge-regulated protein kinase. Science 2011, 332, 1433–1435.
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233.
- Ross, F.A.; Jensen, T.E.; Hardie, D.G. Differential regulation by AMP and ADP of AMPK complexes containing different γ subunit isoforms. Biochem. J. 2016, 473, 189–199.
- Zhang, C.S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.W.; Zhu, M.; et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 2017, 548, 112–116.
- Zong, Y.; Zhang, C.S.; Li, M.; Wang, W.; Wang, Z.; Hawley, S.A.; Ma, T.; Feng, J.W.; Tian, X.; Qi, Q.; et al. Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Res. 2019, 29, 460–473.
- Davies, S.P.; Helps, N.R.; Cohen, P.T.; Hardie, D.G. 5’-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995, 377, 421–425.
- Suter, M.; Riek, U.; Tuerk, R.; Schlattner, U.; Wallimann, T.; Neumann, D. Dissecting the role of 5’-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 2006, 281, 32207–32216.
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Averet, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228.
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614.
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369.
- Bridges, H.R.; Jones, A.J.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014, 462, 475–487.
- Ouyang, J.; Parakhia, R.A.; Ochs, R.S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 2011, 286, 1–11.
- Meng, S.; Cao, J.; He, Q.; Xiong, L.; Chang, E.; Radovick, S.; Wondisford, F.E.; He, L. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J. Biol. Chem. 2015, 290, 3793–3802.
- He, L.; Wondisford, F.E. Metformin action: Concentrations matter. Cell Metab. 2015, 21, 159–162.
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569.
- Sliwinska, A.; Drzewoski, J. Molecular action of metformin in hepatocytes: An updated insight. Curr. Diabetes Rev. 2015, 11, 175–181.
- Karnewar, S.; Neeli, P.K.; Panuganti, D.; Kotagiri, S.; Mallappa, S.; Jain, N.; Jerald, M.K.; Kotamraju, S. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: Relevance in age-associated vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1115–1128.
- Rattan, R.; Giri, S.; Hartmann, L.C.; Shridhar, V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J. Cell. Mol. Med. 2011, 15, 166–178.
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654.
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224.
- Motoshima, H.; Goldstein, B.J.; Igata, M.; Araki, E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 2006, 574, 63–71.
- Choi, Y.K.; Park, K.G. Metabolic roles of AMPK and metformin in cancer cells. Mol. Cells 2013, 36, 279–287.
- Gao, F.; Chen, J.; Zhu, H. A potential strategy for treating atherosclerosis: Improving endothelial function via AMP-activated protein kinase. Sci. China Life Sci. 2018, 61, 1024–1029.
- Lyons, C.L.; Roche, H.M. Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int. J. Mol. Sci. 2018, 19, 3092.
- Viollet, B.; Foretz, M. Revisiting the mechanisms of metformin action in the liver. Ann. Endocrinol. 2013, 74, 123–129.
- Johanns, M.; Lai, Y.C.; Hsu, M.F.; Jacobs, R.; Vertommen, D.; Van Sande, J.; Dumont, J.E.; Woods, A.; Carling, D.; Hue, L.; et al. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat. Commun. 2016, 7, 10856.
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260.
- He, L.; Sabet, A.; Djedjos, S.; Miller, R.; Sun, X.; Hussain, M.A.; Radovick, S.; Wondisford, F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009, 137, 635–646.
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546.
- Madiraju, A.K.; Qiu, Y.; Perry, R.J.; Rahimi, Y.; Zhang, X.M.; Zhang, D.; Camporez, J.G.; Cline, G.W.; Butrico, G.M.; Kemp, B.E.; et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat. Med. 2018, 24, 1384–1394.
- Cuyàs, E.; Verdura, S.; Llorach-Pares, L.; Fernández-Arroyo, S.; Luciano-Mateo, F.; Cabré, N.; Stursa, J.; Werner, L.; Martin-Castillo, B.; Viollet, B.; et al. Metformin directly targets the H3K27me3 demethylase KDM6A/UTX. Aging Cell. 2018, 17, e12772.
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858.
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735.
- Duca, F.A.; Côté, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015, 21, 506–511.
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–1188.
- Huang, N.L.; Chiang, S.H.; Hsueh, C.H.; Liang, Y.J.; Chen, Y.J.; Lai, L.P. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int. J. Cardiol. 2009, 134, 169–175.
- Okamura, H.; Yoshida, K.; Sasaki, E.; Qiu, L.; Amorim, B.R.; Morimoto, H.; Haneji, T. Expression of PTEN and Akt phosphorylation in lipopolysaccharide-treated NIH3T3 cells. Cell Biol. Int. 2007, 31, 119–125.
- Lee, S.K.; Lee, J.O.; Kim, J.H.; Kim, S.J.; You, G.Y.; Moon, J.W.; Jung, J.H.; Park, S.H.; Uhm, K.O.; Park, J.M.; et al. Metformin sensitizes insulin signaling through AMPK-mediated PTEN down-regulation in preadipocyte 3T3-L1 cells. J. Cell. Biochem. 2011, 112, 1259–1267.
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708.
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic ovary syndrome. Lancet 2007, 370, 685–697.
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e25.
- Abbott, D.H.; Dumesic, D.A.; Levine, J.E. Hyperandrogenic origins of polycystic ovary syndrome—Implications for pathophysiology and therapy. Expert Rev. Endocrinol. Metab. 2019, 14, 131–143.
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573.
- Alvarez-Blasco, F.; Botella-Carretero, J.I.; San Millán, J.L.; Escobar-Morreale, H.F. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch. Intern. Med. 2006, 166, 2081–2086.
- Legro, R.S.; Barnhart, H.X.; Schlaff, W.D.; Carr, B.R.; Diamond, M.P.; Carson, S.A.; Steinkampf, M.P.; Coutifaris, C.; McGovern, P.G.; Cataldo, N.A.; et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2007, 356, 551–566.
