Acute respiratory distress syndrome (ARDS) is a serious clinical illness, defined by severe hypoxemic respiratory failure, which continues to be associated with significant morbidity, mortality, and healthcare resource utilization.
- ARDS, COVID-19, ventilatory support
- ARDS
- ventilatory support
- COVID-19
definition
Acute respiratory distress syndrome (ARDS) is a serious clinical illness, defined by severe hypoxemic respiratory failure, which continues to be associated with significant morbidity, mortality, and healthcare resource utilization.
1. Introduction
ARDS comprises 7–10% of admissions and 15–25% of mechanically ventilated patients in the intensive care unit (ICU), is fatal in 30–50% of patients, and costs on average over USD 90,000 per patient’s ICU stay [1–5][1][2][3][4][5].
ARDS has been intensively investigated for more than 50 years, resulting in our current understanding of a clinical-physiologic syndrome of lung inflammation and injury, biologically driven by a plethora of inflammatory cells and soluble molecules (i.e., cytokines). Despite greater understanding and multiple international clinical practice guidelines, ARDS remains under-recognized, the clinical importance is under-appreciated, and management is sub-optimal [2][2]. As such, many patients continue to suffer more severe, prolonged ARDS and worse clinical outcomes including higher mortality. Moreover, novel causes of ARDS, like coronavirus disease 2019 (COVID-19) are contributing to significant human disease and will undoubtedly continue to do so in the future. The global COVID-19 pandemic offers an important opportunity for all physicians to update their understanding of ARDS.
2. Causes of ARDS
The most common clinical conditions associated with development of ARDS include severe pneumonia (30–50%) and sepsis (25–30%; Figure 1), as confirmed in many large single- and multi-centred cohorts, including the Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNGSAFE) registry, the largest cross-sectional study of ARDS patients admitted to intensive care units (ICUs) [2,6][2][6].
Pneumonia-associated ARDS is frequently due to bacterial infection (e.g., Streptococcus pneumoniae, Staphylococcus aureus), but also develops with viral (e.g., influenza A) and fungal (e.g., Pneumocystis jirovecii) infections [6][6]. Coronavirus (CoV) causes of pneumonia and resulting ARDS have been recognized since the 2003 pandemic of severe acute respiratory syndrome (SARS). SARS-CoV-2 is a novel human coronavirus responsible for the pandemic known as COVID-19, first described in Wuhan, China in December 2019 [7][7]. Since then, more than 40 million COVID-19 cases globally have resulted in over 1 million deaths [8][8]. Although most infected individuals are asymptomatic or exhibit only mild symptoms, a significant minority of COVID-19 patients develop severe illness requiring hospitalization (10–14%), typically manifested as pneumonia [9,10][9][10].
Sepsis has long been recognized as a common and clinically important cause of ARDS. For example, sepsis was the primary cause of ARDS in 16% of cases in the LUNGSAFE registry and approximately 18% of patients with septic shock developed ARDS [2][2]. Moreover, sepsis-induced ARDS can have a worse prognosis than other causes of ARDS, typically because of the presence of co-morbid illnesses and higher risk of multiple organ dysfunction syndrome (MODS) [11,12][11][12].
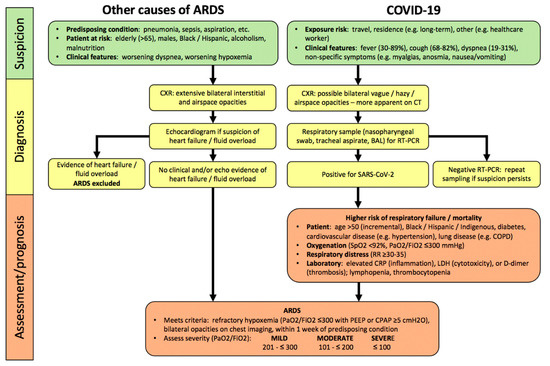
Figure 1. Algorithm for hospitalized patients at risk for acute respiratory distress syndrome (ARDS) and coronavirus disease 2019 (COVID-19). In contrast, to COVID-19, there are no specific lab abnormalities which adequately assess severity or predict prognosis in other causes of ARDS. Abbreviations: PaO2, partial pressure of oxygen in arterial blood; FiO2, inspired oxygen fraction; RR, respiratory rate; CRP, C-reactive protein; LDH, lactate dehydrogenase; PEEP, positive end-expiratory pressure; CPAP, continuous positive airway pressure.
3. Clinical Pathophysiology of ARDS
ARDS is characterized by the rapid development of severe lung inflammation causing damage to alveolar epithelial cells (AEC) and pulmonary microvascular endothelial cells (EC). Dysfunction of the alveolar-capillary endothelial barrier results in diffuse alveolar damage (DAD), which includes an initial exudative phase characterized by high-permeability, proteinaceous pulmonary interstitial and alveolar edema associated with the injury and death of EC as well as AEC desquamation, and a delayed fibroproliferative phase comprising fibrosis in intraluminal and interstitial compartments, and type II AEC proliferation (Table 1) [13,14][13][14]. Pathologic DAD is found in about half of patients with ARDS, and is associated with more severe hypoxemia and higher mortality [13][13].
Severe hypoxemia in ARDS is exacerbated by concomitant pathophysiologic disturbances, including surfactant dysfunction (reducing lung compliance causing atelectasis), pulmonary microvascular thrombosis (due to EC injury), higher physiological dead space, and increased shunt fraction due to impairment of hypoxic pulmonary vasoconstriction [15–18][15][16][17][18]. In addition, patients’ respiratory distress and strong inspiratory efforts can increase negative pleural pressure swings, increasing lung inflation stress, pulmonary blood flow and vascular pressures, potentially worsening pulmonary edema, collectively termed patient self-induced lung injury (P-SILI) [19][19].
The pathology of COVID-19-associated ARDS (COVID-ARDS) is also largely characterized by DAD, with some key differences such as lymphocyte rather than neutrophil predominance (Table 1). Some studies have highlighted more severe pulmonary microvascular EC injury associated with extensive microvascular thrombosis [20[20][21],21], for example, pulmonary vascular clot burden was up to nine times greater in COVID-ARDS versus influenza-associated ARDS [20][20]. However, such pulmonary microvascular findings have not been consistently observed in other pathologic descriptions [22–24][22][23][24]. The pathophysiology of respiratory failure in most patients with COVID-ARDS is also similar to other causes of ARDS, including atelectasis, low respiratory compliance, and intrapulmonary shunt [25,26][25][26]. It has been suggested that some COVID-ARDS patients manifest a different phenotype characterized by consolidation without atelectasis, preserved respiratory compliance, and more striking perfusion dysregulation, which may have treatment implications [27–29][27][28][29]. This remains an area of controversy and active clinical and physiologic research.
Table 1.
