Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Mazhar Sher.
Biofilms are complex communities of microorganisms that can form on various surfaces, including medical devices, industrial equipment, and natural environments. The presence of biofilms can lead to a range of problems, including infections, reduced efficiency and failure of equipment, biofouling or spoilage, and environmental damage. As a result, there is a growing need for tools to measure and monitor levels of biofilms in various biomedical, pharmaceutical, and food processing settings.
- biofilms
- electrochemical impedance spectroscopy
- biosensors
1. Introduction
Different electrochemical techniques have been developed to examine biofilm sensing throughout this broad range of uses including electrochemical impedance spectroscopy (EIS), chronopotentiometry, chronoamperometry, cyclic voltammetry, and scanning electrochemical microscopy [31,32][1][2].
An electrochemical reaction at an electrode surface can be broken down mechanistically into several steps processes (charge transfer, adsorption, and mass transport processes), each happening at a different rate. Different steps might happen at various timescales and are time-dependent. To make the evaluation of electrochemical systems easier more rapid approaches such as EIS are necessary. The EIS enables the examination of the time-dependent mechanism through the electrochemical system’s response (current or potential) recorded at specific frequencies. The EIS technique is useful because it can explain the electrochemical mechanisms taking place on the surface of the electrode in only one measurement. These mechanisms include the operating of electrochemical biosensors, the operating of industrial batteries, and the corrosion of alloys and metals.
2. Electrochemical Impedance Spectroscopy for Sensing Purpose
Electrochemical biosensors are a famous type of sensor that are commercially successful to be developed [33][3]. According to the operational principle and mechanism of measurement, these are generally classified into two types: impedimetric (non-faradaic) transducers and faradaic (amperometric, potentiometric). The non-faradaic electrochemical impedance spectroscopy (EIS) is a non-destructive method; thus, it allows repeated measurements on the same sample. EIS also measures the impedance over a range of frequencies, and it is highly sensitive to very small changes in the biofilm. The EIS does not require direct current. It does not involve redox reactions and there is no need for a reference electrode. As a result, the EIS-based methods for biofilm detection are more amenable to miniaturization compared to faradaic methods. To analyze EIS data, some knowledge of electrochemistry and biofilm physics is required. EIS experiments are time-consuming as multiple measurements at different frequencies are required. EIS of biofilms is well-suited for applications where highly sensitive, non-destructive, and time-dependent measurements are required. EIS is an excellent choice for studying the overall dynamic behavior of biofilms. It provides an opportunity to investigate the biofilm responses to various conditions. EIS would be the preferred choice to monitor biofilm growth over the passage of time.
The faradaic methods of biofilm detection focus on specific electrochemical reactions. Thus, they provide specific information about biofilm components. As faradaic approaches do not require multiple frequency sweeps, they provide faster measurements. Faradaic methods typically involve simpler data analysis compared to the more complex impedance spectra of EIS. The Faradic techniques often perturb the system with electrochemical reactions, which can destroy the biofilm. Destructive Faradaic approaches might not be as sensitive to rapid small changes in the biofilm as EIS. Faradaic electrochemical approaches can be utilized where non-destructive measurements are not a priority and researchers want to obtain specific information about biofilm components or processes. Faradaic approaches are less time-consuming and require simple data analysis.
The ultimate choice between EIS and faradaic electrochemical methods for biofilm detection will depend on the specific application where researchers can choose one based on their specific requirements and considering sensitivity, data complexity, and non-destructive nature.
2.1.1. Impedimetric Biosensors
To detect and quantify bacteria, impedance-based methods have been employed as a transduction mechanism. Impedance microbiology (IM) has been utilized for years to identify bacteria from the samples of the environment, food sector, healthcare, etc. In this method, a pair of electrodes is immersed in the culture media. The idea is to measure the change in impedance. Capacitance or impedance and total or relative change in conductivity of the solutions are evaluated at a specific temperature, to identify bacterial development in real-time. Direct or indirect measurement techniques are used in conventional impedance microbiology to measure the impedance change of the media. The overall impedance change due to bacterial development is composed of two elements that could be monitored at various ranges of frequency: (i) change in impedance by the media and (ii) change in impedance caused by the electrolyte/electrode interaction, typically called as the double electrochemical layer (EDL). The impedimetric procedure used to measure electrochemical impedance is typically non-faradaic. The impedance of the growing medium will become more effective on frequencies over 10 kHz, whereas the EDL impedance is more prominent at lower frequencies (usually < 10 kHz). A basic circuit involving two EDL capacitors with value Cdl and a resistor Rs connected in series can be utilized to explain how both impedances’ frequency impacts total impedance. Equation (1) below can be used to numerically show the circuit’s impedance Z:
where
f = Frequency
Rs = Solution resistance
Cdl = EDL capacitance at the electrode.