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488.
- Li, R.; Zhang, Q.; Yang, D.; Li, S.; Lu, S.; Wu, X.; Wei, Z.; Song, X.; Wang, X.; Fu, S.; et al. Prevalence of polycystic ovary syndrome in women in China: A large community-based study. Hum. Reprod. 2013, 28, 2562–2569.
- Kollmann, M.; Klaritsch, P.; Martins, W.P.; Guenther, F.; Schneider, V.; Herzog, S.A.; Craciunas, L.; Lang, U.; Obermayer-Pietsch, B.; Lerchbaum, E.; et al. Maternal and neonatal outcomes in pregnant women with PCOS: Comparison of different diagnostic definitions. Hum. Reprod. 2015, 30, 2396–2403.
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631.
- Spritzer, P.M. Polycystic ovary syndrome: Reviewing diagnosis and management of metabolic disturbances. Arq. Bras. Endocrinol. Metabol. 2014, 58, 182–187.
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525.
- Faure, M.; Bertoldo, M.J.; Khoueiry, R.; Bongrani, A.; Brion, F.; Giulivi, C.; Dupont, J.; Froment, P. Metformin in Reproductive Biology. Front. Endocrinol. 2018, 9, 675.
- Jones, M.R.; Goodarzi, M.O. Genetic determinants of polycystic ovary syndrome: Progress and future directions. Fertil. Steril. 2016, 106, 25–32.
- Liu, H.; Zhao, H.; Chen, Z.J. Genome-Wide Association Studies for Polycystic Ovary Syndrome. Semin. Reprod. Med. 2016, 34, 224–229.
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260.
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132.
- Xita, N.; Tsatsoulis, A. Review: Fetal programming of polycystic ovary syndrome by androgen excess: Evidence from experimental, clinical, and genetic association studies. J. Clin. Endocrinol. Metab. 2006, 91, 1660–1666.
- Ilie, I.R.; Georgescu, C.E. Polycystic Ovary Syndrome-Epigenetic Mechanisms and Aberrant MicroRNA. Adv. Clin. Chem. 2015, 71, 25–45.
- Yu, Y.Y.; Sun, C.X.; Liu, Y.K.; Li, Y.; Wang, L.; Zhang, W. Genome-wide screen of ovary-specific DNA methylation in polycystic ovary syndrome. Fertil. Steril. 2015, 104, 145–153.
- Wang, S.; Alvero, R. Racial and ethnic differences in physiology and clinical symptoms of polycystic ovary syndrome. Semin. Reprod. Med. 2013, 31, 365–369.
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24.
- Abdolahian, S.; Tehrani, F.R.; Amiri, M.; Ghodsi, D.; Yarandi, R.B.; Jafari, M.; Majd, H.A.; Nahidi, F. Effect of lifestyle modifications on anthropometric, clinical, and biochemical parameters in adolescent girls with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 71.
- Abbott, D.H.; Barnett, D.K.; Bruns, C.M.; Dumesic, D.A. Androgen excess fetal programming of female reproduction: A developmental etiology for polycystic ovary syndrome? Hum. Reprod. Update 2005, 11, 357–374.
- Abbott, D.H.; Bacha, F. Ontogeny of polycystic ovary syndrome and insulin resistance in utero and early childhood. Fertil. Steril. 2013, 100, 2–11.
- De Melo, A.S.; Dias, S.V.; Cavalli, R.d.C.; Cardoso, V.C.; Bettiol, H.; Barbieri, M.A.; Ferriani, R.A.; Vieira, C.S. Pathogenesis of polycystic ovary syndrome: Multifactorial assessment from the foetal stage to menopause. Reproduction 2015, 150, 1–24.
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017, 11, CD003053.
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: ; Practice Committee of the American Society for Reproductive Medicine. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): A guideline. Fertil. Steril. 2017, 108, 426–441.
- Abdalla, M.A.; Deshmukh, H.; Atkin, S.; Sathyapalan, T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11.
- Bordewijk, E.M.; Nahuis, M.; Costello, M.F.; Van der Veen, F.; Tso, L.O.; Mol, B.W.; van Wely, M. Metformin during ovulation induction with gonadotrophins followed by timed intercourse or intrauterine insemination for subfertility associated with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2017, 1, CD009090.
- Sharpe, A.; Morley, L.C.; Tang, T.; Norman, R.J.; Balen, A.H. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 12, CD013505.
- Gadalla, M.A.; Norman, R.J.; Tay, C.T.; Hiam, D.S.; Melder, A.; Pundir, J.; Thangaratinam, S.; Teede, H.J.; Mol, B.W.J.; Moran, L.J. Medical and Surgical Treatment of Reproductive Outcomes in Polycystic Ovary Syndrome: An Overview of Systematic Reviews. Int. J. Fertil. Steril. 2020, 13, 257–270.
- Wu, Y.; Tu, M.; Huang, Y.; Liu, Y.; Zhang, D. Association of Metformin with Pregnancy Outcomes in Women With Polycystic Ovarian Syndrome Undergoing In Vitro Fertilization: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2011995.
- Rojas, J.; Chávez-Castillo, M.; Bermúdez, V. The Role of Metformin in Metabolic Disturbances during Pregnancy: Polycystic Ovary Syndrome and Gestational Diabetes Mellitus. Int. J. Reprod. Med. 2014, 2014, 797681.
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018, 89, 251–268.
- Gormsen, L.C.; Søndergaard, E.; Christensen, N.L.; Brøsen, K.; Jessen, N.; Nielsen, S. Metformin increases endogenous glucose production in non-diabetic individuals and individuals with recent-onset type 2 diabetes. Diabetologia 2019, 62, 1251–1256.
- McCreight, L.J.; Mari, A.; Coppin, L.; Jackson, N.; Umpleby, A.M.; Pearson, E.R. Metformin increases fasting glucose clearance and endogenous glucose production in non-diabetic individuals. Diabetologia 2020, 63, 444–447.