Pulmonary pathology features of COVID-19-associated ARDS (COVID-ARDS) versus other causes of acute respiratory distress syndrome (ARDS).
|
Pathology |
ARDS |
COVID-ARDS |
|
Diffuse Alveolar |
Early/Exudative: - interstitial/alveolar edema - “hyaline” membranes - neutrophil infiltration - AEC desquamation - pulmonary microvascular thrombosis Late/Fibroproliferative: - alveolar/interstitial fibrosis - type II AEC hyperplasia
|
Similar to ARDS except: - paucity of neutrophils - interstitial/alveolar lymphocytic infiltration - possibly increased pulmonary microvascular thrombi relative to other causes
|
|
Other features |
- organizing pneumonia (fibrosis) - alveolar haemorrhage - viral pneumonia |
- occasional viral cytopathic changes (multinucleated syncytial cells, atypical enlarged AEC) - viral inclusions in AEC |
Abbreviations: AEC, alveolar epithelial cells.
- Diagnosis of ARDS
4. Diagnosis of ARDS
34.1. Clinical Assessment
By definition, ARDS develops within one week of onset or worsening of a predisposing condition (Figure 1), most commonly (>90%) within 48 h [1,2][1][2]. Demographic risks for developing ARDS are recognized (e.g., greater age, male sex, non-Caucasian ethnicity) [30,31][30][31]. ARDS diagnosis in hospitalized patients requires a clinical suspicion, based upon predisposing conditions, worsening oxygenation and dyspnea, bilateral interstitial and/or alveolar opacities consistent with pulmonary edema on chest radiograph (CXR), and exclusion of common causes of pulmonary edema (e.g., heart failure, fluid overload) clinically or with echocardiography [1][1].
A significant care-gap exists in the diagnosis of ARDS, especially mild ARDS which was unrecognized in 50% of patients in the large, global LUNGSAFE registry [2]. Indeed, mild ARDS is not a benign illness, as less than 20% of patients recovered within a week and overall in-hospital mortality was 29.7%. In addition, more than 40% of mild ARDS progressed to moderate–severe ARDS which was associated with higher mortality of 35–42.9% [32][32].
Most COVID-19 patients develop symptoms of fever, cough, and dyspnea within 5 days of infection (Figure 1). Hospitalized patients can deteriorate quite rapidly within hours to a few days, manifesting worsening hypoxemia and respiratory distress as features of severe pneumonia, and 20–30% develop COVID-ARDS [9,10,33,34][9][10][33][34]. Compared to patients with other causes of ARDS, pulmonary opacities are less obvious on CXR (54–76%) in COVID-ARDS patients [35,36][35][36]. Chest CT scan is clearly more sensitive to the presence of abnormalities in patients with confirmed COVID-19, with a sensitivity of 93.1% (95% CI: 90.2–95.0) in a meta-analysis (65 studies; 5759 patients) [37][37], but abnormalities are poorly specific for a diagnosis of COVID-19 compared to other respiratory infections.
34.2. Assessment of Severity
ARDS severity is assessed by the degree of hypoxemia, quantified by the ratio of arterial partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) as per the Berlin criteria, which is strongly predictive of worsening survival (Figure 1) [1][1]. In addition, the presence of hypercapnia (PaCO2 >50 mmHg) was independently associated with more organ dysfunction and higher mortality[38] [38]. Other laboratory abnormalities have not been shown to assess severity or predict prognosis in ARDS, but key investigations can identify prognostically-important complications of non-pulmonary organ dysfunction, e.g., cardiac, renal, and potentially MODS[39] [39]
In patients with COVID-19, severity of pneumonia and respiratory failure is also assessed by the degree of hypoxemia, including arterial oxygen saturation by pulse oximetry (SpO2), and the PaO2/FiO2 ratio[26][40] [26,40] . COVID-19 is associated with distinct laboratory abnormalities which predict greater risk of respiratory failure and worse clinical outcomes including higher mortality, independent of the severity of ARDS. These include markers of inflammation (elevated C-reactive protein (CRP)), cytotoxicity (increased lactate dehydrogenase (LDH)), and both macrovascular and microvascular thrombosis in systemic and pulmonary circulations (higher D-dimer levels), as well as lymphopenia (Figure 1) [41,42][41][42]. It has been suggested that these markers should be assessed at baseline in hospitalized COVID-19 patients [40[40][43],43], however the clinical utility of serial monitoring has not yet been established.
Given the presence of multiple physiologic and laboratory abnormalities in COVID-19, there may be more robust prognostic value in assessing a combination of parameters. For example, in a multicentre, observational retrospective study of patients being assessed in the ED, a model developed through machine-learning, the Quick COVID-19 Severity Index comprising three respiratory parameters (FiO2, SpO2, respiratory rate (RR)) was predictive of the risk of respiratory failure within the first 24 h of admission[44] [44]. Following hospital admission, another machine-learning composite score, which included age, lymphocyte count and levels of inflammatory markers (e.g., LDH, CRP), was found to best predict the risk of severe hypoxemic respiratory failure, need for ICU admission and/or invasive respiratory support, and mortality in hospitalized COVID-19 patients [45][45]. Finally, in COVID-19 patients with ARDS, a multicentre, observational study identified the highest risk of mortality was associated with both reduced respiratory compliance and higher D-dimer levels [46][46].
4. Management of Patients with ARDS
4.1. General Approach
Management of ARDS remains largely supportive, including treatment of the predisposing condition, as there are no specific medical therapies that address the lung inflammation and alveolo-capillary injury. Standard care for ICU-admitted patients includes early nutritional support, appropriate analgesia, sedation, thromboprophylaxis, semi-recumbent position, gastric ulcer prophylaxis, and glycaemic control (FASTHUG) [47][47]. In ARDS patients, the frequent presence of non-pulmonary organ dysfunction or development of MODS contributes to severity of illness, intensity of required care, and mortality [12,48][12][48]. Similarly, thirty-to-fifty percent of critically-ill COVID-19 patients will develop non-pulmonary organ dysfunction leading to MODS, which is the most common cause of mortality [34,36,49][34][36][49].
Many respiratory support modalities are high-risk aerosol-generating medical procedures requiring specific attention, during the care of COVID-19 patients, to minimization of unnecessary staff exposure, appropriate contact precautions, and airway management expertise. Physicians are encouraged to follow local guidelines for safe application and monitoring of all respiratory support and associated procedures, e.g., high-flow nasal-cannula O2 (HFNO), non-invasive positive pressure ventilation (NIPPV), intubation, mechanical ventilation (MV), bronchoscopy [40][40].
4.2. Respiratory Support of Mild ARDS
Initial respiratory support of patients with hypoxemia consists of supplemental O2 [50][50]. Specific SpO2 targets in various patient populations remain uncertain, given competing goals of addressing persistent hypoxemia as well as avoiding hyperoxia, both of which may be associated with increased mortality [51,52][51][52]. In ARDS, permissive hypoxemia is not recommended [53–56][53][54][55][56]. For example, conservative O2 (SpO2 88–92%) was associated with a non-significant higher risk of 28-day mortality, but higher 90-day mortality and more intestinal ischemia than more liberal O2 (SpO2 ≥ 96%) [53]. In persistent hypoxemic respiratory failure despite maximal supplemental O2 by facemask, various non-invasive respiratory support modalities may be considered, and clearly are being commonly employed recently in COVID-19 patients [57–59][57][58][59].
