Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Jessie Wu and Version 4 by Peter Tang.
Direct liquid fuel cells (DLFCs) operate directly on liquid fuel instead of hydrogen, as in typical proton-exchange membrane fuel cells (PEMFCs). DLFCs have the advantages of higher energy densities and fewer issues with the transportation and storage of their fuels compared with compressed hydrogen and are adapted to mobile applications. Among DLFCs, the direct borohydride–hydrogen peroxide fuel cell (DBPFC) is one of the most promising DLFC technologies. DBPFCs are fed sodium borohydride (NaBH4) as the fuel and hydrogen peroxide (H2O2) as the oxidant.
- direct liquid fuel cells (DLFCs)
- direct borohydride–hydrogen peroxide fuel cells (DBPFCs)
- ion-selective membranes
- bipolar membrane
1. Introduction
The quest for sustainable energy sources that are efficient, renewable, and environmentally benign is now more imperative than ever. The massive use of fossil fuels leads to enormous carbon emissions, which have resulted in climate change (e.g., greenhouse effect and sea-level rise) [1]. Therefore, the concerns over energy sustainability and environmental issues are gaining increasing importance. Power generation from renewable and clean energy resources will definitely become the main energy source of future energy strategy [2]. Fuel cells have emerged as a viable alternative to conventional energy sources. The genesis of fuel cells traces back to 1839 when William Robert Grove discovered these electrochemical energy conversion devices. Traditional fuel cells primarily utilize hydrogen as their fuel source. Still, the challenges related to the storage and supply of hydrogen, primarily due to safety and efficiency concerns, have led to the exploration of other alternatives [3][4]. Among the diverse options, sodium borohydride (NaBH4) and methanol have shown promise as liquid fuels for direct liquid fuel cells (DLFCs), operating directly on liquid fuel instead of hydrogen, as in proton-exchange membrane fuel cells (PEMFCs). DLFCs have the advantages of higher energy densities and fewer issues with the transportation and storage of their fuels compared with compressed hydrogen and are adapted to mobile applications.
Specifically, direct borohydride fuel cells (DBFCs) employ (NaBH4) as a fuel and have distinct advantages in terms of energy density. The success of DBFCs, like other fuel cells, heavily relies on the electrochemical processes within the cell. A critical component that influences these processes is the separator or membrane used in the fuel cell. Initially, cation-exchange membranes (CEMs) were presumed to be more effective, particularly in controlling the crossover of negatively charged borohydride ions (BH4−), as opposed to anion-exchange membranes (AEMs) [5]. Nafion, a proton-exchange membrane (PEM), a subset of CEM, gained prominence as an effective separator for direct borohydride–hydrogen peroxide fuel cells (DBPFCs). However, as the research landscape evolves, Nafion faces competition from alternative membrane materials. This reflects a growing dynamism and diversity in membrane technologies that aim to address the challenges and limitations of conventional materials. The choice of the membrane is paramount, as it affects not only the efficiency but also the environmental impact and economic feasibility of the fuel cell applications [6][7][8].
CEM and AEM have served pivotal roles in the progression of DBPFCs, yet their inherent limitations necessitate a more refined approach. Bipolar membranes (BPMs) have appeared as an advanced combination of CEMs and AEMs. BPMs bring unique features to the maintenance of the critical pH gradient between alkaline BH4− anolytes and acidic hydrogen peroxide (H2O2) catholytes, a requirement paramount to the effectiveness of electrochemical reactions at the electrodes. This ability is not only an incremental advance but also a fundamental enhancement that has the potential to redefine fuel cell performance and efficiency standards.
The adoption of BPMs is not without challenges, and current research endeavors aim to optimize the balance between stability and selectivity in BPMs. These challenges are multifaceted and encompass various aspects. Firstly, manufacturing complexity presents a significant hurdle, as producing high-quality BPMs demands precise fabrication techniques and specialized materials, resulting in elevated production costs. Secondly, maintaining membrane stability under diverse operating conditions, such as high temperatures, corrosive environments, and exposure to reactive species, becomes paramount in ensuring long-term effectiveness. Achieving the desired selectivity for proton and hydroxide ion transport while minimizing the crossover of other species is an intricate task that affects the efficiency of BPMs in facilitating ion transport and separation. Furthermore, operating at high current densities (>1.5 A cm−2) poses a crucial challenge, requiring stable ion exchange performance for optimal fuel cell and electrolysis system functionality.
