Printing technologies represent powerful tools to combine simpler and more customizable fabrication of electrochemical biosensors with high resolution, miniaturization and integration with more complex microfluidic and electronics systems. Considering this framework, this review provides an overview of the opportunities offered by printed electrochemical biosensors in terms of transducing principles, metrological characteristics and enlargement of the application field, as well as of the main metrological challenges related.
- printed biosensors
- printing technologies
- electrochemistry
- point-of-care
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
In recent decades, printed electronics, which include all the additive manufacturing techniques to fabricate sensors, circuits, and active and passive electronical components, has gained increasing attention due to advantages in terms of process flexibility, cost and time effectiveness [1], [2][1][2]. Focusing on the biomedical area, the potential of printed electronics has recently been exploited for the fabrication of bio-sensing electrodes and their conditioning circuits. In this framework, printed electrochemical biosensors have acquired widely recognized relevance in various fields ranging from basic laboratory research to commercially available point-of-care. Thus, the possibility to obtain a sensitive analysis with a time and cost-effective approach, relying on disposable materials and on user-friendly protocols for transduction, is highly demanded by medical personnel, biologists and biotechnologists [3].
Moreover, in basic laboratory research, the possibility given by electrochemical biosensors to correlate electrical quantifiable signals with cell functions or with biomolecule/pathogen concentrations represents an interesting tool for improving the investigation of cellular pathophysiological processes and of their interaction with pathogens [4]. In hospital-based medicine, non-invasive and sensitive bio-sensing gives the possibility to improve the care of patients through ad hoc monitoring during hospitalization, contributing to better detection of bacterial infections [5], and to adjust treatment due to sensitive feedback about patient status [6]. In diagnostics, the possibility to enable the reliable detection of very low concentrations of pathology-related biomarkers, with reduced time and costs with respect to actual biochemical and molecular assays, could bring a revolution in the early diagnosis of pathologies like cancer, cardiac or neurodegenerative diseases [7,8][7][8]. Finally, the possibility to integrate those biosensors in standalone platforms (e.g., wearable, point-of-care), usable even by non-experts at home, could provide a powerful contribution to eHealth and telemedicine [9–11][9][10][11].
Recent advances in the development of micro- and nanoscale bio-transducers capable of detecting changes down to the molecular level, enabled by technological advances, have strongly accelerated the improvement of the metrological issues still affecting electrochemical biosensors. Those metrological characteristics encompass sensitivity (slope of the calibration plot, given by the ratio between output and input signals), selectivity (ability to correlate changes to a specific analyte, reducing the cross-sensitivity), signal-to-noise ratio (SNR, ratio between the signal of interest and background noise), repeatability (stability of the results among multiple analysis performed under the same conditions) and stability (repeatability in long-term monitoring) [12]. Another relevant useful quantity commonly adopted to compare results in chemistry/biology sensing is the limit of detection (LOD), which express the lowest quantity of an analyte that can be distinguished from the absence of that substance (a blank value) with a stated confidence level (generally 99%). It is estimated from the mean of the blank, the standard deviation (SD) of the blank, the slope (analytical sensitivity) of the calibration plot and a defined confidence factor (usually 3SD) [13,14][13][14]. It can also be considered as an indicator of the resolution of the system obtained with a statistical approach, since it is taking into consideration both the contribution of uncertainty and of resolution [13].
Looking at electrochemical biosensors from a metrological perspective, it is undeniable that their characteristics need to be discussed and compared with really competitive counterparts: mass-based and optical biosensors [15,16][15][16]. Mass-based devices also referred to as gravimetric biosensors, apply the basic principle of a response to a change in mass, using piezoelectric crystals, in the form of resonating or as surface acoustic wave devices [17]. Their main advantage is their high sensitivity to minimal mass changes, especially for molecules that are neither electroactive nor fluorescent [18,19][18][19]. Optical biosensors, both label free and label based, are based on the interaction of optical fields with biorecognition elements, showing well-known levels of sensitivity and specificity [20,21][20][21]. Despite those clear advantages and emerging trends in the area of fiber optics [22], both mass-based and optical biosensors show significant challenges in terms of their lack of repeatability, high dependency upon contour variables, high cost, high fragility, limited flexibility, and the portability and integrability of the overall readout system with more complex systems (e.g., point-of-care) [23]. Thus, compared to mass-based [24] and optical [25] biosensors, electrochemical sensors are easier to fabricate and miniaturize, facilitating the possibility of their integration on the same sensing substrate and also customized readout circuits [26]. Regarding metrological performances, despite recent advances in nanostructures, nano-printing strategies and hybrid nano-molecules that have strongly improved the LOD, the main challenges for electrochemical biosensors concern selectivity, repeatability and stability [27]. Recent advances in the area of printing technologies combined with advances in bio- and electrochemistry, nanostructures, solid-state and surface material physics, integrated circuits, microfluidics and data processing offered the possibility to address a whole new generation of electrochemical biosensors [28]; however, these biosensors require attention in relation to their metrological performance.
Compared to the most commonly adopted techniques to fabricate electrochemical biosensors, such as subtractive manufacturing, thin film, vacuum, lithography and electro-based deposition, printing technologies offer unique opportunities in terms of miniaturization, integration in complex systems and ease of customization (Table 1) [29].
Table 1. Main fabrication techniques for electrochemical biosensors: advantages and challenges (referenced articles are limited to the recent literature focusing on critical evaluation of positive and challenging aspects of the reported techniques).