4.2.1. High-Flow Nasal-Cannula O2 (HFNO)
This is a novel technique which can improve oxygenation in hypoxemic respiratory failure (Figure 2), through several mechanisms including higher inspired O2 concentrations ≤90%, decreased dead space, and increased lung volume through generation of a low-level of continuous positive airway pressure (CPAP) [60,61][60][61]. In the largest randomized controlled trial (RCT) of HFNO vs. standard O2 therapy in patients with hypoxemic respiratory failure (the absence of use of CPAP meant that ARDS could not be formally diagnosed based on Berlin criteria), HFNO reduced 90-day mortality by 50% but there was no difference in the need for invasive respiratory support through intubation/MV [60][60]. A retrospective review and two meta-analyses have concluded that HFNO was associated with 15–24% reduced risk of subsequent intubation in hypoxemic respiratory failure, but did not reduce duration of hospital or ICU admission or improve survival [62–64][62][63][64].
HFNO has more commonly been used in the management of hypoxemic respiratory failure in COVID-19 patients, depending on geography and access to other respiratory support measures [57,65,66][57][65][66]. For example, 5–64% of moderate–severe hypoxemic COVID-19 patients in Italy, China, and the US were initially supported with HFNO[42][67][68][69]. [42,67–69] In a retrospective review of the largest single-centre series of 104 COVID-19 patients with moderate–severe hypoxemia, 64% of those treated with HFNO avoided intubation and had mortality of 2.9%, compared to 14.4% in those requiring subsequent intubation/MV [63][63].
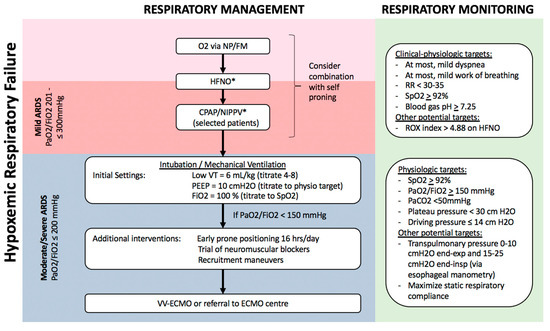
Figure 2. Algorithm for respiratory management of patients with hypoxemic respiratory failure. Patients with hypoxemia despite supplemental O2, including those who meet criteria for mild ARDS, can potentially be managed with non-invasive respiratory support, including HFNO and NIPPV, possibly in combination with self-proning. Sequential escalation of non-invasive respiratory support modalities should be considered unless clinical-physiologic targets are met, depending on local critical care expertise and resources. Patients with moderate–severe ARDS require invasive mechanical ventilation with monitoring and adjustment of ventilatory parameters to minimize ventilator-induced lung injury (VILI), and may benefit from additional measures to improve oxygenation such as prone positioning, recruitment manoeuvres, and potentially veno-venous extra-corporeal membrane oxygenation (VV-ECMO). Notes: a. Non-invasive respiratory support with HFNO/CPAP/NIPPV requires careful monitoring for lack of improvement or persistent respiratory distress, and consideration of intubation/mechanical ventilation. b. All non-invasive and invasive respiratory support modalities are high-risk aerosol-generating medical procedures which should be carried out by experts in airway management, with appropriate precautions (e.g., minimal staff in room, N95, negative pressure room). c. Tidal volume is referenced to predicted body weight. d. Recruitment manoeuvres requires sustained inflation, e.g., inspiratory hold at 35–40 cm H2O for set time (e.g., 40 s). Stepwise recruitment (with incremental levels of PEEP) is not recommended. e. ROX index = SpO2/FiO2/Respiratory Rate. f. Plateau pressure = airway pressure after 0.5 s pause at end-inspiration. g. Driving pressure = plateau pressure – PEEP. h. Transpulmonary pressure = airway pressure – pleural pressure (under zero flow conditions). i. Static respiratory compliance = tidal volume/(plateau pressure – PEEP). Abbreviations: NP, nasal prongs; FM, facemask; HFNO, high-flow nasal oxygen; CPAP, continuous positive airway pressure; NIPPV, non-invasive positive pressure ventilation; RR, respiratory rate; VT, tidal volume; PEEP, positive end-expiratory pressure; ECMO, extra-corporeal membrane oxygenation. * These interventions, while increasingly being used globally, especially during the COVID-19 pandemic, are not yet supported by robust evidence in patients with ARDS.
4.2.2. Continuous Positive Airway Pressure (CPAP)/Non-Invasive Positive Pressure Ventilation (NIPPV)
In patients with persistent hypoxemia despite maximal supplemental O2 by either facemask or HFNO, a trial of either CPAP (via nasal/facemask or hood/helmet) or NIPPV via facemask can be considered. CPAP/NIPPV may be beneficial in improving oxygenation and respiratory distress, decreasing FiO2 requirements, and possibly reducing the need for invasive support through intubation/MV [70,71][70][71]. For example, the above-cited network meta-analysis of non-invasive respiratory support in hypoxemic respiratory failure reported that both helmet NIPPV (RR 0.26, 95%CI 0.14–0.46) and facemask NIPPV (RR 0.76, 95%CI 0.62–0.90) reduced the risk of subsequent intubation and were both associated with reduced risk of death compared to supplemental O2 [62,72][62][72].
CPAP/NIPPV is commonly being used globally for patients with hypoxemic respiratory failure including ARDS, e.g., 15.5% of ARDS patients in the global LUNGSAFE registry [70][70]. However, there is a clear risk of failure of such non-invasive respiratory support, as 22.2% of mild and 42–47% of moderate–severe ARDS patients failed CPAP/NIPPV trial within 2 days, experiencing lack of improvement or worsening of respiratory distress and/or hypoxemia [70][70]. During NIPPV trials, careful respiratory monitoring is essential because clinical outcomes are worse in patients who fail NIPPV, possibly because of delayed definitive management of respiratory failure with intubation and MV [73,74][73][74]. For example, patients with hypoxemic respiratory failure who failed NIPPV had longer ICU and hospital stay, as well as more than four-fold higher mortality [74][74]. Thus, NIPPV may be beneficial in patients with mild ARDS (Figure 2), but this specific respiratory support measure has not been specifically recommended in recent guidelines [48,75][48][75].