- Bryrup, T.; Thomsen, C.W.; Kern, T.; Allin, K.H.; Brandslund, I.; Jørgensen, N.R.; Vestergaard, H.; Hansen, T.; Hansen, T.H.; Pedersen, O.; et al. Metformin-induced changes of the gut microbiota in healthy young men: Results of a non-blinded, one-armed intervention study. Diabetologia 2019, 62, 1024–1035.
- Derkach, K.V.; Kuznetsova, L.A.; Sharova, T.S.; Ignat’eva, P.A.; Bondareva, V.M.; Shpakov, A.O. The effect of prolonged metformin treatment on the activity of the adenylate cyclase system and NO-synthase in the brain and myocardium of obese rats. Cell Tissue Biol. 2015, 9, 385–394.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil. Steril. 2008, 89, 505–522.
- Lautatzis, M.E.; Goulis, D.G.; Vrontakis, M. Efficacy and safety of metformin during pregnancy in women with gestational diabetes mellitus or polycystic ovary syndrome: A systematic review. Metabolism 2013, 62, 1522–1534.
- Sivalingam, V.N.; Myers, J.; Nicholas, S.; Balen, A.H.; Crosbie, E.J. Metformin in reproductive health, pregnancy and gynaecological cancer: Established and emerging indications. Hum. Reprod. Update 2014, 20, 853–868.
- Feng, L.; Lin, X.F.; Wan, Z.H.; Hu, D.; Du, Y.K. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2015, 31, 833–839.
- Sinai Talaulikar, V.; Tang, T.; Yasmin, E. Role of Metformin in Women’s Health: Review of Its Current Place in Clinical Practice and Emerging Indications for Future. Obstet. Gynecol. Surv. 2016, 71, 307–317.
- Tan, X.; Li, S.; Chang, Y.; Fang, C.; Liu, H.; Zhang, X.; Wang, Y. Effect of metformin treatment during pregnancy on women with PCOS: A systematic review and meta-analysis. Clin. Investig. Med. 2016, 39, 120–131.
- Zeng, X.L.; Zhang, Y.F.; Tian, Q.; Xue, Y.; An, R.F. Effects of metformin on pregnancy outcomes in women with polycystic ovary syndrome: A meta-analysis. Medicine 2016, 95, e4526.
- Løvvik, T.S.; Carlsen, S.M.; Salvesen, Ø.; Steffensen, B.; Bixo, M.; Gómez-Real, F.; Lønnebotn, M.; Hestvold, K.V.; Zabielska, R.; Hirschberg, A.L.; et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 256–266.
- Baillargeon, J.P.; Jakubowicz, D.J.; Iuorno, M.J.; Jakubowicz, S.; Nestler, J.E. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil. Steril. 2004, 82, 893–902.
- Carmina, E.; Lobo, R.A. Does metformin induce ovulation in normoandrogenic anovulatory women? Am. J. Obstet. Gynecol. 2004, 191, 1580–1584.
- Tang, T.; Lord, J.M.; Norman, R.J.; Yasmin, E.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2010, 1, CD003053.
- Kjøtrød, S.B.; Carlsen, S.M.; Rasmussen, P.E.; Holst-Larsen, T.; Mellembakken, J.; Thurin-Kjellberg, A.; Haapaniemikouru, K.; Morin-Papunen, L.; Humaidan, P.; Sunde, A.; et al. Use of metformin before and during assisted reproductive technology in non-obese young infertile women with polycystic ovary syndrome: A prospective, randomized, double-blind, multi-centre study. Hum. Reprod. 2011, 26, 2045–2053.
- Morin-Papunen, L.; Rantala, A.S.; Unkila-Kallio, L.; Tiitinen, A.; Hippeläinen, M.; Perheentupa, A.; Tinkanen, H.; Bloigu, R.; Puukka, K.; Ruokonen, A.; et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): A multicenter, double-blind, placebo-controlled randomized trial. J. Clin. Endocrinol. Metab. 2012, 97, 1492–1500.
- Tang, T.; Lord, J.M.; Norman, R.J.; Yasmin, E.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2012, 5, CD003053.
- Tso, L.O.; Costello, M.F.; Albuquerque, L.E.; Andriolo, R.B.; Macedo, C.R. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2014, 2014, CD006105.
- Kollmann, M.; Martins, W.P.; Lima, M.L.; Craciunas, L.; Nastri, C.O.; Richardson, A.; Raine-Fenning, N. Strategies for improving outcome of assisted reproduction in women with polycystic ovary syndrome: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2016, 48, 709–718.
- Palomba, S.; Falbo, A.; Carrillo, L.; Villani, M.T.; Orio, F.; Russo, T.; Di Cello, A.; Cappiello, F.; Capasso, S.; Tolino, A.; et al. Metformin reduces risk of ovarian hyperstimulation syndrome in patients with polycystic ovary syndrome during gonadotropin-stimulated in vitro fertilization cycles: A randomized, controlled trial. Fertil. Steril. 2011, 96, 1384–1390.
- Palomba, S.; Falbo, A.; La Sala, G.B. Effects of metformin in women with polycystic ovary syndrome treated with gonadotrophins for in vitro fertilisation and intracytoplasmic sperm injection cycles: A systematic review and meta-analysis of randomised controlled trials. BJOG 2013, 120, 267–276.
- Costello, M.F.; Chapman, M.; Conway, U. A systematic review and meta-analysis of randomized controlled trials on metformin co-administration during gonadotrophin ovulation induction or IVF in women with polycystic ovary syndrome. Hum. Reprod. 2006, 21, 1387–1399.
- Tso, L.O.; Costello, M.F.; Albuquerque, L.E.; Andriolo, R.B.; Freitas, V. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2009, 2, CD006105.
- Palomba, S.; Falbo, A.; Zullo, F.; Orio, F., Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: A comprehensive review. Endocr. Rev. 2009, 30, 1–50.