|
Fabrication Techniques |
Advantages |
Challenges |
Refs |
|
Bulk Electrodes |
higher stability, |
no possibility of miniaturization, large volumes of sample needed, low customization possibility |
[30] [31] |
|
Printing Technologies |
miniaturization, low cost, |
stability, |
[4] [8] [32] [25] |
|
Thin Film (Vacuum-Based, Spin Coating) |
fine control of the thickness, low costs, |
high temperatures, |
[33] [34] [35] |
|
Lithography |
high resolution, |
long process, |
[15] [36] [37] |
|
Electrospray, Electrospinning |
good control of fibers, |
low lateral resolution, |
[38] [39] [40] |
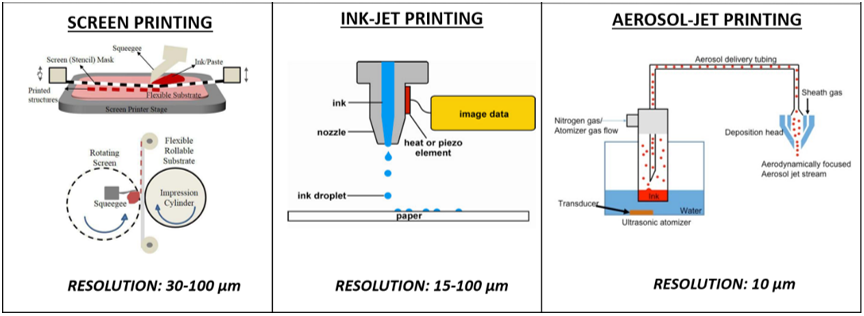
The available equipment for printing technologies ranges from economic devices ensuring very low-cost production, which are ideal for rapid prototyping, to the most expensive ones providing a greater geometrical resolution, which are in some way comparable with standard lithographic methods, but without the need for clean rooms and/or multiple step processes with sacrificial layers [2,25,41][2][25][41]. Overall, the printing technologies employed for fabricating electrochemical sensors can be classified between contact printing (gravure, flexographic, offset, micro-contact dispensing and screen printing (SP)) and non-contact printing (inkjet (IP), aerosol jet printing (AJP), laser-induced forward transfer (LIFT), micro and nano-pen printing). Contact printing encompasses all the mask-based techniques in which patterned structures with inked surfaces and substrate are in physical contact. These techniques ensure high throughput and thus are often (e.g., SP) the most frequently adopted for low-cost and rapidly fabricated biosensors [42]. However, since they are characterized by high material waste, limited resolution and a limited range of materials (substrates, inks and solvents), increasing attention has recently been paid to non-contact printing techniques (also defined as maskless techniques). These technologies are based on ink dispensed through openings or nozzles and define structures by moving the stage in a pre-programmed pattern. Thus, they allow for a reduction in material waste, the simplification of the printing process, an improvement in its control and flexibility and also enable improved resolution, miniaturization and more complex patterns (Figure 1) [43,44][43][44].
Figure 1. Comparison among fabrication processes to print electrochemical biosensors, in terms of ink dispensing and resolution achieved. Reproduced with permission according to the terms of the Creative Commons Attribution 3.0 license from [43–45][43][44][45].
Along with the advantages discussed, challenges in terms of compatibility among the wide variety of materials used in the fabrication of sensors represent a predominant issue that must be faced to ensure the feasibility and metrological performances of the printed devices. The most recent emerging non-contact techniques [46] are aiming to optimize the processes of ink deposition, reducing the dimensions of droplets (micro- or nano-pen printings [47,48][47][48]), through the finest control of printed track width using lasers (LIFT) or by focusing aerosol ink through a stream of gas (AJP) [49]. Additionally, novel sintering methods (e.g., photonic curing) are under investigation to optimize ink post-processing. These emerging techniques are thus trying to face the challenges in terms of conductivity, repeatability and standardization that are still openly affecting printed biosensors when compared with their bulk counterparts [50]. Additionally, the possibility to combine and customize different materials and to exploit novel curing methods with respect to other traditional techniques (e.g., laser cutting, machining) opens the way for the effective integration of biosensing with directly printed microfluidic circuits (e.g., paper based, polymer based) and embedded electronics (insulating layer and conductive tracks), with consequently improved costs and time effectiveness [4,9,51][4][9][51].
2. Transducing Principles of Printed Electrochemical Biosensors
The transducing principles of printed electrochemical biosensors can be grouped into three main classes: amperometric, impedimetric and potentiometric [52]. Common advantages brought by printing technologies to all three classes are related to the miniaturization of the electrodes, to the use of nanostructured inks, to printed microfluidic paths and via the extension to non-conventional substrates.
Thanks to printing technologies, both three-electrode (for amperometric) and two-electrode (for impedimetric and potentiometric) conformations, traditionally implemented with solid electrodes in a baker containing several milliliters of samples, can be easily miniaturized onto a small substrate, ensuring a reduction in the required sample volume from milliliters to a variable range within picoliters and microliters [49]. Moreover, the capacitive background current associated with the charging of the double layer is reduced proportionally to the reduction in the surface area of the conductive electrodes. The resistive drop in the electrode–solution system is reduced by shortening the ionic current path in miniaturized cells. Overall, those elements contribute to reducing the interfering noise coupled to the electrodes. The reduced time constant coming from reduced capacitance and resistance enables faster electron transfer kinetics monitoring.
Printing technologies enable an easier fabrication of microfluidic circuits. This possibility, combined with high-resolution nanostructured coatings, enhances the accuracy and sensitivity. In fact, thanks to the high accuracy of sample delivery to the sensing area and to the presence of nanowires and nanospheres, the interaction between the analyte and the electrode active area is enhanced, changing it from a 1D planar diffusion to a more uniform 2D or 3D diffusion. The use of nanoinks allows to increase the surface to volume ratio, increasing the active area useful for redox current detection, for impedance variation or charge accumulation detection, bringing an improvement in terms of overall sensitivity. Furthermore, the highest control obtained in these microsystems in terms of sample dispensing, ink and coating deposition can also improve the repeatability of the electrochemical measurements [53,54][53][54]. Overall, the combination of the reduction in the interference noise processes and the enhancement of the transducing effect of the measurand achievable in printed miniaturized integrated biosensors increases the signal-to-noise ratio of such bioanalytical systems [55,56][55][56].
In addition to working electrodes (WE), the potential of printing techniques also needs to be exploited for improving counter (CE) and reference electrodes (RE), which require particular attention when aiming for electrochemical cell miniaturization [57]. CE represents the element required to complete the circuit with the WE, thus allowing the charge coming from the reaction on WE to flow and be read [58]. Consequently, its size should be much larger than the WE to ensure no current limitations arise. Thus, nanostructures and complex geometries made available by emerging printing are under investigation to increase the surface to volume ratio and to guarantee proper control of the electrical parameters of the cell during the analysis [59]. Regarding RE, it is the element that needs to be kept at a constant potential during all the analyses, to control the potential of WE (e.g., in voltammetry) or to allow measurement of an indicator electrode (e.g., in potentiometry). Thus, attention is being paid to novel materials and curing strategies to improve the stability of RE and limit the influence of surrounding conditions [60].
Despite these common advantages, due to significative differences in terms of speed, sensitivity and selectivity among amperometric, impedimetric and potentiometric biosensors, the specific potential offered by printing technologies for each class needs to be discussed, considering their intrinsic characteristics (Table 2) [15].
Next, the basic working principles, advantages and disadvantages of amperometric, impedimetric and potentiometric biosensors will be overviewed, focusing on the specific contribution and improvement brought by the printing approach to each method. For more extensive and theoretical details of each electrochemical technique, out of the scope of this review, we suggest that the reader deepens their knowledge of this theoretical topic in the related literature [61].