Non-invasive respiratory support with CPAP/NIPPV is also being increasingly instituted in COVID-19 patients, especially under local conditions of constrained ICU resources [59][59]. For example, 3–56% of hypoxemic COVID-19 were treated with CPAP/NIPPV, with higher rates of usage in critically ill and moderate–severe patients [7,10,33,49,66–69,76,77][7][10][33][49][66][67][68][69][76][77]. Several uncontrolled reports suggested a reduced need for intubation, but only a single controlled study has addressed this, using a retrospective, historical time period-controlled cohort design, reporting significantly higher intubation-free survival at 7 days with CPAP [78][78]. While such non-invasive respiratory support measures may be appropriate in some COVID-19 patients with hypoxemic respiratory failure, specifically those who either do not yet meet criteria for ARDS or have mild ARDS (Figure 2), current guidelines do not provide any specific recommendations in the absence of more robust data [40,43,59,79][40][44][59][79].
4.2.3. Prone Positioning
Based on strong evidence for improved clinical outcomes in ARDS patients who are intubated and ventilated (see Section 3.3. Respiratory Support of Moderate–Severe ARDS below[80] [80]), prone positioning is being increasingly used to improve oxygenation in spontaneously-breathing non-intubated patients with hypoxemic respiratory failure, including COVID-19. For example, in a small prospective cohort study of 20 patients with ARDS, prone positioning combined with either HFNO or NIPPV was associated with reduced need for intubation/MV only in patients with moderate ARDS, not in those with severe ARDS [81][81].
Several uncontrolled series have reported that self-proning may improve oxygenation in spontaneously breathing COVID-19 patients receiving supplemental O2 or other non-invasive respiratory support (e.g., HFNO, CPAP/NIPPV) [65,82–85][65][82][83][84][85]. In the first reported series of 50 COVID-19 patients managed in the emergency department, oxygenation improved from an average of 84% on supplemental O2 to 94% after self-proning for 5 min [82][82]. In addition, 64% of patients with unspecified repeated self-proning sessions recovered to hospital discharge without intubation/MV. Self-proning was not tolerated, including worsening oxygenation and/or respiratory distress, in 13–25% of patients [84,86,87][84][86][87]. Moreover, although oxygenation improves in most patients when prone, the improvement is maintained in only about 50% of patients when resuming the supine position, with some evidence that proning may be more effective earlier in the hospital course and specifically in patients with higher inflammatory markers (e.g., CRP, LDH) [84][84]. Early oxygenation improvement has been associated with reduced need for subsequent intubation/MV in some studies[87][88] [87,88] but not in others [84][84]. In summary, self-proning is currently widely employed in the management of COVID-19 patients globally in the absence of strong evidence of improved outcomes and there are no clear recommendations regarding specifics of patient selection, duration and frequency of proning sessions. Self-proning is not feasible or tolerable for all patients, is associated with clear risks, including inadequate respiratory support in patients with respiratory distress and/or high work of breathing which are associated with higher risk of P-SILI and progressive lung injury [19,89,90][19][89][90]. As such, prone positioning in spontaneously breathing patients mandates rigorous clinical and respiratory monitoring for lack of improvement and/or persistent respiratory distress in order to facilitate timely intubation/MV.
4.3. Respiratory Support of Moderate–Severe ARDS
In moderate–severe ARDS patients, respiratory management is similar for ARDS from all causes including COVID-19 (Figure 2) [26,40,48,75,79,91][26][40][48][75][79][91]. Invasive respiratory support through endotracheal intubation and MV is strongly recommended for worsening or persistent respiratory distress, persistent hypoxemia (SpO2 < 92%), or progressive hypercapnia. In patients requiring MV, specific ventilatory modalities and parameters are guidelines-recommended based on improved outcomes in multiple RCTs (Figure 2) [48,75,91][48][75][91]. The goal is to use a lung protective strategy to prevent excessive lung tidal-inflation stress (volutrauma) and cyclic atelectasis-recruitment (atelectrauma), reducing the risk of ventilation-induced lung injury (VILI) [92][92]. The most important measure is MV using low-tidal volumes, specifically a target of 4–8 mL/kg predicted body weight [91,93][91][93].
The application of positive end-expiratory pressure (PEEP) is essential in order to reduce atelectasis and maximize respiratory compliance, and PEEP is optimally selected to avoid excessive plateau and driving pressures (Figure 2) [91,93,94][91][93][94]. Novel physiologic monitoring using oesophageal manometry may allow optimization of PEEP in individual patients, although the benefit of such an approach in terms of clinical outcomes remains uncertain [95,96][95][96]. Additionally, early prone positioning should be implemented as a lung protective measure, as it has been shown to reduce 28-day mortality by 16% when implemented 12–24 h after initiation of MV [80][80].
Several weak recommendations suggest approaches for management of persistent hypoxemia, patient-ventilator dyssynchrony, or low lung compliance with high plateau or driving pressures (Figure 2). These include short courses of neuromuscular blockade-induced paralysis, and specific recruitment manoeuvres [91,97,98][91][97][98]. Refractory hypoxemia not responding to conventional therapy warrants consideration of veno-venous extra-corporeal membrane oxygenation (VV-ECMO). Besides directly improving hypoxemia and related multiple organ dysfunction, ECMO may offer more homogeneous, ultraprotective ventilation. In brief, ECMO should be considered when patients have (a) persistent PaO2/FiO2 <50 mmHg for >3 hrs or <80 mmHg for >6 h despite FiO2 >80% and PEEP >10, or (b) pH < 7.25 with PaCO2 > 60 mmHg for >6 h. If ECMO is not available locally, patients with severe respiratory failure should be considered for transfer to a high-volume facility with ECMO expertise, if clinically feasible. VV-ECMO achieves similar outcomes in all causes of ARDS, including COVID-ARDS [99,100][99][100].
4.4. Medical Approaches to ARDS Therapy
Given the central contribution of alveolo-capillary injury and high-permeability pulmonary edema to refractory hypoxemia in ARDS, conservative fluid management after initial resuscitation may reduce edema, improve gas-exchange, and improve clinical outcomes such as decreased duration of MV and ICU length of stay (Table 2) [91,101][91][101]. Regardless of the primary cause of ARDS, the presence of concomitant bacterial infection should be investigated, and broad-spectrum antibiotic therapy considered. Limited evidence indicates early systemic steroids may reduce duration of MV and mortality, but there are conflicting recommendations regarding dose, timing, and consideration in individual patients (Table 2) [91,102][91][102]. A multitude of RCTs of various anti-inflammatory and pathophysiology-based therapies have failed to improve clinical outcomes, such that there is no specific medical therapy currently indicated or recommended for lung inflammation and injury in ARDS patients.
There is active research into various anti-viral and anti-inflammatory therapies specifically for SARS-CoV-2 infection resulting in COVID-19 pneumonia and/or ARDS (Table 2) [79,103][79][103]. Strong evidence supports that corticosteroids (i.e., dexamethasone) reduce the need for ICU admission and intubation/MV in hospitalized, hypoxemic COVID-19 patients. Moreover, in COVID-ARDS patients, corticosteroids shorten the duration of MV and reduce mortality [104][104]. As such, corticosteroids are strongly recommended for hypoxemic COVID-19 patients [104,105][104][105]. Remdesivir is the first antiviral drug found to have some clinical benefit, namely in reducing time to recovery [106][106]. Many putative therapies are in ongoing clinical trials with some promise of preventing or treating COVID-19, including human convalescent plasma, systemic anticoagulation, and 25-hydroxy vitamin D [107–109][107][108][109]. A number of other medical therapies have been considered but have shown no benefit, including lopanivir/ritonavir and hydroxychloroquine [110,111][110][111]. There is concern around the routine clinical use of unproven experimental therapies, including high risk of drug–drug interactions given that the majority of hospitalized COVID-19 patients are older with multiple co-morbidities requiring treatment with many other medications [112][112].