- Abdalmageed, O.S.; Farghaly, T.A.; Abdelaleem, A.A.; Abdelmagied, A.E.; Ali, M.K.; Abbas, A.M. Impact of Metformin on IVF Outcomes in Overweight and Obese Women With Polycystic Ovary Syndrome: A Randomized Double-Blind Controlled Trial. Reprod. Sci. 2019, 26, 1336–1342.
- Barbieri, R.L. Metformin for the treatment of polycystic ovary syndrome. Obstet. Gynecol. 2003, 101, 785–793.
- Abu Hashim, H.; Shokeir, T.; Badawy, A. Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: A randomized controlled trial. Fertil. Steril. 2010, 94, 1405–1409.
- Bjelica, A.; Trninić-Pjević, A.; Mladenović-Segedi, L.; Cetković, N.; Petrović, D. Comparison of the efficiency of clomiphene citrate and letrozole in combination with metformin in moderately obese clomiphene citrate-resistant polycystic ovarian syndrome patients. Srp. Arh. Celok. Lek. 2016, 144, 146–150.
- Nemati, M.; Nemati, S.; Taheri, A.M.; Heidari, B. Comparison of metformin and N-acetyl cysteine, as an adjuvant to clomiphene citrate, in clomiphene-resistant women with polycystic ovary syndrome. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 579–585.
- Yu, Y.; Fang, L.; Zhang, R.; He, J.; Xiong, Y.; Guo, X.; Du, Q.; Huang, Y.; Sun, Y. Comparative effectiveness of 9 ovulation-induction therapies in patients with clomiphene citrate-resistant polycystic ovary syndrome: A network meta-analysis. Sci. Rep. 2017, 7, 3812.
- Sawant, S.; Bhide, P. Fertility Treatment Options for Women with Polycystic Ovary Syndrome. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119890867.
- Maged, A.M.; Elsawah, H.; Abdelhafez, A.; Bakry, A.; Mostafa, W.A. The adjuvant effect of metformin and N-acetylcysteine to clomiphene citrate in induction of ovulation in patients with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2015, 31, 635–638.
- Rezk, M.; Shaheen, A.E.; Saif El-Nasr, I. Clomiphene citrate combined with metformin versus letrozole for induction of ovulation in clomiphene-resistant polycystic ovary syndrome: A randomized clinical trial. Gynecol. Endocrinol. 2018, 34, 298–300.
- Chang, H.H.; Hsueh, Y.S.; Cheng, Y.W.; Ou, H.T.; Wu, M.H. Association between Polymorphisms of OCT1 and Metabolic Response to Metformin in Women with Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2019, 20, 1720.
- Hoeger, K.; Davidson, K.; Kochman, L.; Cherry, T.; Kopin, L.; Guzick, D.S. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J. Clin. Endocrinol. Metab. 2008, 93, 4299–4306.
- Palomba, S.; Materazzo, C.; Falbo, A.; Orio, F.; La Sala, G.B.; Sultan, C. Metformin, oral contraceptives or both to manage oligo-amenorrhea in adolescents with polycystic ovary syndrome? A clinical review. Gynecol. Endocrinol. 2014, 30, 335–340.
- Stefanaki, C.; Bacopoulou, F.; Kandaraki, E.; Boschiero, D.; Diamandi-Kandarakis, E. Lean Women on Metformin and Oral Contraceptives for Polycystic Ovary Syndrome Demonstrate a Dehydrated Osteosarcopenic Phenotype: A Pilot Study. Nutrients 2019, 11, 2055.
- Douchi, T.; Yamamoto, S.; Oki, T.; Maruta, K.; Kuwahata, R.; Nagata, Y. Serum androgen levels and muscle mass in women with polycystic ovary syndrome. Obstet. Gynecol. 1999, 94, 337–340.
- Cetrone, M.; Mele, A.; Tricarico, D. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr. Diabetes Rev. 2014, 10, 231–237.
- Kim, K.H.; Lee, M.S. FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti-diabetic drugs. J. Endocrinol. 2015, 226, 1–16.
- Gupta, A.; Jelinek, H.F.; Al-Aubaidy, H. Glucagon like peptide-1 and its receptor agonists: Their roles in management of Type 2 diabetes mellitus. Diabetes Metab. Syndr. 2017, 11, 225–230.
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756.
- Gentilella, R.; Pechtner, V.; Corcos, A.; Consoli, A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: Are they all the same? Diabetes Metab. Res. Rev. 2019, 35, e3070.
- Gillani, S.W.; Moosvi, A.F. Clinical Review: Safety and Efficacy Comparison between Sulfonylureas and Dipeptidyl Peptidase-4 Inhibitors as Second-Line Therapies in Type 2 Diabetes Mellitus. Curr. Pharm. Des. 2020, 26, 4315–4322.
- Pani, A.; Gironi, I.; Di Vieste, G.; Mion, E.; Bertuzzi, F.; Pintaudi, B. From Prediabetes to Type 2 Diabetes Mellitus in Women with Polycystic Ovary Syndrome: Lifestyle and Pharmacological Management. Int. J. Endocrinol. 2020, 2020, 6276187.
- Romualdi, D.; Versace, V.; Lanzone, A. What is new in the landscape of insulin-sensitizing agents for polycystic ovary syndrome treatment. Ther. Adv. Reprod. Health 2020, 14, 2633494120908709.
- Jensterle, M.; Kravos, N.A.; Goričar, K.; Janez, A. Short-term effectiveness of low dose liraglutide in combination with metformin versus high dose liraglutide alone in treatment of obese PCOS: Randomized trial. BMC Endocr. Disord. 2017, 17, 5.
- Tao, T.; Wu, P.; Wang, Y.; Liu, W. Comparison of glycemic control and β-cell function in new onset T2DM patients with PCOS of metformin and saxagliptin monotherapy or combination treatment. BMC Endocr. Disord. 2018, 18, 14.