Table 2. Review of main advantages and challenges of the three main groups of electrochemical techniques (referenced articles are limited to the recent literature focusing on critical evaluation of positive and challenging aspects of the reported techniques).
|
Detectable Analyte Concentration |
Advantages |
Challenges |
Ref |
|
|
Amperometry/ |
lower than |
highest sensitivity, high specificity, continuous monitoring, possibility to detect many compounds with different characteristic potentials in one measurement |
required electroactivity, current production, interferences, effect of surrounding environment, |
[49] [61] [62] [63] |
|
Impedance spectroscopy / Conductometry |
~10−8 M (some recent example down to ~10−12 M) |
miniaturization, |
need nanotechnologies to improve sensitivities, |
[64] [65] [66] |
|
Potentiometry |
~10−8 M |
simple conditioning, |
intrinsic non-specificity, |
[62] [67] [68] [69] |
|
Detectable Analyte Concentration |
Advantages |
Challenges |
Ref |
|
|
Amperometry/ |
lower than |
highest sensitivity, high specificity, continuous monitoring, possibility to detect many compounds with different characteristic potentials in one measurement |
required electroactivity, current production, interferences, effect of surrounding environment, |
[49] [61] [62] [63] |
|
Impedance spectroscopy / Conductometry |
~10−8 M (some recent example down to ~10−12 M) |
miniaturization, |
need nanotechnologies to improve sensitivities, |
[64] [65] [66] [53], [54] |
|
Potentiometry |
~10−8 M |
simple conditioning, |
intrinsic non-specificity, |
[62] [67] [68] [69] |
2.1. Amperometric
In amperometry, a three-electrode conformation is used, comprising a WE, CE and RE electrode. WE potential is controlled through a signal from a generator and the current resulting from the oxidation and/or the reduction reaction of electroactive molecules exchanging electrons with the WE conductive surface are then measured in the loop closed by the cell. If the signal coming from the generator is varied, then the methods belong to the sub-class of voltammetry [61].
The main challenges of printed amperometric biosensors still refer to cross-sensitivity, the interferences of the buffer composition and the effect of the surrounding environment [70]. Concerning the influence of contour variables, the most challenging aspects refer to interfering molecules (inks, mediators, labels) with similar potential. Concerning implantable electrodes or analyses performed on biological fluids, a relevant issue is the electrode fouling by non-target proteins and biomolecules, which can limit direct electrode exchange. Furthermore, the accuracy and stability of the currents measured are particularly challenging for both short and long-term measurements [71]. Static measurements, in the absence of stirring and without proper fluidics, can be easily affected by saturation due to species accumulation, by difficult low current detection due to double-layer capacitance or by a decrease in electrode performances due to the degradation of ink or of the ink–substrate bonding [72].
A smart combination of high-resolution nanostructure direct printing with peculiar techniques able to enhance low faradaic currents and not background processes (e.g., differential pulse voltammetry) can help to face those issues, reaching LOD < 10−12 M, the lowest among electrochemical techniques [52]. Finally, focusing on biosensor selectivity, cross-sensitivity of different species can be improved thanks to the flexibility in ink preparation. The possibility to directly print selective electroactive labels allows to enhance the selectivity of currents resulting from voltammetries using nanoparticles and nanostructures as electroactive labels (limiting the need for additional markers) [73] and to improve repeatability due to better control of the deposition process.
2.2. Impedimetric
Impedimetric biosensors are based on the direct correlation of impedance changes with changes in terms of target analyte concentration, without requiring additional labels or biomolecule electroactivity. After applying an alternate voltage to the two electrodes (WE and CE), with a constant amplitude (usually between 5 and 10 mV) and a defined frequency range -, the resulting alternate current is measured and the overall impedance (Z) correlated with analyte concentration [69]. They provide the result directly, without requiring the electroactivity of the target analyte. Impedimetric biosensors based on the principle that biomolecules bound onto a printed conductive surface are acting as insulators (e.g., adherent cells proliferation monitoring) fall in the subclass of reactive [74]; the ones based on the measurement of electrolytic conductivity to monitor the progress of a chemical ionic reaction instead fall in the class of conductometric [68].
Among the most important advantages of impedimetric biosensors compared to other classes are the low voltages employed, which do not damage or disturb most bio-recognition layers [75]. From the point of view of the target analyte, the small excitation signals adopted cause small amplitude perturbations from the steady state, which makes this method optimal to monitor in real time the dynamics of biomolecule interactions and the pathophysiological processes of living cells, without significant alterations to the ionic balance in the extracellular space [53,54,76][53][54][76]. Furthermore, from the point of view of materials, this low invasiveness gives the possibility to explore novel non-conventional organic conductive materials (e.g., conductive functionalized polymers or small molecule organic semiconductors) with peculiar surface modifications that can enhance the sensitivity and the LOD of the analysis [77].
The challenging aspects of impedimetric biosensors are the strong influence of pH, temperature, buffer characteristics or non-reacting ions on measurement accuracy and repeatability [76[76][78],78], the worse detection limits compared to potentiometric or amperometric methods (usually around 10−8 M) and the sources of error due to double-layer capacitance and electrode polarization [68]. Furthermore, the wide spectrum of frequencies of the applied voltage implies a very small power at each frequency and, consequently, a limited SNR of the impedance measurement with respect to other electrochemical techniques [79].
The opportunities of the printing approach for impedimetric biosensing mainly refer to the possibility to exploit novel nanostructured inks to enhance SNR and to the availability of biocompatible organic inks to improve the integration of sensing elements in biological environments. Thus, due to the limited invasiveness of the technique, printable organic and degradable inks can also be deposited on the electrode to investigate live cells, allowing impedimetric monitoring during a long-term culture both in 2D and 3D environments [80,81][80][81].
2.3. Potentiometric
In potentiometric biosensors, the measurement is performed in zero-current conditions, with a two-electrode structure, without the need for a generator or current measurement device. The voltage across WE and RE is measured with a high-input impedance device, to minimize the contribution of the ohmic potential drop to the total difference in potential. The potential of WE, thanks to an accumulation of charged molecules (ions), exclusively depends on the analytical concentration of the analyte in the gas or solution phase, while the RE is needed to provide a defined reference potential [62].
Those biosensors can be easily miniaturized and integrated in all printed devices since they require low-cost measurement instrumentation. Due to the simple electronic conditioning circuit, potentiometric biosensors show a rapid response, ease of use and robustness. On the contrary, their main intrinsic challenges are related to their non-specificity, to the influence on temperature variation, to the need for frequent re-calibration and to false positives due to interfering charged molecules in solution [60,61,82][60][61][82].
Thanks to the progress of additive manufacturing, printed potentiometric biosensors are undergoing a renaissance, with improvements in the detection limits (down to ~10−8 M) and selectivity enabled by the introduction of novel materials and the integrability of these sensing concepts with wearable and implantable devices [67]. The possibility to fabricate miniaturized electrodes with customized inks could provide improvements in terms of the stability of RE, tuning the ink composition [57,60][57][60] and the selectivity of the approach, directly printing selective coatings to substitute for the selective membranes that are traditionally adopted [83]. Other great opportunities provided by potentiometric measurements combined with printing technologies refer to the possibility to realize innovative sensors on degradable or biological substrates (directly on the skin or implanted in the human body) due to the sensing principle at zero current, which limits the possible perturbation in the sensing area [84].