Table 2.
Medical treatment approaches for ARDS and specifically for COVID-19-associated ARDS.
|
Intervention |
ARDS |
COVID-ARDS |
|
Fluid management |
||
|
Conservative fluid strategy |
Weak recommendation post initial resuscitation (SCCM[48], FICM-ICS[75]) |
Weak recommendation (SSC [40]) |
|
Anti-inflammatory therapy |
||
|
Steroid |
Weak recommendation - Methylprednisolone 1–2 mg/kg/d with 14 d taper (FICM-ICS[75], SCCM-ESICM[102] |
Recommended - Dexamethasone 6 mg/d for 10 d (WHO[33], IDSA[79], CMAJ[103]) |
|
Other (Physiologic/Biologic) |
Not recommended - β2-agonists - Exogenous surfactant - Anti-IL1β - Statins |
Not recommended - Hydroxychloroquine/chloroquine - Lopanivir/ritonavir |
|
Experimental |
Current trials - Anti-tissue factor antibody fragment - MAPK inhibitor - Stem cell therapies - Complement inhibitor - JAK inhibitor |
Current trials - Intravenous Immunoglobulin - IL-6 inhibitor (e.g., tocilizumab) - IL-1 inhibitor (e.g., anakinra) - Anti-GM-CSF (e.g., mavrilimumab) - Anticoagulants (e.g., Low molecular weight heparin) - Fibrinolytics (e.g., tPA) - 25-OH vitamin D |
|
Anti-microbials |
||
|
Antibiotics |
Strong recommendation - If ARDS due to pneumonia or sepsis (SCCM[48]) - If evidence of ventilator-associated pneumonia (SCCM[48]]) |
Weak recommendation - In patients requiring MV (SSC[40], IDSA[79]) - If concomitant bacterial pneumonia (SSC[40], IDSA[79])) |
|
Antivirals |
Specific viral targeted therapy indicated - If viral infection identified (e.g., influenza, RSV) |
Specific viral targeted therapy indicated - If evidence of concomitant viral pneumonia (e.g., influenza, RSV) SARS-CoV-2 targeted therapy - Remdesivir (IDSA[79]) |
|
Intervention |
ARDS |
COVID-ARDS |
|
Fluid management |
||
|
Conservative fluid strategy |
Weak recommendation post initial resuscitation (SCCM [48], FICM-ICS [75]) |
Weak recommendation (SSC [40]) |
|
Anti-inflammatory therapy |
||
|
Steroid |
Weak recommendation - Methylprednisolone 1–2 mg/kg/d with 14 d taper (FICM-ICS [75], SCCM-ESICM [102] |
Recommended - Dexamethasone 6 mg/d for 10 d (WHO [33], IDSA [79], CMAJ [103]) |
|
Other (Physiologic/Biologic) |
Not recommended - β2-agonists - Exogenous surfactant - Anti-IL1β - Statins |
Not recommended - Hydroxychloroquine/chloroquine - Lopanivir/ritonavir |
|
Experimental |
Current trials - Anti-tissue factor antibody fragment - MAPK inhibitor - Stem cell therapies - Complement inhibitor - JAK inhibitor |
Current trials - Intravenous Immunoglobulin - IL-6 inhibitor (e.g., tocilizumab) - IL-1 inhibitor (e.g., anakinra) - Anti-GM-CSF (e.g., mavrilimumab) - Anticoagulants (e.g., Low molecular weight heparin) - Fibrinolytics (e.g., tPA) - 25-OH vitamin D |
|
Anti-microbials |
||
|
Antibiotics |
Strong recommendation - If ARDS due to pneumonia or sepsis (SCCM [48]) - If evidence of ventilator-associated pneumonia (SCCM [48]) |
Weak recommendation - In patients requiring MV (SSC [40], IDSA [79]) - If concomitant bacterial pneumonia (SSC [40], IDSA [79])) |
|
Antivirals |
Specific viral targeted therapy indicated - If viral infection identified (e.g., influenza, RSV) |
Specific viral targeted therapy indicated - If evidence of concomitant viral pneumonia (e.g., influenza, RSV) SARS-CoV-2 targeted therapy - Remdesivir (IDSA [79]) |
References
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533.
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800.
- Brun-Buisson, C.; Minelli, C.; Bertolini, G.; Brazzi, L.; Pimentel, J.; Lewandowski, K.; Bion, J.; Romand, J.-A.; Villar, J.; Thorsteinsson, A.; et al. Epidemiology and outcome of acute lung injury in European intensive care units. Intensive Care Med. 2004, 30, 51–61.
- Cheung, A.; Tansey, C.M.; Tomlinson, G.; Diaz-Granados, N.; Matte, A.; Barr, A.; Mehta, S.; Mazer, C.D.; Guest, C.B.; Stewart, T.E.; et al. Two-Year Outcomes, Health Care Use, and Costs of Survivors of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 538–544.
- Pham, T.; Rubenfeld, G.D. Fifty Years of Research in ARDS.The Epidemiology of Acute Respiratory Distress Syndrome. A 50th Birthday Review. Am. J. Respir. Crit. Care Med. 2017, 195, 860–870.
- De Prost, N.; Pham, T.; Carteaux, G.; Dessap, A.M.; Brun-Buisson, C.; Fan, E.; Bellani, G.; Laffey, J.G.; Mercat, A.; Brochard, L.; et al. Etiologies, diagnostic work-up and outcomes of acute respiratory distress syndrome with no common risk factor: A prospective multicenter study. Ann. Intensive Care 2017, 7, 69.
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513.
- World Health Organization. Coronavirus Disease (COVID-19) Situation Reports [Internet]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Luo, L.; Shaver, C.M.; Zhao, Z.; Koyama, T.; Calfee, C.S.; Bastarache, J.A.; Ware, L.B. Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. Chest 2017, 151, 755–763.
- Sheu, C.C.; Gong, M.N.; Zhai, R.; Chen, F.; Bajwa, E.K.; Clardy, P.F.; Gallagher, D.C.; Thompson, B.T.; Christiani, D.C. Clinical Characteristics and Outcomes of Sepsis-Related vs. Non-Sepsis-Related ARDS. Chest 2010, 138, 559–567.