- Elkind-Hirsch, K.E.; Paterson, M.S.; Seidemann, E.L.; Gutowski, H.C. Short-term therapy with combination dipeptidyl peptidase-4 inhibitor saxagliptin/metformin extended release (XR) is superior to saxagliptin or metformin XR monotherapy in prediabetic women with polycystic ovary syndrome: A single-blind, randomized, pilot study. Fertil. Steril. 2017, 107, 253–260.
- Lamos, E.M.; Malek, R.; Davis, S.N. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert. Rev. Clin. Pharmacol. 2017, 10, 401–408.
- Tzotzas, T.; Karras, S.N.; Katsiki, N. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in the Treatment of Obese Women with Polycystic Ovary Syndrome. Curr. Vasc. Pharmacol. 2017, 15, 218–229.
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709.
- Livadas, S.; Androulakis, I.; Angelopoulos, N.; Lytras, A.; Papagiannopoulos, F.; Kassi, G. Liraglutide administration improves hormonal/metabolic profile and reproductive features in women with HAIR-AN syndrome. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 19–0150.
- Nestler, J.E.; Jakubowicz, D.J. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N. Engl. J. Med. 1996, 335, 617–623.
- Chou, K.H.; von Eye Corleta, H.; Capp, E.; Spritzer, P.M. Clinical, metabolic and endocrine parameters in response to metformin in obese women with polycystic ovary syndrome: A randomized, double-blind and placebo-controlled trial. Horm. Metab. Res. 2003, 35, 86–91.
- Allen, H.F.; Mazzoni, C.; Heptulla, R.A.; Murray, M.A.; Miller, N.; Koenigs, L.; Reiter, E.O. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 2005, 18, 761–768.
- Bridger, T.; MacDonald, S.; Baltzer, F.; Rodd, C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch. Pediatr. Adolesc. Med. 2006, 160, 241–246.
- Diamanti-Kandarakis, E.; Christakou, C.D.; Kandaraki, E.; Economou, F.N. Metformin: An old medication of new fashion: Evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur. J. Endocrinol. 2010, 162, 193–212.
- Al-Zubeidi, H.; Klein, K.O. Randomized clinical trial evaluating metformin versus oral contraceptive pills in the treatment of adolescents with polycystic ovarian syndrome. J. Pediatr. Endocrinol. Metab. 2015, 28, 853–858.
- Al Khalifah, R.A.; Florez, I.D.; Dennis, B.; Thabane, L.; Bassilious, E. Metformin or Oral Contraceptives for Adolescents With Polycystic Ovarian Syndrome: A Meta-analysis. Pediatrics 2016, 137, e20154089.
- Sam, S.; Ehrmann, D.A. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia 2017, 60, 1656–1661.
- Kupreeva, M.; Diane, A.; Lehner, R.; Watts, R.; Ghosh, M.; Proctor, S.; Vine, D. Effect of metformin and flutamide on insulin, lipogenic and androgen-estrogen signaling, and cardiometabolic risk in a PCOS-prone metabolic syndrome rodent model. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E16–E33.
- Diri, H.; Bayram, F.; Simsek, Y.; Caliskan, Z.; Kocer, D. Comparison of finasteride, metformin, and finasteride plus metformin in PCOS. Acta Endocrinol. 2017, 13, 84–89.
- Kurzthaler, D.; Hadziomerovic-Pekic, D.; Wildt, L.; Seeber, B.E. Metformin induces a prompt decrease in LH-stimulated testosterone response in women with PCOS independent of its insulin-sensitizing effects. Reprod. Biol. Endocrinol. 2014, 12, 98.
- Barber, T.M.; Wass, J.A.; McCarthy, M.I.; Franks, S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: Implications for the management of polycystic ovary syndrome. Clin. Endocrinol. 2007, 66, 513–517.
- Adams, J.M.; Taylor, A.E.; Crowley, W.F., Jr.; Hall, J.E. Polycystic ovarian morphology with regular ovulatory cycles: Insights into the pathophysiology of polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 4343–4350.
- Attia, G.R.; Rainey, W.E.; Carr, B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001, 76, 517–524.
- Mansfield, R.; Galea, R.; Brincat, M.; Hole, D.; Mason, H. Metformin has direct effects on human ovarian steroidogenesis. Fertil. Steril. 2003, 79, 956–962.
- Tosca, L.; Chabrolle, C.; Uzbekova, S.; Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5’ monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007, 76, 368–378.
- Tosca, L.; Froment, P.; Solnais, P.; Ferré, P.; Foufelle, F.; Dupont, J. Adenosine 5′-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells. Endocrinology 2005, 146, 4500–4513.
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753.
- Tosca, L.; Crochet, S.; Ferré, P.; Foufelle, F.; Tesseraud, S.; Dupont, J. AMP-activated protein kinase activation modulates progesterone secretion in granulosa cells from hen preovulatory follicles. J. Endocrinol. 2006, 190, 85–97.
- Downs, S.M.; Chen, J. Induction of meiotic maturation in mouse oocytes by adenosine analogs. Mol. Reprod. Dev. 2006, 73, 1159–1168.
- LaRosa, C.; Downs, S.M. Meiotic induction by heat stress in mouse oocytes: Involvement of AMP-activated protein kinase and MAPK family members. Biol. Reprod. 2007, 76, 476–486.
- Mayes, M.A.; Laforest, M.F.; Guillemette, C.; Gilchrist, R.B.; Richard, F.J. Adenosine 5′-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol. Reprod. 2007, 76, 589–597.
- Bilodeau-Goeseels, S. Cows are not mice: The role of cyclic AMP, phosphodiesterases, and adenosine monophosphate-activated protein kinase in the maintenance of meiotic arrest in bovine oocytes. Mol. Reprod. Dev. 2011, 78, 734–743.
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235.