References
- Saengchairat: N.; Tran, T.; Chua, C.-K. A review: Additive manufacturing for active electronic components. Virtual Phys. Prototyp. 2016, 12, 1–16.
- Tan, H.W.; Tran, T.; Chua, C.K. A review of printed passive electronic components through fully additive manufacturing methods. Virtual Phys. Prototyp. 2016, 11, 271–288.
- McEachern, F.; Harvey, E.; Merle, G. Emerging Technologies for the Electrochemical Detection of Bacteria. J. 2020, 15, 2000140.
- Abdalla, A.; Patel, B.A. 3D-printed electrochemical sensors: A new horizon for measurement of biomolecules. Opin. Electrochem. 2020, 20, 78–81.
- Munteanu, F.-D.; Titoiu, A.M.; Marty, J.-L.; Vasilescu, A. Detection of antibiotics and evaluation of antibacterial activity with screen-printed electrodes. Sensors 2018, 18, 901.
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O. Screen-printed biosensors in drug analysis. Pharm. Anal. 2017, 13, 169–174.
- Kozitsina, A.N.; Svalova, T.S.; Malysheva, N.N.; Okhokhonin, A.V.; Vidrevich, M.B.; Brainina, K.Z. Sensors based on bio and biomimetic receptors in medical diagnostic, environment, and food analysis. Biosensors 2018, 8, 35.
- Mincu, N.-B.; Lazar, V.; Stan, D.; Mihailescu, C.M.; Iosub, R.; Mateescu, A.L. Screen-Printed Electrodes (SPE) for in vitro diagnostic purpose. Diagnostics 2020, 10, 517.
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Screen-printed electrodes: Promising paper and wearable transducers for (bio)sensing. Biosensors 2020, 10, 76.
- Tuoheti, A.; Aiassa, S.; Criscuolo, F.; Stradolini, F.; Tzouvadaki, I.; Carrara, S.; Demarchi, D. New Approach for Making Standard the Development of Biosensing Devices by a Modular Multi-Purpose Design. IEEE Trans. Nanobiosci. 2020, 19, 339–346.
- Khan, S.; Ali, S.; Bermak, A. Recent developments in printing flexible and wearable sensing electronics for healthcare applications. Sensors 2019, 19, 1230.
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8.
- Lavín, Á.; Vicente, J.d.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaria, B.; Maigler, M.V.; Hernandez, A.L.; Ramirez, Y. On the Determination of Uncertainty and Limit of Detection in Label-Free Biosensors. Sensors 2018, 18, 2038.
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Biochem. Rev. 2008, 29 (Suppl. 1), S49–52.
- Kokkinos, C.; Economou, A. Recent advances in voltammetric, amperometric and ion-selective (bio)sensors fabricated by microengineering manufacturing approaches. Opin. Electrochem. 2020, 23, 21–25.
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A.; Mohamed, A.M.A.; Kahraman, R. A novel classification of prostate specific antigen (PSA) biosensors based on transducing elements. Talanta 2017, 168, 52–61.
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.Z.; Latif, U.; Feroz, S. Gravimetric viral diagnostics: QCM based biosensors for early detection of viruses. Chemosensors 2017, 5, 7.
- Nolan, P.; Auer, S.; Spehar, A.; Oplatowska-Stachowiak, M.; Campbell, K. Evaluation of Mass Sensitive Micro-Array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 2020, 222, 12152.
- Arlett, J.; Myers, E.B.; Roukes, M. Comparative Advantages of Mechanical Biosensors. Nanotechnol. 2011, 6, 203–215.
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Bioelectron. 2020, 167, 112511.
- Sharma, S.; Kumari, R.; Varshney, S.K.; Lahiri, B. Optical biosensing with electromagnetic nanostructures. Phys. 2020, 5, 100044.
- Tabassum, S.; Kumar, R. Advances in Fiber-Optic Technology for Point-of-Care Diagnosis and In Vivo Biosensing. Mater. Technol. 2020, 5, 19000792.
- Méjard, R.; Griesser, H.J.; Thierry, B. Optical biosensing for label-free cellular studies. TrAC - Trends Anal. Chem. 2014, 53, 178–186.
- Lucarelli, F.; Tombelli, S.; Minunni, M.; Marrazza, G.; Mascini, M. Electrochemical and piezoelectric DNA biosensors for hybridisation detection. Chim. Acta 2008, 609, 139–159.
- Muñoz, J.; Pumera, M. 3D-printed biosensors for electrochemical and optical applications. TrAC Trends Anal. Chem. 2020, 128, 115933.
- Yu, H.L.L.; Maslova, A.; Hsing, I.-M. Rational Design of Electrochemical DNA Biosensors for Point-of-Care Applications. Electro. Chem. 2017, 4, 795–805.
- Menon, S.; Mathew, M.R.; Sam, S.; Keerthi, K.; Kumar, K.G. Recent advances and challenges in electrochemical biosensors for emerging and re-emerging infectious diseases. Electroanal. Chem. 2020, 878, 114596.
- Han, Y.; Dong, J. Electrohydrodynamic printing for advanced micro/nanomanufacturing: Current progresses, opportunities, and challenges. Micro Nano-Manuf. 2018,DOI: 10.1115/1.4041934
- Kamanina, O.A.; Kamanin, S.S.; Kharkova, A.S.; Arlyapov, V.A. Glucose biosensor based on screen-printed electrode modified with silicone sol–gel conducting matrix containing carbon nanotubes. 3 Biotech. 2019, 9, 290.
- Soni, D.; Ahmad, R.; Dubey, S. Biosensor for the detection of Listeria monocytogenes: Emerging trends. Rev. Microbiol. 2018, 44, 590–608.
- Brett, A.M.O.; Serrano, S.H.P.; Gutz, I.G.R.; La-Scalea, M.A. Comparison of the Voltammetric Behavior of Metronidazole at a DNA-Modified Glassy Carbon Electrode, a Mercury Thin Film Electrode and a Glassy Carbon Electrode. Electroanalysis 1997, 9, 110–114.
- Nesaei, S.; Song, Y.; Wang, Y.; Ruan, X.; Du, D.; Gozen, A.; Lin, Y. Micro additive manufacturing of glucose biosensors: A feasibility study. Chim. Acta 2018, 1043, 142–149.