2. Processes Occurring in the Direct Borohydride–Hydrogen Peroxide Fuel Cell
Renewable energy sources play a critical role in addressing environmental challenges associated with conventional energy production by offering a sustainable and cleaner alternative. Unlike fossil fuels, which contribute to greenhouse gas emissions and climate change, renewable energy provides a greener option. However, the widespread adoption of hydrogen fuel cells, which offer renewable and green energy, faces obstacles such as cost and safety with its transport requirements. A potential solution lies in DLFCs utilizing methanol or ethanol, simplifying fuel storage for portable devices. DBPFCs, as low-temperature DLFCs utilizing NaBH4 and H2O2, offer advantages in terms of lower cost and lower emissions compared with ethanol and methanol fuel cells. However, they do not surpass hydrogen fuel cells in terms of energy density, with H2/air fuel cells able to generate 1 W cm−2 without harmful emissions. Despite slightly lower energy density, DBPFCs are still well-suited for space and underwater applications due to their cost-effectiveness, simplified fuel/oxidant management, and reduced emissions among DLFCs [8]. In a DBPFC, the borohydride oxidation reaction (BOR) at the anode is given by Equation (1):
BH4− + 8OH− → BO2− + 6H2O + 8e− → E0 = −1.24 V vs. SHE (1)
where E0 represents the standard electrode potential of BOR against the standard hydrogen electrode (SHE). As there is always some production of hydrogen gas due to the unwanted partial hydrolysis of BH4− (Equation (2)), the possibility of this reaction occurring as a parasitic reaction cannot be ignored. Thus, the borohydride oxidation considering the parasitic BH4− hydrolysis can be written as Equation (3):
BH4− + H2O → H2 + BH3OH− (2)
BH4− + xH2O → B(OH)4− + (x − 4) H2O + (4 − x/2) H2 + xe− (3)
where “x” is the coulombic number. The x value can be obtained experimentally using typical electrochemical methods, like cyclic voltammetry, rotating disk electrode (RDE), and rotating ring-disk electrode (RRDE) measurements, or by measuring the volume of hydrogen gas produced [9]; x values can range between 0 and 8, usually being 4 when using platinum-based electrodes [10].
Gold-based electrocatalysts are usually utilized to prevent BH4− hydrolysis and were long considered the most faradaic efficient. In alkaline media, H2O2 exists as HO2− (Equation (4)), which can be reduced via a two-electron process to hydroxyl ions, as shown in Equation (5). The cathodic reaction in a DBPFC is influenced by the pH conditions described in Equations (5) and (6) for alkaline and acidic media, respectively. At this high pH, AEMs are more adequate than CEM because the OH− ions generated during the hydrogen peroxide reduction reaction (HPRR) pass through the AEM and are consumed in the BH4− oxidation reaction.
H2O2 + OH− → HO2− + H2O (4)
HO2− + H2O + 2e− → 3OH− → E0 = 0.87 V vs. SHE (5)
Although this seems like an elegant concept, the low stability of H2O2 in alkaline media leads to its spontaneous decomposition. Thus, the system’s efficiency must be increased by keeping the oxidant solution at low pH. However, under acidic conditions, H2O2 is adsorbed onto the electrode and is reduced according to Equation (6).
H2O2 + 2H+ + 2e− → 2H2O E0 = 1.77 V vs. SHE (6)
Combining Equations (1) and (6) leads to the net cell reaction of a typical alkaline–acidic DBPFC, as given by (Equation (7)), with a theoretical cell voltage of 3.01 V at 25 °C [8].