- Reverchon, M.; Cornuau, M.; Cloix, L.; Ramé, C.; Guerif, F.; Royère, D.; Dupont, J. Visfatin is expressed in human granulosa cells: Regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013, 19, 313–326.
- Bertoldo, M.J.; Guibert, E.; Faure, M.; Ramé, C.; Foretz, M.; Viollet, B.; Dupont, J.; Froment, P. Specific deletion of AMP-activated protein kinase (α1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS ONE 2015, 10, e0119680.
- Tosca, L.; Solnais, P.; Ferré, P.; Foufelle, F.; Dupont, J. Metformin-induced stimulation of adenosine 5’ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol. Reprod. 2006, 75, 342–351.
- Tosca, L.; Ramé, C.; Chabrolle, C.; Tesseraud, S.; Dupont, J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction 2010, 139, 409–418.
- Xu, Y.; Gao, Y.; Huang, Z.; Zheng, Y.; Teng, W.; Zheng, D.; Zheng, X. LKB1 suppresses androgen synthesis in a mouse model of hyperandrogenism via IGF-1 signaling. FEBS Open Bio 2019, 9, 1817–1825.
- Jiang, Z.Z.; Hu, M.W.; Ma, X.S.; Schatten, H.; Fan, H.Y.; Wang, Z.B.; Sun, Q.Y. LKB1 acts as a critical gatekeeper of ovarian primordial follicle pool. Oncotarget 2016, 7, 5738–5753.
- Morris, D.V.; Falcone, T. The relationship between insulin sensitivity and insulin-like growth factor-binding protein-1. Gynecol. Endocrinol. 1996, 10, 407–412.
- Landay, M.; Huang, A.; Azziz, R. Degree of hyperinsulinemia, independent of androgen levels, is an important determinant of the severity of hirsutism in PCOS. Fertil. Steril. 2009, 92, 643–647.
- Firmansyah, A.; Chalid, M.T.; Farid, R.B.; Nusratuddin, N. The correlation between insulin-like growth factor binding protein 1 (IGFBP-1) and homeostasis model assessment of insulin resistance (HOMA-IR) in polycystic ovarian syndrome with insulin resistance. Int. J. Reprod. Biomed. 2018, 16, 679–682.
- Huang, Y.; Yu, Y.; Gao, J.; Li, R.; Zhang, C.; Zhao, H.; Zhao, Y.; Qiao, J. Impaired oocyte quality induced by dehydroepiandrosterone is partially rescued by metformin treatment. PLoS ONE 2015, 10, e0122370.
- Jin, J.; Ma, Y.; Tong, X.; Yang, W.; Dai, Y.; Pan, Y.; Ren, P.; Liu, L.; Fan, H.Y.; Zhang, Y.; et al. Metformin inhibits testosterone-induced endoplasmic reticulum stress in ovarian granulosa cells via inactivation of p38 MAPK. Hum. Reprod. 2020, 35, 1145–1158.
- Wu, L.L.; Russell, D.L.; Norman, R.J.; Robker, R.L. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol. Endocrinol. 2012, 26, 562–573.
- Harada, M.; Nose, E.; Takahashi, N.; Hirota, Y.; Hirata, T.; Yoshino, O.; Koga, K.; Fujii, T.; Osuga, Y. Evidence of the activation of unfolded protein response in granulosa and cumulus cells during follicular growth and maturation. Gynecol. Endocrinol. 2015, 31, 783–787.
- Park, H.J.; Park, J.Y.; Kim, J.W.; Yang, S.G.; Jung, J.M.; Kim, M.J.; Kang, M.J.; Cho, Y.H.; Wee, G.; Yang, H.Y.; et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal Res. 2018, 64, e12458.
- Tobiume, K.; Matsuzawa, A.; Takahashi, T.; Nishitoh, H.; Morita, K.; Takeda, K.; Minowa, O.; Miyazono, K.; Noda, T.; Ichijo, H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001, 2, 222–228.
- Kim, H.S.; Kim, Y.; Lim, M.J.; Park, Y.G.; Park, S.I.; Sohn, J. The p38-activated ER stress-ATF6α axis mediates cellular senescence. FASEB J. 2019, 33, 2422–2434.
- Azhary, J.M.K.; Harada, M.; Takahashi, N.; Nose, E.; Kunitomi, C.; Koike, H.; Hirata, T.; Hirota, Y.; Koga, K.; Wada-Hiraike, O.; et al. Endoplasmic Reticulum Stress Activated by Androgen Enhances Apoptosis of Granulosa Cells via Induction of Death Receptor 5 in PCOS. Endocrinology 2019, 160, 119–132.
- Rice, S.; Pellatt, L.; Ramanathan, K.; Whitehead, S.A.; Mason, H.D. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology 2009, 150, 4794–4801.
- Rice, S.; Elia, A.; Jawad, Z.; Pellatt, L.; Mason, H.D. Metformin inhibits follicle-stimulating hormone (FSH) action in human granulosa cells: Relevance to polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 1491–1500.
- Fuhrmeister, I.P.; Branchini, G.; Pimentel, A.M.; Ferreira, G.D.; Capp, E.; Brum, I.S.; von Eye Corleta, H. Human granulosa cells: Insulin and insulin-like growth factor-1 receptors and aromatase expression modulation by metformin. Gynecol. Obstet. Investig. 2014, 77, 156–162.
- Catteau-Jonard, S.; Jamin, S.P.; Leclerc, A.; Gonzalès, J.; Dewailly, D.; di Clemente, N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4456–4461.
- González-Fernández, R.; Peña, Ó.; Hernández, J.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Patients with endometriosis and patients with poor ovarian reserve have abnormal follicle-stimulating hormone receptor signaling pathways. Fertil. Steril. 2011, 95, 2373–2378.
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724.
- Geng, Y.; Sui, C.; Xun, Y.; Lai, Q.; Jin, L. MiRNA-99a can regulate proliferation and apoptosis of human granulosa cells via targeting IGF-1R in polycystic ovary syndrome. J. Assist. Reprod. Genet. 2019, 36, 211–221.