- Cardinal-Fernández, P.; Bajwa, E.K.; Dominguez-Calvo, A.; Menéndez, J.M.; Papazian, L.; Thompson, B.T. The Presence of Diffuse Alveolar Damage on Open Lung Biopsy Is Associated with Mortality in Patients with Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Chest 2016, 149, 1155–1164.
- Guerin, C.; Bayle, F.; Leray, V.; Debord, S.; Stoian, A.; Yonis, H.; Roudaut, J.; Bourdin, G.; Devouassoux-Shisheboran, M.; Bucher, E.; et al. Open lung biopsy in nonresolving ARDS frequently identifies diffuse alveolar damage regardless of the severity stage and may have implications for patient management. Intensive Care Med. 2015, 41, 222–230.
- Benzing, A.; Mols, G.; Brieschal, T.; Geiger, K. Hypoxic Pulmonary Vasoconstriction in Nonventilated Lung Areas Contributes to Differences in Hemodynamic and Gas Exchange Responses to Inhalation of Nitric Oxide. Anesthesiology 1997, 86, 1254–1261.
- Nuckton, T.J.; Alonso, J.A.; Kallet, R.H.; Daniel, B.M.; Pittet, J.F.; Eisner, M.D. Matthay, M.A. Pulmonary Dead-Space Fraction as a Risk Factor for Death in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2002, 346, 1281–1286.
- Tomashefski, J.F.; Davies, P.; Boggis, C.; Greene, R.; Zapol, W.M.; Reid, L.M. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am. J. Pathol. 1983, 112, 112–126.
- Zapol, W.M.; Kobayashi, K.; Snider, M.T.; Greene, R.; Lover, M.B. Vascular Obstruction Causes Pulmonary Hypertension in Severe Acute Respiratory Failure. Chest 1977, 71, 306–307.
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2016, 195, 438–442.
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Werlein, C.; Stark, H.; Tzankov, A.; Li, W.W.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128.
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686.
- Barton, L.M.; Duval, E.J.; Stroberg, E.; Ghosh, S.; Mukhopadhyay, S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020, 153, 725–733.
- Schaller, T.; Hirschbühl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Märkl, B.; Claus, R. Postmortem Examination of Patients with COVID-19. JAMA 2020, 323, 2518–2520.
- Zhang, Y.; Zhou, P.; Yanqiu, W.; Yue, H.; Wang, Y.; Hu, M.; Zhang, S.; Cao, T.; Yang, C.; Li, M.; et al. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient with COVID-19. Ann. Intern. Med. 2020, 172, 629–632.
- Haudebourg, A.F.; Perier, F.; Tuffet, S.; de Prost, N.; Razazi, K.; Mekontso Dessap, A.; Carteaux, G. Respiratory Mechanics of COVID-19 vs. Non-COVID-19 Associated Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 287–290.
- Fan, E.; Beitler, J.R.; Brochard, L.; Calfee, C.S.; Ferguson, N.D.; Slutsky, A.S.; Brodie, D. COVID-19-associated acute respiratory distress syndrome: Is a different approach to management warranted? Lancet Respir. Med. 2020, 8, 816–821.
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102.
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330.
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360.
- Zilberberg, M.D.; Epstein, S.K. Acute Lung Injury in the Medical ICU. Am. J. Respir. Crit. Care Med. 1998, 157, 1159–1164.
- Moss, M.; Mannino, D.M. Race and gender differences in acute respiratory distress syndrome deaths in the United States: An analysis of multiple-cause mortality data (1979–1996). Crit. Care Med. 2002, 30, 1679–1685.
- Pham, T.; Neto, A.S.; Pelosi, P.; Laffey, J.G.; Haro, C.D.; Lorente, J.A.; Bellani, G.; Fan, E.; Brochard, L.J.; Pesenti, A.; et al. Outcomes of Patients Presenting with Mild Acute Respiratory Distress Syndrome: Insights from the LUNG SAFE Study. J. Am. Soc. Anesthesiol. 2019, 130, 263–283.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062.
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, 32–40.
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059.
- Salameh, J.P.; Leeflang, M.M.; Hooft, L.; Islam, N.; McGrath, T.A.; Pol, C.B.; Frank, R.A.; Prager, R.; Hare, S.S.; Dennie, C.; et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2020, 9.
- Nin, N.; Muriel, A.; Peñuelas, O.; Brochard, L.; Lorente, J.A.; Ferguson, N.D.; Raymondos, K.; Rios, F.; Violi, D.A.; Thille, A.W.; et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 200–208.
- Kallet, R.H.; Lipnick, M.S.; Zhuo, H.; Pangilinan, L.P.; Gomez, A. Characteristics of Nonpulmonary Organ Dysfunction at Onset of ARDS Based on the Berlin Definition. Respir. Care 2019, 64, 493–501.
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887.
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients with COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089.
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020, 180, 934–943.
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When COVID-19 is Suspected [Internet]. 2020. Available online: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- Haimovich, A.D.; Ravindra, N.G.; Stoytchev, S.; Young, H.P.; Wilson, F.P.; van Dijk, D.; Schulz, W.L.; Taylor, R.A. Development and Validation of the Quick COVID-19 Severity Index: A Prognostic Tool for Early Clinical Decompensation. Ann. Emerg. Med. 2020, 76, 442–453.
- Wu, G.; Yang, P.; Xie, Y.; Woodruff, H.C.; Rao, X.; Guiot, J.; Frix, A.; Louis, R.; Moutschen, M.; Li, J.; et al. Development of a Clinical Decision Support System for Severity Risk Prediction and Triage of COVID-19 Patients at Hospital Admission: An International Multicenter Study. Eur. Respir. J. 2020, 56, 2001104.
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020.
- Vincent, J.L. Give your patient a fast hug (at least) once a day. Crit. Care Med. 2005, 33, 1225–1229.
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552.
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581.
- O’Driscoll, B.R.; Howard, L.S.; Earis, J.; Mak, V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax 2017, 72 (Suppl. 1), 1–90.
- Chu, D.K.; Kim, L.H.Y.; Young, P.J.; Zamiri, N.; Almenawer, S.A.; Jaeschke, R.; Szczeklik, W.; Schünemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705.
- Munshi, L.; Ferguson, N.D. Evolving Issues in Oxygen Therapy in Acute Care Medicine. JAMA 2020, 323, 607–608.
- Barrot, L.; Asfar, P.; Mauny, F.; Winiszewski, H.; Montini, F.; Badie, J.; Quenot, J.; Pili-Floury, S.; Bouhemad, B.; Louis, G.; et al. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2020, 382, 999–1008.
- Girardis, M.; Busani, S.; Damiani, E.; Donati, A.; Rinaldi, L.; Marudi, A.; Morelli, A.; Antonelli, M.; Singer, M. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA 2016, 316, 1583.
- Mikkelsen, M.E.; Anderson, B.; Christie, J.D.; Hopkins, R.O.; Lanken, P.N. Can We Optimize Long-Term Outcomes in Acute Respiratory Distress Syndrome by Targeting Normoxemia? Ann. Am. Thorac. Soc. 2014, 11, 613–618.