BH4− + 4H2O2 → BO2− + 6H2O E0 = 3.01 V (7)
Šljukić and Santos have argued that the preferred path for H2O2 electroreduction in fuel should be in acidic media (Equation (6)), involving the direct pathway and faster kinetics [8]. Additionally, the direct pathway yields a higher cell voltage due to the higher standard electrode potential of the HPRR. However, the indirect mechanism, which occurs in alkaline media, presents added requirements and greater complexity in constructing the fuel cell system, primarily due to gas management considerations. All three mass-transfer processes occur within a DBPFC’s electrolyte: (electro)migration, convection, and diffusion. In the case of a CEM, the consumption of OH− in the BOR leads to a decrease in the number of anions on the anode side.
Cations must be sent away to compensate for this, resulting in Na+ ions crossing the membrane from the anode to the cathode compartment (Figure 1a). This process is based on diffusion. Additionally, a concentration gradient of other species, such as BH4−, is present, and by diffusion, BH4− ions tend to move to the other side. However, the presence of a CEM only allows the passage of cations, not anions, thus preventing the BH4− crossover to the other side. The presence of an AEM allows only the passage of OH− and Cl− [9] (Figure 1b). Those are based on the Donnan exclusion principle, which states that only ions with opposite charges can be transferred, while transferring ions with the same charge as the membrane-immobilized group is largely prohibited. Therefore, the migration of ions across a membrane is caused by the electric field that arises from the anodic and cathodic reactions. The type of membrane separator used determines the charge carrier. As discussed previously, in the case of an AEM, OH− and Cl− anions move from the cathodic to the anodic compartment to maintain the cell’s charge balance. At the same time, Na+ cations cross the CEM in the cathodic direction to achieve the same result. Additionally, neutral species such as H2O2, gases, and organic compounds may diffuse from one compartment to the other due to concentration gradients [11].