- He, T.; Liu, Y.; Zhao, S.; Liu, H.; Wang, Z.; Shi, Y. Comprehensive assessment the expression of core elements related to IGFIR/PI3K pathway in granulosa cells of women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 134–140.
- He, T.; Sun, Y.; Zhang, Y.; Zhao, S.; Zheng, Y.; Hao, G.; Shi, Y. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod. Biol. Endocrinol. 2019, 17, 68.
- Kadoura, S.; Alhalabi, M.; Nattouf, A.H. Effect of Calcium and Vitamin D Supplements as an Adjuvant Therapy to Metformin on Menstrual Cycle Abnormalities, Hormonal Profile, and IGF-1 System in Polycystic Ovary Syndrome Patients: A Randomized, Placebo-Controlled Clinical Trial. Adv. Pharmacol. Sci. 2019, 2019, 9680390.
- Mason, H.D.; Margara, R.; Winston, R.M.; Seppala, M.; Koistinen, R.; Franks, S. Insulin-like growth factor-I (IGF-I) inhibits production of IGF-binding protein-1 while stimulating estradiol secretion in granulosa cells from normal and polycystic human ovaries. J. Clin. Endocrinol. Metab. 1993, 76, 1275–1279.
- Mason, H.D.; Willis, D.S.; Holly, J.M.; Franks, S. Insulin preincubation enhances insulin-like growth factor-II (IGF-II) action on steroidogenesis in human granulosa cells. J. Clin. Endocrinol. Metab. 1994, 78, 1265–1267.
- Willis, D.S.; Mason, H.D.; Watson, H.; Franks, S. Developmentally regulated responses of human granulosa cells to insulin-like growth factors (IGFs): IGF-I and IGF-II action mediated via the type-I IGF receptor. J. Clin. Endocrinol. Metab. 1998, 83, 1256–1259.
- Palomba, S.; Falbo, A.; Orio, F., Jr.; Manguso, F.; Russo, T.; Tolino, A.; Annamaria, C.; Dale, B.; Zullo, F. A randomized controlled trial evaluating metformin pre-treatment and co-administration in non-obese insulin-resistant women with polycystic ovary syndrome treated with controlled ovarian stimulation plus timed intercourse or intrauterine insemination. Hum. Reprod. 2005, 20, 2879–2886.
- Huang, X.; Wang, P.; Tal, R.; Lv, F.; Li, Y.; Zhang, X. A systematic review and meta-analysis of metformin among patients with polycystic ovary syndrome undergoing assisted reproductive technology procedures. Int. J. Gynaecol. Obstet. 2015, 131, 111–116.
- Piltonen, T.; Morin-Papunen, L.; Koivunen, R.; Perheentupa, A.; Ruokonen, A.; Tapanainen, J.S. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum. Reprod. 2005, 20, 1820–1826.
- Foroozanfard, F.; Samimi, M.; Almadani, K.H.; Sehat, M. Effect of metformin on the anti-Müllerian hormone level in infertile women with polycystic ovarian syndrome. Electron. Physician 2017, 9, 5969–5973.
- Fallat, M.E.; Siow, Y.; Marra, M.; Cook, C.; Carrillo, A. Müllerian-inhibiting substance in follicular fluid and serum: A comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil. Steril. 1997, 67, 962–965.
- Pigny, P.; Merlen, E.; Robert, Y.; Cortet-Rudelli, C.; Decanter, C.; Jonard, S.; Dewailly, D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: Relationship to the ovarian follicle excess and to the follicular arrest. J. Clin. Endocrinol. Metab. 2003, 88, 5957–5962.
- Laven, J.S.; Mulders, A.G.; Visser, J.A.; Themmen, A.P.; De Jong, F.H.; Fauser, B.C. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J. Clin. Endocrinol. Metab. 2004, 89, 318–323.
- Knight, P.G.; Glister, C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206.
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M.; Brown, K.; Simpson, E.R.; Mason, H.D. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil. Steril. 2011, 96, 1246–1251.
- Broer, S.L.; Broekmans, F.J.; Laven, J.S.; Fauser, B.C. Anti-Müllerian hormone: Ovarian reserve testing and its potential clinical implications. Hum. Reprod. Update 2014, 20, 688–701.
- Homburg, R.; Ray, A.; Bhide, P.; Gudi, A.; Shah, A.; Timms, P.; Grayson, K. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: A prospective cohort study. Hum. Reprod. 2013, 28, 1077–1083.
- Qi, X.; Pang, Y.; Qiao, J. The role of anti-Müllerian hormone in the pathogenesis and pathophysiological characteristics of polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 82–87.
- Lv, P.P.; Jin, M.; Rao, J.P.; Chen, J.; Wang, L.Q.; Huang, C.C.; Yang, S.Q.; Yao, Q.P.; Feng, L.; Shen, J.M.; et al. Role of anti-Müllerian hormone and testosterone in follicular growth: A cross-sectional study. BMC Endocr. Disord. 2020, 20, 101.
- Pierre, A.; Peigné, M.; Grynberg, M.; Arouche, N.; Taieb, J.; Hesters, L.; Gonzalès, J.; Picard, J.Y.; Dewailly, D.; Fanchin, R.; et al. Loss of LH-induced down-regulation of anti-Müllerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum. Reprod. 2013, 28, 762–769.
- Garg, D.; Tal, R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod. Biomed. Online 2016, 33, 15–28.
- Sova, H.; Unkila-Kallio, L.; Tiitinen, A.; Hippeläinen, M.; Perheentupa, A.; Tinkanen, H.; Puukka, K.; Bloigu, R.; Piltonen, T.; Tapanainen, J.S.; et al. Hormone profiling, including anti-Müllerian hormone (AMH), for the diagnosis of polycystic ovary syndrome (PCOS) and characterization of PCOS phenotypes. Gynecol. Endocrinol. 2019, 35, 595–600.