- Siemieniuk, R.A.C.; Chu, D.K.; Kim, L.H.Y.; Güell-Rous, M.R.; Alhazzani, W.; Soccal, P.M.; Karanicolas, P.J.; Farhoumand, P.D.; Siemeniuk, J.L.K.; Satia, I.; et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ 2018, 363, k4169.
- Agarwal, A.; Basmaji, J.; Muttalib, F.; Granton, D.; Chaudhuri, D.; Chetan, D.; Hu, M.; Fernando, S.M.; Honarmand, K.; Bakaa, L.; et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: Systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can. J. Anaesth. 2020, 67, 1217–1248.
- Nicholson, T.W.; Talbot, N.P.; Nickol, A.; Chadwick, A.J.; Lawton, O. Respiratory failure and non-invasive respiratory support during the covid-19 pandemic: An update for re-deployed hospital doctors and primary care physicians. BMJ 2020, 369, m2446.
- Winck, J.C.; Ambrosino, N. COVID-19 pandemic and non invasive respiratory management: Every Goliath needs a David. An evidence based evaluation of problems. Pulmonology 2020, 26, 213–220.
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196.
- Messika, J.; Ahmed, K.B.; Gaudry, S.; Miguel-Montanes, R.; Rafat, C.; Sztrymf, B.; Dreyfuss, D.; Ricard, J. Use of High-Flow Nasal Cannula Oxygen Therapy in Subjects with ARDS: A 1-Year Observational Study. Respir. Care 2015, 60, 162–169.
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies with All-Cause Mortality in Adults with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-analysis. JAMA 2020, 324, 57.
- Patel, M.; Gangemi, A.; Marron, R.; Chowdhury, J.; Yousef, I.; Zheng, M.; Mills, N.; Tragesser, L.; Giurintano, L.; Gupta, R.; et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir. Res. 2020, 7, e000650.
- Rochwerg, B.; Granton, D.; Wang, D.X.; Helviz, Y.; Einav, S.; Frat, J.P.; Mekontso-Dessap, A.; Schreiber, A.; Azoulay, E.; Mercat, A.; et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: A systematic review and meta-analysis. Intensive Care Med. 2019, 45, 563–572.
- Sartini, C.; Tresoldi, M.; Scarpellini, P.; Tettamanti, A.; Carcò, F.; Landoni, G.; Zangrillo, A. Respiratory Parameters in Patients with COVID-19 After Using Noninvasive Ventilation in the Prone Position Outside the Intensive Care Unit. JAMA 2020, 323, 2338.
- Wang, K.; Zhao, W.; Li, J.; Shu, W.; Duan, J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann. Intensive Care 2020, 10, 37.
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients with COVID-19 in Washington State. JAMA 2020, 323, 1612.
- Mitra, A.R.; Fergusson, N.A.; Lloyd-Smith, E.; Wormsbecker, A.; Foster, D.; Karpov, A.; Crowe, A.; Haljan, G.; Chittock, D.R.; Kanji, H.D.; et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: A case series. CMAJ 2020, 192, 694–701.
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481.
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2016, 195, 67–77.
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426.
- Patel, B.K.; Kress, J.P.; Hall, J.B. Alternatives to Invasive Ventilation in the COVID-19 Pandemic. JAMA 2020, 324, 43.
- Antonelli, M.; Conti, G.; Moro, M.; Esquinas, A.; Gonzalez-Diaz, G.; Confalonieri, M.; Pelaia, P.; Prinicipi, T.; Gregoretti, C.; Beltrame, F.; et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 2001, 27, 1718–1728.
- Corrêa, T.D.; Sanches, P.R.; de Morais, L.C.; Scarin, F.C.; Silva, E.; Barbas, C.S.V. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: A prospective, observational, cohort study. BMC Pulm. Med. 2015, 15, 144.
- Griffiths, M.J.D.; McAuley, D.F.; Perkins, G.D.; Barrett, N.; Blackwood, B.; Boyle, A.; Chee, N.; Connolly, B.; Dark, P.; Finney, S.; et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir. Res. 2019, 6.
- Argenziano, M.G.; Bruce, S.L.; Slater, C.L.; Tiao, J.R.; Baldwin, M.R.; Barr, R.G.; Chang, B.P.; Chau, K.H.; Choi, J.J.; Gavin, N.; et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020, 369, m1996.
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipvath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022.
- Oranger, M.; Gonzalez-Bermejo, J.; Dacosta-Noble, P.; Llontop, C.; Guerder, A.; Trosini-Desert, V.; Faure, M.; Raux, M.; Decavele, A.; Morélot-Panzini, C.; et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: A two-period retrospective case-control study. Eur. Respir. J. 2020.
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020.
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168.
- Ding, L.; Wang, L.; Ma, W.; He, H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: A multi-center prospective cohort study. Crit. Care 2020, 24, 28.
- Caputo, N.D.; Strayer, R.J.; Levitan, R. Early Self-Proning in Awake, Non-intubated Patients in the Emergency Department: A Single ED’s Experience During the COVID-19 Pandemic. Acad. Emerg. Med. 2020, 27, 375–378.
- Chad, T.; Sampson, C. Prone positioning in conscious patients on medical wards: A review of the evidence and its relevance to patients with COVID-19 infection. Clin. Med. 2020, 20, 97–103.
- Coppo, A.; Bellani, G.; Winterton, D.; Pierro, M.D.; Soria, A.; Faverio, P.; Cairo, M.; Mori, S.; Messinesi, G.; Contro, E.; et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir. Med. 2020, 8, 765–774.
- Elharrar, X.; Trigui, Y.; Dols, A.M.; Touchon, F.; Martinez, S.; Prud’homme, E.; Papazian, L. Use of Prone Positioning in Nonintubated Patients with COVID-19 and Hypoxemic Acute Respiratory Failure. JAMA 2020, 323, 2336–2338.
- Retucci, M.; Aliberti, S.; Ceruti, C.; Santambrogio, M.; Tammaro, S.; Cuccarini, F.; Carai, C.; Grasselli, G.; Oneta, A.M.; Saderi, L.; et al. Prone and Lateral Positioning in Spontaneously Breathing Patients with COVID-19 Pneumonia Undergoing Noninvasive Helmet CPAP Treatment. Chest 2020.
- Thompson, A.E.; Ranard, B.L.; Wei, Y.; Jelic, S. Prone Positioning in Awake, Nonintubated Patients with COVID-19 Hypoxemic Respiratory Failure. JAMA Intern. Med. 2020, 180, 1537.
- Taboada, M.; González, M.; Álvarez, A.; González, I.; García, J.; Eiras, M.; Diaz Vieito, M.; Naveira, A.; Otero, P.; Campaña, O.; et al. Effectiveness of prone positioning in non-intubated ICU patients with moderate to severe ARDS by COVID-19. Anesth Analg 2020.