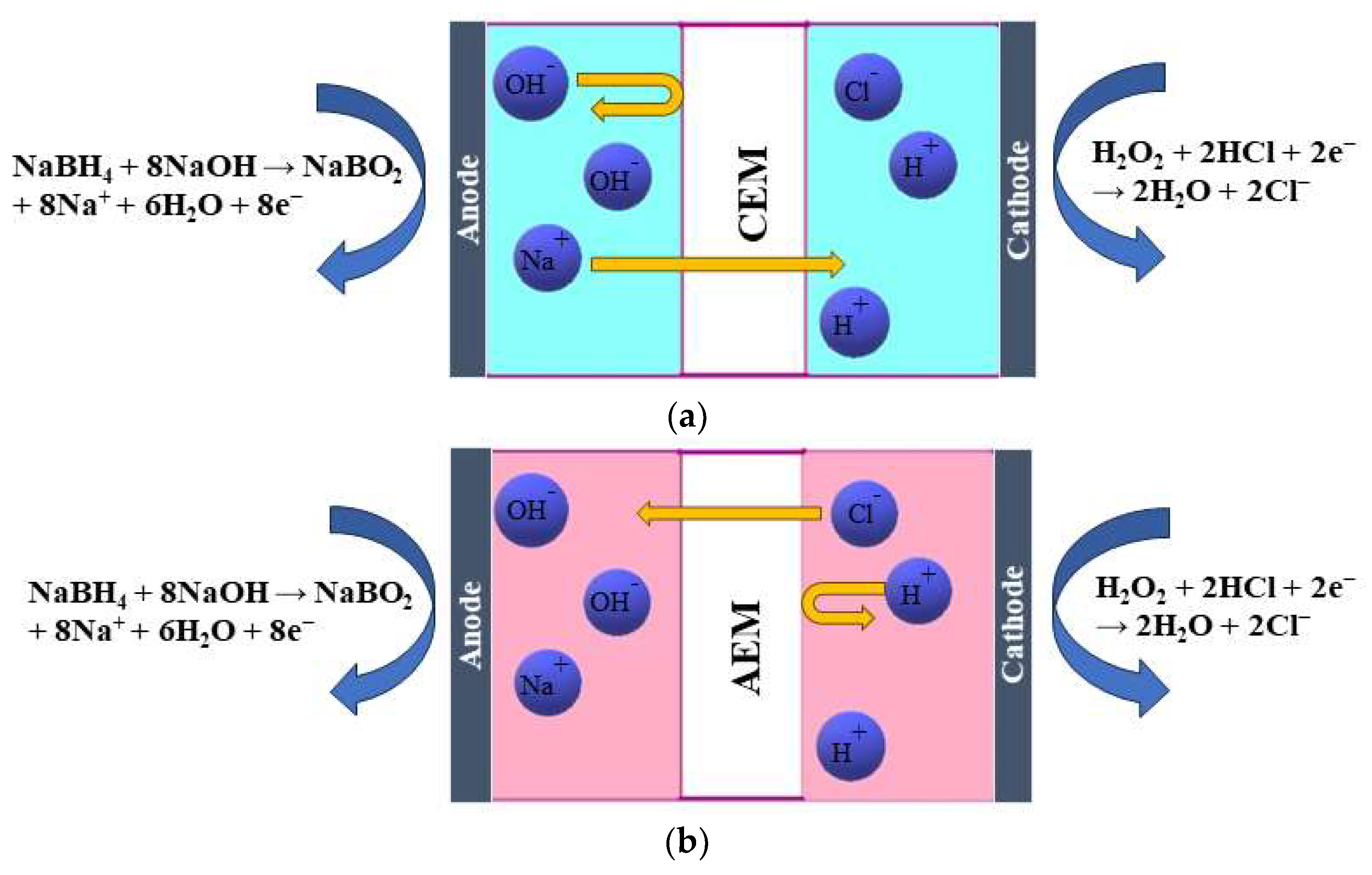
Figure 1. Schematic illustration of the working principle of (a) a cation-exchange membrane (CEM) and (b) an anion-exchange membrane (AEM) in a DBPFC.
References
- Li, C.; Wang, Z.; Liu, H.; Qin, J.; Wei, L. Comparative study on the performance of the application of clean alternative fuels in SOFC/ICE hybrid power systems on electric aircraft. Front. Energy Res. 2023, 11, 1146587.
- Zhang, H.; Sun, C.; Ge, M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202.
- Kamarudin, S.K.; Daud, W.R.W.; Som, A.M.; Mohammad, A.W.; Takriff, S.; Masdar, M.S. The conceptual design of a PEMFC system via simulation. Chem. Eng. J. 2004, 103, 99–113.
- Ahmad, M.M.; Kamarudin, S.K.; Daud, W.R.W.; Yaakub, Z. High power passive μDMFC with low catalyst loading for small power generation. Energy Convers. Manag. 2010, 51, 821–825.
- Santos, D.M.F.; Sequeira, C.A.C. Polymeric Membranes for Direct Borohydride Fuel Cells: A Comparative Study. ECS Trans. 2010, 25, 111–122.
- Li, Z.P.; Liu, B.H.; Arai, K.; Suda, S. A Fuel Cell Development for Using Borohydrides as the Fuel. J. Electrochem. Soc. 2003, 150, A868.
- Cheng, H.; Scott, K.; Lovell, K.V.; Horsfall, J.A.; Waring, S.C. Evaluation of new ion exchange membranes for direct borohydride fuel cells. J. Memb. Sci. 2007, 288, 168–174.
- Šljukić, B.; Santos, D.M.F. Direct borohydride fuel cells (DBFCs). In Direct Liquid Fuel Cells: Fundamentals, Advances and Future; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–232.
- Chatenet, M. Tailoring membranes. Nat. Energy 2019, 4, 261–262.
- Šljukić, B.; Morais, A.; Santos, D.M.F.; Sequeira, C.A.C. Anion- or cation-exchange membranes for NaBH4/H2O2 fuel cells? Membranes 2012, 2, 478–492.
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A comprehensive review on the synthesis and applications of ion exchange membranes. Chemosphere 2021, 282, 130817.
More