- Fleming, R.; Harborne, L.; MacLaughlin, D.T.; Ling, D.; Norman, J.; Sattar, N.; Seifer, D.B. Metformin reduces serum mullerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fertil. Steril. 2005, 83, 130–136.
- Tomova, A.; Deepinder, F.; Robeva, R.; Kirilov, G.; Mechandjiev, Z.; Kumanov, P. Anti-Müllerian hormone in women with polycystic ovary syndrome before and after therapy with metformin. Horm. Metab. Res. 2011, 43, 723–727.
- Neagu, M.; Cristescu, C. Anti-Műllerian hormone—A prognostic marker for metformin therapy efficiency in the treatment of women with infertility and polycystic ovary syndrome. J. Med. Life. 2012, 5, 462–464.
- Wiweko, B.; Susanto, C.A. The Effect of Metformin and Cinnamon on Serum Anti-Mullerian Hormone in Women Having PCOS: A Double-Blind, Randomized, Controlled Trial. J. Hum. Reprod. Sci. 2017, 10, 31–36.
- Chhabra, N.; Malik, S. Effect of Insulin Sensitizers on Raised Serum Anti-mullerian Hormone Levels in Infertile Women with Polycystic Ovarian Syndrome. J. Hum. Reprod. Sci. 2018, 11, 348–352.
- Furat Rencber, S.; Kurnaz Ozbek, S.; Eraldemır, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian Res. 2018, 11, 55.
- Saleh, B.O.; Ibraheem, W.F.; Ameen, N.S. The role of anti-Mullerian hormone and inhibin B in the assessment of metformin therapy in women with polycystic ovarian syndrome. Saudi Med. J. 2015, 36, 562–567.
- Goldman, S.; Shalev, E. MMPS and TIMPS in ovarian physiology and pathophysiology. Front. Biosci. 2004, 9, 2474–2483.
- Lewandowski, K.C.; Komorowski, J.; O’Callaghan, C.J.; Tan, B.K.; Chen, J.; Prelevic, G.M.; Randeva, H.S. Increased circulating levels of matrix metalloproteinase-2 and -9 in women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1173–1177.
- Chen, J.S.; Wang, Q.; Fu, X.H.; Huang, X.H.; Chen, X.L.; Cao, L.Q.; Chen, L.Z.; Tan, H.X.; Li, W.; Bi, J.; et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol. Res. 2009, 39, 177–186.
- Li, X.; Yang, Z.; Song, W.; Zhou, L.; Li, Q.; Tao, K.; Zhou, J.; Wang, X.; Zheng, Z.; You, N.; et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int. J. Oncol. 2013, 43, 793–802.
- Jalving, M.; Gietema, J.A.; Lefrandt, J.D.; de Jong, S.; Reyners, A.K.; Gans, R.O.; de Vries, E.G. Metformin: Taking away the candy for cancer? Eur. J. Cancer 2010, 46, 2369–2380.
- Chen, Z.; Wei, H.; Zhao, X.; Xin, X.; Peng, L.; Ning, Y.; Wang, Y.; Lan, Y.; Zhang, Q. Metformin treatment alleviates polycystic ovary syndrome by decreasing the expression of MMP-2 and MMP-9 via H19/miR-29b-3p and AKT/mTOR/autophagy signaling pathways. J. Cell. Physiol. 2019, 234, 19964–19976.
- Sam, S.; Dunaif, A. Polycystic ovary syndrome: Syndrome XX? Trends Endocrinol. Metab. 2003, 14, 365–370.
- Teede, H.J.; Hutchison, S.K.; Zoungas, S. The management of insulin resistance in polycystic ovary syndrome. Trends Endocrinol. Metab. 2007, 18, 273–279.
- Kai, Y.; Kawano, Y.; Yamamoto, H.; Narahara, H. A possible role for AMP-activated protein kinase activated by metformin and AICAR in human granulosa cells. Reprod. Biol. Endocrinol. 2015, 13, 27.
- Wang, W.; Zheng, J.; Cui, N.; Jiang, L.; Zhou, H.; Zhang, D.; Hao, G. Baicalin ameliorates polycystic ovary syndrome through AMP-activated protein kinase. J. Ovarian Res. 2019, 12, 109.
- Maciel, G.A.; Hayashida, S.A.; da Costa, L.C.; Marcondes, J.A.; da Fonseca, A.M.; Soares, J.M., Jr.; Baracat, E.C. Influence of LH and high-density lipoprotein cholesterol (HDL-C) on metformin response in women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 180–184.
- Gambineri, A.; Tomassoni, F.; Gasparini, D.I.; Di Rocco, A.; Mantovani, V.; Pagotto, U.; Altieri, P.; Sanna, S.; Fulghesu, A.M.; Pasquali, R. Organic cation transporter 1 polymorphisms predict the metabolic response to metformin in women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2010, 95, 204–208.
- Schweighofer, N.; Lerchbaum, E.; Trummer, O.; Schwetz, V.; Pieber, T.; Obermayer-Pietsch, B. Metformin resistance alleles in polycystic ovary syndrome: Pattern and association with glucose metabolism. Pharmacogenomics 2014, 15, 305–317.
- Mofo Mato, E.P.; Guewo-Fokeng, M.; Essop, M.F.; Owira, P.M.O. Genetic polymorphisms of organic cation transporter 1 (OCT1) and responses to metformin therapy in individuals with type 2 diabetes: A systematic review. Medicine 2018, 97, e11349.
- Di Pietro, M.; Velazquez, C.; Matzkin, M.E.; Frungieri, M.B.; Peña, M.G.; de Zúñiga, I.; Pascuali, N.; Irusta, G.; Bianchi, M.S.; Parborell, F.; et al. Metformin has a direct effect on ovarian cells that is dependent on organic cation transporters. Mol. Cell. Endocrinol. 2020, 499, 110591.
More