- Hashim, U.; Salleh, S.; Rahman, S.F.A.; Abdullah, A.R.A.J. Design and fabrication of Nanowire-based conductance biosensor using spacer patterning technique. In 2008 International Conference on Electronic Design, ICED 2008; IEEE: New York, NY, USA, 2008.
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Today 2009, 12, 12–20.
- Raymundo-Pereira, P.A.; Baccarin, M.; Jr, O.N.O.; Janegitz, B.C. Thin Films and Composites Based on Graphene for Electrochemical Detection of Biologically-relevant Molecules. Electroanalysis 2018, 30, 1888–1896.
- Cotte, S.; Baraket, A.; Bessueille, F.; Gout, S.; Yaakoubi, N.; Leonard, D.; Errachid, A. Fabrication of Microelectrodes Using Original ‘Soft Lithography’ Processes. In New Sensors and Processing Chain; Wiley Online Library: Hoboken, NJ, USA, 2014; Volume 9781848216266, pp. 1–9.
- Tran, K.T.M.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. Sci. Adv. Mater. Devices 2017, 2, 1–14.
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Cartoni, A.; Calandra, P.; Tempesta, E.; Giardi, M.T.; Antonacci, A.; Arduini, F.; et al. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Bioelectron. 2020, 163, 112299.
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 1–23.
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Opin. Biomed. Eng. 2020, 13, 174–189.
- Willmann, J.; Stocker, D.; Dörsam, E. Characteristics and evaluation criteria of substrate-based manufacturing. Is roll-to-roll the best solution for printed electronics? Electron. 2014, 15, 1631–1640.
- Tonello, S.; Serpelloni, M.; Lopomo, N.F.; Abate, G.; Uberti, D.L.; Sardini, E. Screen-Printed Biosensors for the Early Detection of Biomarkers Related to Alzheimer Disease: Preliminary Results. In Procedia Engineering; Elsevier: New York, NY, USA, 2016; Volume 168.
- Lau, G.-K.; Shrestha, M. Ink-Jet Printing of Micro-Electro-Mechanical Systems (MEMS). Micromachines 2017, 8, 194.
- Agarwala, S.; Goh, G.L.; Yeong, W.Y. Optimizing aerosol jet printing process of silver ink for printed electronics. IOP Conf. Ser. Mater. Sci. Eng. 2017, 191, 12027.
- Khan, S.; Lorenzelli, L.; Dahiya, R.S. Technologies for printing sensors and electronics over large flexible substrates: A review. IEEE Sens. J. 2015, 15, 3164–3185.
- Mondal, K.; McMurtrey, M.D. Present status of the functional advanced micro-, nano-printings—A mini review. Today Chem. 2020, 17,DOI: 10.1016/j.mtchem.2020.100328
- Grünwald, S. Reproducible dispensing of liquids in the nanolitre range. Adhes. Sealants 2018, 15, 28–31.
- Abas, M.; Salman, Q.; Khan, A.M.; Rahman, K. Direct ink writing of flexible electronic circuits and their characterization. Brazilian Soc. Mech. Sci. Eng. 2019, 41, 563.
- Yang, H.; Rahman, T.; Du, D.; Panat, R.; Lin, Y. 3-D Printed Adjustable Microelectrode Arrays for Electrochemical Sensing and Biosensing. Actuators. B. Chem. 2016, 230, 600–606.
- Hoffman, J.; Hwang, S.; Ortega, A.; Kim, N.-S.; Moon, K.-S. The standardization of printable materials and direct writing systems. Electron. Packag. Trans. ASME 2013, 135,DOI: 10.1115/1.4023809
- Ramasamy, M.; Varadan, V.K. 3D printing of nano-and micro-structures. In Proceedings of SPIE—The International Society for Optical Engineering; International Society for Optics and Photonics: Hague, The Netherland, 2016; Volume 9802.
- Dziąbowska, K.; Czaczyk, E.; Nidzworski, D. Application of Electrochemical Methods in Biosensing Technologies. Technol. Detect. Pathogens-A Prosp. Way Rapid Anal. 2018, DOI: 10.5772/intechopen.72175
- Nagar, B.; Balsells, M.; de la Escosura-Muñiz, A.; Gomez-Romero, P.; Merkoçi, A. Fully printed one-step biosensing device using graphene/AuNPs composite. Bioelectron. 2019, 129, 238–244.
- Wang, Y.; Ye, Z.; Ying, Y. New trends in impedimetric biosensors for the detection of foodborne pathogenic bacteria. Sensors 2012, 12, 3449–3471.
- Soleymani, L.; Li, F. Mechanistic Challenges and Advantages of Biosensor Miniaturization into the Nanoscale. ACS Sensors 2017, 2, 458–467.
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chinese Chem. Lett. 2020, 31, 589–600.
- Manjakkal, L.; Shakthivel, D.; Dahiya, R. Flexible Printed Reference Electrodes for Electrochemical Applications. Mater. Technol. 2018, 3, 1800252.
- Søpstad, S.; Johannessen, E.A.; Imenes, K. Analytical errors in biosensors employing combined counter/pseudo-reference electrodes. Results Chem. 2020, 2, 100028.
- Faria, A.M.; Peixoto, E.B.M.I.; Adamo, C.B.; Flacker, A.; Longo, E.; Mazon, T. Controlling parameters and characteristics of electrochemical biosensors for enhanced detection of 8-hydroxy-2′-deoxyguanosine. Rep. 2019, 9, 7411.
- Sopstad, S.; Imenes, K.; Johannessen, E.A. Chloride and pH Determination on a Wireless, Flexible Electrochemical Sensor Platform. IEEE Sens. J. 2020, 20, 599–609.
- Diamond, D. Analytical electrochemistry—Analytical Electrochemistry, by Joseph Wang, VCH, Weinheim, 1994, xii + 198 pages, DM 98.00, ISBN 1-56081-572-2. Trends Anal. Chem. 1996, 15, X–XI.
- Thapliyal, N.; Chiwunze, T.; Karpoormath, R.; Goyal, R.; Patel, H.; Srinivasulu, C. Research Progress in Electroanalytical Techniques for Determination of Antimalarial Drugs in Pharmaceutical and Biological Samples. RSC Adv. 2016, 6, 57580–57602.
- Gwon, K.; Lee, S.; Nam, H.; Shin, J.H. Disposable strip-type biosensors for amperometric determination of galactose. Electrochem. Sci. Technol. 2020, 11, 310–317.
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Microbiol. Immunol. 2020, 209, 343–362.
- Zehani, N.; Dzyadevych, S.; Kherrat, R.; Jaffrezic-Renault, N. Sensitive impedimetric biosensor for direct detection of diazinon based on lipases. Chem. 2014, 2, 44.
- Ariffin, E.Y.; Heng, L.Y.; Tan, L.L.; Karim, N.H.A.; Hasbullah, S.A. A highly sensitive impedimetric DNA biosensor based on hollow silica microspheres for label-free determination of E. Coli. Sensors 2020, 20, 1279.