- Cruces, P.; Retamal, J.; Hurtado, D.E.; Erranz, B.; Iturrieta, P.; González, C.; Diaz, F. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit. Care 2020, 24, 494.
- Koeckerling, D.; Barker, J.; Mudalige, N.L.; Oyefeso, O.; Pan, D.; Pareek, M.; Thompson, J.P.; Ng, G.A. Awake prone positioning in COVID-19. Thorax 2020, 75, 833–834.
- Eddy Fan; Lorenzo Del Sorbo; Ewan C. Goligher; C.L. Hodgson; Laveena Munshi; Allan J. Walkey; Neill K. J. Adhikari; Marcelo B. P. Amato; Richard Branson; Roy G. Brower; et al.Niall D. FergusonOgnjen GajicLuciano GattinoniDean HessJordi ManceboMaureen O. MeadeDaniel F. McAuleyAntonio PesentiV. Marco RanieriGordon RubenfeldEileen RubinMaureen SeckelArthur S. SlutskyDaniel S. TalmorB. Taylor ThompsonHannah WunschElizabeth UlerykJan BrozekLaurent Brochard An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. American Journal of Respiratory and Critical Care Medicine 2017, 195, 1253-1263, 10.1164/rccm.201703-0548st.
- Arthur S. Slutsky; V. Marco Ranieri; Ventilator-Induced Lung Injury. New England Journal of Medicine 2013, 369, 2126-2136, 10.1056/nejmra1208707.
- Acute Respiratory Distress Syndrome Network; Roy G Brower; Michael A Matthay; Alan Morris; David Schoenfeld; B Taylor Thompson; Arthur Wheeler; Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2000, 342, 1301-1308, 10.1056/nejm200005043421801.
- Marcelo B. P. Amato; Maureen O. Meade; Arthur S. Slutsky; Laurent Brochard; Eduardo L.V. Costa; David A. Schoenfeld; Thomas E. Stewart; Matthias Briel; Daniel S. Talmor; Alain Mercat; et al.Jean-Christophe Marie RichardCarlos R.R. CarvalhoRoy G. Brower Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2015, 372, 747-755, 10.1056/nejmsa1410639.
- Talmor, D.; Sarge, T.; Malhotra, A.; O’Donnell, C.R.; Ritz, R.; Lisbon, A.; Novack, V.; Loring, S.H. Mechanical Ventilation Guided by Esophageal Pressure in Acute Lung Injury. N. Engl. J. Med. 2008, 359, 2095–2104.
- Beitler, J.R.; Sarge, T.; Banner-Goodspeed, V.M.; Gong, M.N.; Cook, D.; Novack, V.; Loring, S.H.; Talmor, D. Effect of Titrating Positive End-Expiratory Pressure (PEEP) with an Esophageal Pressure–Guided Strategy vs an Empirical High PEEP-FiO2 Strategy on Death and Days Free from Mechanical Ventilation Among Patients with Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019, 321, 846–857.
- Moss, M.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; Hite, R.D.; et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2019, 380, 1997–2008.
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.D.; Combes, A.; Dreyfuss, D.; Forel, J.; Guerin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69.
- Munshi, L.; Walkey, A.; Goligher, E.; Pham, T.; Uleryk, E.M.; Fan, E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir. Med. 2019, 7, 163–172.
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Herbert P Wiedemann; Arthur P Wheeler; Gordon R Bernard; B Taylor Thompson; Douglas Hayden; Ben Deboisblanc; Alfred F Connors; R Duncan Hite; Andrea L Harabin; et al. Comparison of Two Fluid-Management Strategies in Acute Lung Injury. New England Journal of Medicine 2006, 354, 2564-2575, 10.1056/nejmoa062200.
- Djilalli Annane; Stephen M. Pastores; Bram Rochwerg; Wiebke Arlt; Robert A. Balk; Albertus Beishuizen; Josef Briegel; Joseph Carcillo; Mirjam Christ-Crain; Mark S. Cooper; et al.Paul E. MarikGianfranco Umberto MeduriKeith M. OlsenSophia C. RodgersJames A. RussellGreet Van Den Berghe Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I). Critical Care Medicine 2017, 45, 2078-2088, 10.1097/ccm.0000000000002737.
- Zhikang Ye; Bram Rochwerg; Ying Wang; Neill K. Adhikari; Srinivas Murthy; François Lamontagne; Robert A. Fowler; Haibo Qiu; Li Wei; Ling Sang; et al.Mark LoebNing ShenMinhua HuangZhaonan JiangYaseen M. ArabiLuis Enrique Colunga-LozanoLi JiangYounsuck KohDong LiuFang LiuJason PhuaAizong ShenTianyi HuoBin DuSuodi ZhaiGordon H. Guyatt Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. Canadian Medical Association Journal 2020, 192, E536-E545, 10.1503/cmaj.200648.
- The RECOVERY Collaborative Group; Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. New England Journal of Medicine 2020, null, null, 10.1056/nejmoa2021436.
- Zhikang Ye; Ying Wang; Luis Enrique Colunga-Lozano; Manya Prasad; Wimonchat Tangamornsuksan; Bram Rochwerg; Liang Yao; Shahrzad Motaghi; Rachel J. Couban; Maryam Ghadimi; et al.Malgorzata M. BalaHuda GomaaFang FangYingqi XiaoGordon H. Guyatt Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. Canadian Medical Association Journal 2020, 192, E756-E767, 10.1503/cmaj.200645.
- John H. Beigel; Kay M. Tomashek; Lori E. Dodd; Aneesh K. Mehta; Barry S. Zingman; Andre C. Kalil; Elizabeth Hohmann; Helen Y. Chu; Annie Luetkemeyer; Susan Kline; et al.Diego Lopez De CastillaRobert W. FinbergKerry DierbergVictor TapsonLanny HsiehThomas F. PattersonRoger ParedesDaniel A. SweeneyWilliam R. ShortGiota TouloumiDavid Chien LyeNorio OhmagariMyoung-Don OhGuillermo M. Ruiz-PalaciosThomas BenfieldGerd FätkenheuerMark G. KortepeterRobert L. AtmarC. Buddy CreechJens LundgrenAbdel G. BabikerSarah PettJames D. NeatonTimothy H. BurgessTyler BonnettMichelle GreenMat MakowskiAnu OsinusiSeema NayakH. Clifford Lane Remdesivir for the Treatment of Covid-19 — Final Report. New England Journal of Medicine 2020, 386, 1813–1826, 10.1056/nejmoa2007764.
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124.
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2020.
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid. Biochem. Mol. Biol. 2020, 203.
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799.
- Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; Prudon, B.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020.
- Catherine Hodge; Fiona Marra; Catia Marzolini; Alison Boyle; Sara Gibbons; Marco Siccardi; David Burger; David Back; Saye Khoo; Drug interactions: a review of the unseen danger of experimental COVID-19 therapies. Journal of Antimicrobial Chemotherapy 2020, 75, 3417-3424, 10.1093/jac/dkaa340.