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803.
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric Microbiosensors for Environmental Monitoring. Sensors 2008, 8, 2569–2588.
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80.
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V.; et al. Analytical Problems in Exposing Amperometric Enzyme Biosensors to Biological Fluids. Sensors 2016, 16, 780.
- Pemberton, R.M.; Xu, J.; Pittson, R.; Drago, G.A.; Griffiths, J.; Jackson, S.K.; Hart, J.P. A screen-printed microband glucose biosensor system for real-time monitoring of toxicity in cell culture. Bioelectron. 2011, 26, 2448–2453.
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A review on amperometric-type immunosensors based on screen-printed electrodes. Analyst 2014, 139, 2289–2311.
- Alarcon-Angeles, G., Álvarez-Romero, G.A., Merkoçi, A., Electrochemical biosensors: Enzyme kinetics and role of nanomaterials, (2018) Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry, pp. 140-155., DOI: 10.1016/B978-0-12-409547-2.13477-8.
- Pal, K., Kraatz, H.-B., Khasnobish, A., Bag, S., Banerjee, I., Kuruganti, U.,Bioelectronics and medical devices: From materials to devices - fabrication, applications and reliability(2019) Bioelectronics and Medical Devices: From Materials to Devices, DOI: 10.1016/C2017-0-00496-2
- Li, H.; Liu, X.; Li, L.; Mu, X.; Genov, R.; Mason, A.J. CMOS Electrochemical Instrumentation for Biosensor Microsystems: A Review. Sensors 2016, 17.
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Cells, Nanomedicine, Biotechnol. 2016, 44, 248–262.
- Hopkins, J.; Fidanovski, K.; Lauto, A.; Mawad, D. All-Organic Semiconductors for Electrochemical Biosensors: An Overview of Recent Progress in Material Design. Bioeng. Biotechnol. 2019, 7, 237.
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of Electrochemical Impedance Spectroscopy in Protein Biosensing. Chem. 2009, 81, 3944–3949.
- Carminati, M.; Ferrari, G.; Bianchi, D.; Sampietro, M. Impedance Spectroscopy for Biosensing: Circuits and Applications; Springer: New York, NY,USA, 2015; pp. 1–24.
- Tonello, S.; Bianchetti, A.; Braga, S.; Almici, C.; Marini, M.; Piovani, G.; Guindani, M.; Dey, K.; Sartore, L.; Re, F.; et al. Impedance-based monitoring of mesenchymal stromal cell three-dimensional proliferation using aerosol jet printed sensors: A tissue engineering application. Materials 2020, 13, 2231.
- Aggas, J.R.; Abasi, S.; Phipps, J.F.; Podstawczyk, D.A.; Guiseppi-Elie, A. Microfabricated and 3-D printed electroconductive hydrogels of PEDOT:PSS and their application in bioelectronics. Bioelectron. 2020, 168, 112568.
- Li, J.; Qin, W. An integrated all-solid-state screen-printed potentiometric sensor based on a three-dimensional self-assembled graphene aerogel. J. 2020, 159, 105453.
- Tehrani, Z.; Whelan, S.P.; Mostert, A.B.; Paulin, J.V.; Ali, M.M.; Ahmadi, E.D.; Graeff, C.F.O.; Guy, O.J.; Gethin, D.T. Printable and flexible graphene pH sensors utilising thin film melanin for physiological applications. 2D Mater. 2020, 7, 24008.
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sensors Actuators B Chem. 2018, 273, 966–972.
References
- Saengchairat: N.; Tran, T.; Chua, C.-K. A review: Additive manufacturing for active electronic components. Virtual Phys. Prototyp. 2016, 12, 1–16.
- Tan, H.W.; Tran, T.; Chua, C.K. A review of printed passive electronic components through fully additive manufacturing methods. Virtual Phys. Prototyp. 2016, 11, 271–288.
- McEachern, F.; Harvey, E.; Merle, G. Emerging Technologies for the Electrochemical Detection of Bacteria. J. 2020, 15, 2000140.
- Abdalla, A.; Patel, B.A. 3D-printed electrochemical sensors: A new horizon for measurement of biomolecules. Opin. Electrochem. 2020, 20, 78–81.
- Munteanu, F.-D.; Titoiu, A.M.; Marty, J.-L.; Vasilescu, A. Detection of antibiotics and evaluation of antibacterial activity with screen-printed electrodes. Sensors 2018, 18, 901.
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O. Screen-printed biosensors in drug analysis. Pharm. Anal. 2017, 13, 169–174.
- Kozitsina, A.N.; Svalova, T.S.; Malysheva, N.N.; Okhokhonin, A.V.; Vidrevich, M.B.; Brainina, K.Z. Sensors based on bio and biomimetic receptors in medical diagnostic, environment, and food analysis. Biosensors 2018, 8, 35.
- Mincu, N.-B.; Lazar, V.; Stan, D.; Mihailescu, C.M.; Iosub, R.; Mateescu, A.L. Screen-Printed Electrodes (SPE) for in vitro diagnostic purpose. Diagnostics 2020, 10, 517.
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Screen-printed electrodes: Promising paper and wearable transducers for (bio)sensing. Biosensors 2020, 10, 76.
- Tuoheti, A.; Aiassa, S.; Criscuolo, F.; Stradolini, F.; Tzouvadaki, I.; Carrara, S.; Demarchi, D. New Approach for Making Standard the Development of Biosensing Devices by a Modular Multi-Purpose Design. IEEE Trans. Nanobiosci. 2020, 19, 339–346.
- Khan, S.; Ali, S.; Bermak, A. Recent developments in printing flexible and wearable sensing electronics for healthcare applications. Sensors 2019, 19, 1230.
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8.
- Lavín, Á.; Vicente, J.d.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaria, B.; Maigler, M.V.; Hernandez, A.L.; Ramirez, Y. On the Determination of Uncertainty and Limit of Detection in Label-Free Biosensors. Sensors 2018, 18, 2038.
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Biochem. Rev. 2008, 29 (Suppl. 1), S49–52.
- Kokkinos, C.; Economou, A. Recent advances in voltammetric, amperometric and ion-selective (bio)sensors fabricated by microengineering manufacturing approaches. Opin. Electrochem. 2020, 23, 21–25.
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A.; Mohamed, A.M.A.; Kahraman, R. A novel classification of prostate specific antigen (PSA) biosensors based on transducing elements. Talanta 2017, 168, 52–61.
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.Z.; Latif, U.; Feroz, S. Gravimetric viral diagnostics: QCM based biosensors for early detection of viruses. Chemosensors 2017, 5, 7.
- Nolan, P.; Auer, S.; Spehar, A.; Oplatowska-Stachowiak, M.; Campbell, K. Evaluation of Mass Sensitive Micro-Array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 2020, 222, 12152.
- Arlett, J.; Myers, E.B.; Roukes, M. Comparative Advantages of Mechanical Biosensors. Nanotechnol. 2011, 6, 203–215.
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Bioelectron. 2020, 167, 112511.
- Sharma, S.; Kumari, R.; Varshney, S.K.; Lahiri, B. Optical biosensing with electromagnetic nanostructures. Phys. 2020, 5, 100044.
- Tabassum, S.; Kumar, R. Advances in Fiber-Optic Technology for Point-of-Care Diagnosis and In Vivo Biosensing. Mater. Technol. 2020, 5, 19000792.
- Méjard, R.; Griesser, H.J.; Thierry, B. Optical biosensing for label-free cellular studies. TrAC - Trends Anal. Chem. 2014, 53, 178–186.
- Lucarelli, F.; Tombelli, S.; Minunni, M.; Marrazza, G.; Mascini, M. Electrochemical and piezoelectric DNA biosensors for hybridisation detection. Chim. Acta 2008, 609, 139–159.
- Muñoz, J.; Pumera, M. 3D-printed biosensors for electrochemical and optical applications. TrAC Trends Anal. Chem. 2020, 128, 115933.
- Yu, H.L.L.; Maslova, A.; Hsing, I.-M. Rational Design of Electrochemical DNA Biosensors for Point-of-Care Applications. Electro. Chem. 2017, 4, 795–805.
- Menon, S.; Mathew, M.R.; Sam, S.; Keerthi, K.; Kumar, K.G. Recent advances and challenges in electrochemical biosensors for emerging and re-emerging infectious diseases. Electroanal. Chem. 2020, 878, 114596.
- Han, Y.; Dong, J. Electrohydrodynamic printing for advanced micro/nanomanufacturing: Current progresses, opportunities, and challenges. Micro Nano-Manuf. 2018,DOI: 10.1115/1.4041934
- Kamanina, O.A.; Kamanin, S.S.; Kharkova, A.S.; Arlyapov, V.A. Glucose biosensor based on screen-printed electrode modified with silicone sol–gel conducting matrix containing carbon nanotubes. 3 Biotech. 2019, 9, 290.
- Soni, D.; Ahmad, R.; Dubey, S. Biosensor for the detection of Listeria monocytogenes: Emerging trends. Rev. Microbiol. 2018, 44, 590–608.
- Brett, A.M.O.; Serrano, S.H.P.; Gutz, I.G.R.; La-Scalea, M.A. Comparison of the Voltammetric Behavior of Metronidazole at a DNA-Modified Glassy Carbon Electrode, a Mercury Thin Film Electrode and a Glassy Carbon Electrode. Electroanalysis 1997, 9, 110–114.
- Nesaei, S.; Song, Y.; Wang, Y.; Ruan, X.; Du, D.; Gozen, A.; Lin, Y. Micro additive manufacturing of glucose biosensors: A feasibility study. Chim. Acta 2018, 1043, 142–149.
- Hashim, U.; Salleh, S.; Rahman, S.F.A.; Abdullah, A.R.A.J. Design and fabrication of Nanowire-based conductance biosensor using spacer patterning technique. In 2008 International Conference on Electronic Design, ICED 2008; IEEE: New York, NY, USA, 2008.
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Today 2009, 12, 12–20.
- Raymundo-Pereira, P.A.; Baccarin, M.; Jr, O.N.O.; Janegitz, B.C. Thin Films and Composites Based on Graphene for Electrochemical Detection of Biologically-relevant Molecules. Electroanalysis 2018, 30, 1888–1896.
- Cotte, S.; Baraket, A.; Bessueille, F.; Gout, S.; Yaakoubi, N.; Leonard, D.; Errachid, A. Fabrication of Microelectrodes Using Original ‘Soft Lithography’ Processes. In New Sensors and Processing Chain; Wiley Online Library: Hoboken, NJ, USA, 2014; Volume 9781848216266, pp. 1–9.
- Tran, K.T.M.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. Sci. Adv. Mater. Devices 2017, 2, 1–14.
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Cartoni, A.; Calandra, P.; Tempesta, E.; Giardi, M.T.; Antonacci, A.; Arduini, F.; et al. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Bioelectron. 2020, 163, 112299.
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 1–23.
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Opin. Biomed. Eng. 2020, 13, 174–189.
- Willmann, J.; Stocker, D.; Dörsam, E. Characteristics and evaluation criteria of substrate-based manufacturing. Is roll-to-roll the best solution for printed electronics? Electron. 2014, 15, 1631–1640.
- Tonello, S.; Serpelloni, M.; Lopomo, N.F.; Abate, G.; Uberti, D.L.; Sardini, E. Screen-Printed Biosensors for the Early Detection of Biomarkers Related to Alzheimer Disease: Preliminary Results. In Procedia Engineering; Elsevier: New York, NY, USA, 2016; Volume 168.
- Lau, G.-K.; Shrestha, M. Ink-Jet Printing of Micro-Electro-Mechanical Systems (MEMS). Micromachines 2017, 8, 194.
- Agarwala, S.; Goh, G.L.; Yeong, W.Y. Optimizing aerosol jet printing process of silver ink for printed electronics. IOP Conf. Ser. Mater. Sci. Eng. 2017, 191, 12027.
- Khan, S.; Lorenzelli, L.; Dahiya, R.S. Technologies for printing sensors and electronics over large flexible substrates: A review. IEEE Sens. J. 2015, 15, 3164–3185.
- Mondal, K.; McMurtrey, M.D. Present status of the functional advanced micro-, nano-printings—A mini review. Today Chem. 2020, 17,DOI: 10.1016/j.mtchem.2020.100328
- Grünwald, S. Reproducible dispensing of liquids in the nanolitre range. Adhes. Sealants 2018, 15, 28–31.
- Abas, M.; Salman, Q.; Khan, A.M.; Rahman, K. Direct ink writing of flexible electronic circuits and their characterization. Brazilian Soc. Mech. Sci. Eng. 2019, 41, 563.
- Yang, H.; Rahman, T.; Du, D.; Panat, R.; Lin, Y. 3-D Printed Adjustable Microelectrode Arrays for Electrochemical Sensing and Biosensing. Actuators. B. Chem. 2016, 230, 600–606.
- Hoffman, J.; Hwang, S.; Ortega, A.; Kim, N.-S.; Moon, K.-S. The standardization of printable materials and direct writing systems. Electron. Packag. Trans. ASME 2013, 135,DOI: 10.1115/1.4023809
- Ramasamy, M.; Varadan, V.K. 3D printing of nano-and micro-structures. In Proceedings of SPIE—The International Society for Optical Engineering; International Society for Optics and Photonics: Hague, The Netherland, 2016; Volume 9802.
- Dziąbowska, K.; Czaczyk, E.; Nidzworski, D. Application of Electrochemical Methods in Biosensing Technologies. Technol. Detect. Pathogens-A Prosp. Way Rapid Anal. 2018, DOI: 10.5772/intechopen.72175
- Nagar, B.; Balsells, M.; de la Escosura-Muñiz, A.; Gomez-Romero, P.; Merkoçi, A. Fully printed one-step biosensing device using graphene/AuNPs composite. Bioelectron. 2019, 129, 238–244.
- Wang, Y.; Ye, Z.; Ying, Y. New trends in impedimetric biosensors for the detection of foodborne pathogenic bacteria. Sensors 2012, 12, 3449–3471.
- Soleymani, L.; Li, F. Mechanistic Challenges and Advantages of Biosensor Miniaturization into the Nanoscale. ACS Sensors 2017, 2, 458–467.
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chinese Chem. Lett. 2020, 31, 589–600.
- Manjakkal, L.; Shakthivel, D.; Dahiya, R. Flexible Printed Reference Electrodes for Electrochemical Applications. Mater. Technol. 2018, 3, 1800252.
- Søpstad, S.; Johannessen, E.A.; Imenes, K. Analytical errors in biosensors employing combined counter/pseudo-reference electrodes. Results Chem. 2020, 2, 100028.
- Faria, A.M.; Peixoto, E.B.M.I.; Adamo, C.B.; Flacker, A.; Longo, E.; Mazon, T. Controlling parameters and characteristics of electrochemical biosensors for enhanced detection of 8-hydroxy-2′-deoxyguanosine. Rep. 2019, 9, 7411.
- Sopstad, S.; Imenes, K.; Johannessen, E.A. Chloride and pH Determination on a Wireless, Flexible Electrochemical Sensor Platform. IEEE Sens. J. 2020, 20, 599–609.
- Diamond, D. Analytical electrochemistry—Analytical Electrochemistry, by Joseph Wang, VCH, Weinheim, 1994, xii + 198 pages, DM 98.00, ISBN 1-56081-572-2. Trends Anal. Chem. 1996, 15, X–XI.
- Thapliyal, N.; Chiwunze, T.; Karpoormath, R.; Goyal, R.; Patel, H.; Srinivasulu, C. Research Progress in Electroanalytical Techniques for Determination of Antimalarial Drugs in Pharmaceutical and Biological Samples. RSC Adv. 2016, 6, 57580–57602.
- Gwon, K.; Lee, S.; Nam, H.; Shin, J.H. Disposable strip-type biosensors for amperometric determination of galactose. Electrochem. Sci. Technol. 2020, 11, 310–317.
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Microbiol. Immunol. 2020, 209, 343–362.
- Zehani, N.; Dzyadevych, S.; Kherrat, R.; Jaffrezic-Renault, N. Sensitive impedimetric biosensor for direct detection of diazinon based on lipases. Chem. 2014, 2, 44.
- Ariffin, E.Y.; Heng, L.Y.; Tan, L.L.; Karim, N.H.A.; Hasbullah, S.A. A highly sensitive impedimetric DNA biosensor based on hollow silica microspheres for label-free determination of E. Coli. Sensors 2020, 20, 1279.
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803.
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric Microbiosensors for Environmental Monitoring. Sensors 2008, 8, 2569–2588.
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80.
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V.; et al. Analytical Problems in Exposing Amperometric Enzyme Biosensors to Biological Fluids. Sensors 2016, 16, 780.
- Pemberton, R.M.; Xu, J.; Pittson, R.; Drago, G.A.; Griffiths, J.; Jackson, S.K.; Hart, J.P. A screen-printed microband glucose biosensor system for real-time monitoring of toxicity in cell culture. Bioelectron. 2011, 26, 2448–2453.
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A review on amperometric-type immunosensors based on screen-printed electrodes. Analyst 2014, 139, 2289–2311.
- Alarcon-Angeles, G., Álvarez-Romero, G.A., Merkoçi, A., Electrochemical biosensors: Enzyme kinetics and role of nanomaterials, (2018) Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry, pp. 140-155., DOI: 10.1016/B978-0-12-409547-2.13477-8.
- Pal, K., Kraatz, H.-B., Khasnobish, A., Bag, S., Banerjee, I., Kuruganti, U.,Bioelectronics and medical devices: From materials to devices - fabrication, applications and reliability(2019) Bioelectronics and Medical Devices: From Materials to Devices, DOI: 10.1016/C2017-0-00496-2
- Li, H.; Liu, X.; Li, L.; Mu, X.; Genov, R.; Mason, A.J. CMOS Electrochemical Instrumentation for Biosensor Microsystems: A Review. Sensors 2016, 17.
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Cells, Nanomedicine, Biotechnol. 2016, 44, 248–262.
- Hopkins, J.; Fidanovski, K.; Lauto, A.; Mawad, D. All-Organic Semiconductors for Electrochemical Biosensors: An Overview of Recent Progress in Material Design. Bioeng. Biotechnol. 2019, 7, 237.
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of Electrochemical Impedance Spectroscopy in Protein Biosensing. Chem. 2009, 81, 3944–3949.
- Carminati, M.; Ferrari, G.; Bianchi, D.; Sampietro, M. Impedance Spectroscopy for Biosensing: Circuits and Applications; Springer: New York, NY,USA, 2015; pp. 1–24.
- Tonello, S.; Bianchetti, A.; Braga, S.; Almici, C.; Marini, M.; Piovani, G.; Guindani, M.; Dey, K.; Sartore, L.; Re, F.; et al. Impedance-based monitoring of mesenchymal stromal cell three-dimensional proliferation using aerosol jet printed sensors: A tissue engineering application. Materials 2020, 13, 2231.
- Aggas, J.R.; Abasi, S.; Phipps, J.F.; Podstawczyk, D.A.; Guiseppi-Elie, A. Microfabricated and 3-D printed electroconductive hydrogels of PEDOT:PSS and their application in bioelectronics. Bioelectron. 2020, 168, 112568.
- Li, J.; Qin, W. An integrated all-solid-state screen-printed potentiometric sensor based on a three-dimensional self-assembled graphene aerogel. J. 2020, 159, 105453.
- Tehrani, Z.; Whelan, S.P.; Mostert, A.B.; Paulin, J.V.; Ali, M.M.; Ahmadi, E.D.; Graeff, C.F.O.; Guy, O.J.; Gethin, D.T. Printable and flexible graphene pH sensors utilising thin film melanin for physiological applications. 2D Mater. 2020, 7, 24008.
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sensors Actuators B Chem. 2018, 273, 966–972.