The electrochemical impedance of biofilms is produced by the extracellular matrix (ECM) and the cells inside of the biofilm act as dielectric materials, which change with metabolic state, composition, and time. It is possible to model the bacterial biofilms that have developed on the surface of microelectrodes as an electrical circuit. Figure 1a–c shows such an electronic circuit model. A sterile electrical model growth medium without bacteria is shown in Figure 1a. ECM and biofilm are produced between the two electrodes; a simple series and corresponding parallel electrical model is shown in Figure 1b,c. The following are the parameters in the circuit stand for: The EDL capacitance is denoted by the Cdl, the media resistance without bacterial cells are denoted by the Rsol, and the resistance and capacitance of biofilm are denoted by Rbio and Cbio, respectively. The impedimetric responses of the culture change accordingly when the first two variables Cdl and Rsol are affected by bacterial metabolism [34][4]. Equations (2)–(4) can be used to figure out the magnitude of the impedance of the three electrical circuits illustrated in Figure 2a–c.
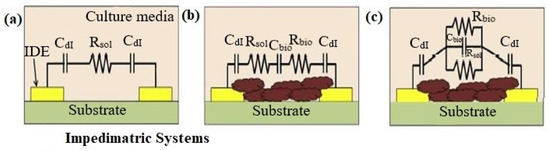
Figure 1. (a) Model of sterile culture media before bacterial cells are inoculated. (b) Model of series circuits after ECM and biofilm development (c) models of parallel circuits after ECM and biofilm development.
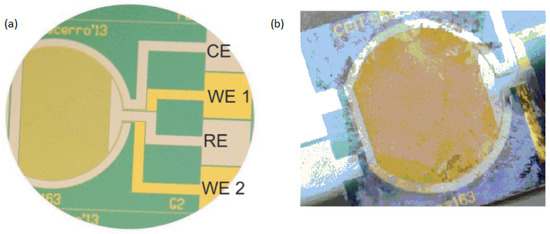
Figure 2. (a) An image of the thin-film electrochemical biosensor taken before biofilm growth. (b) The Actual image of electrochemical biosensor after the experiment of biofilm formation.
These formulas were used to evaluate experimental data for Staphylococcus epidermis biofilms, and researchers were able to determine the numerical values of the different parameters [34][4]. Specific electrical properties of the system can be monitored and applied to precisely detect the initiation of biofilm development as well as the development with respect to time by applying these or other similar models to the experimental data. For instance, Liu et al. used a similar circuit model and an interdigitated microelectrodes (IDE)-based impedance sensor to monitor variations in the capacitance and resistance of Salmonella and E. coli biofilms with time [36][5]. Microelectrode arrays have also been used to measure the capacitive and resistive features of E. coli growth [37][6]. Impedimetric techniques are among the most widely used approaches for detecting and characterizing biofilms due to their benefits, which include a low resistance, good signal-to-noise ratio, decrease in sample volume, low power requirement, and quick establishment of a steady state [38][7].
2.2. Potentiometric and Amperometric Biosensors
For real-time biofilm sensing, faradic electrochemical approaches including amperometric and potentiometric techniques have also been used. To measure the faradaic current produced through the oxidation and reduction of a redox species with the interaction of a solid electrode is accomplished by these measurements. It has been demonstrated that charge transmission takes place among the cells and the medium through the early phases of bacterial biofilms [39,40][8][9]. Different types of molecules produced by bacterial cells, such as pyocyanin and phenazine-1-carboxylic acid, include electrochemically active groups which can connect with the free electrons of a surface. Electrochemical methods allow for the observation of this phenomenon, their analysis, and subsequent detection of bacterial existence at the early phases of adhesive and biofilm development. Beccero et al., 2016 created a thin-film sensor [41][10]. It was developed by differential pulse voltammetry (DPV) and cyclic voltammetry (CV). The four-microelectrode configuration as shown in Figure 2 was used by the researchers consists of a pseudo-reference electrode made of platinum, a counter electrode made of platinum, and two gold working electrodes. In comparison to the two-electrode arrangement, this method showed high sensitivity, a faster time to steady-state current, and a smaller ohmic drop [42,43][11][12].
The existence of a Staphylococcus epidermidis biofilm was discovered after 2 h of the first inoculation by CV and 1 h through DPV. There was an increase in both the current signal and the redox peaks were observed proportionally to biofilm growth.
3. Electrochemical Impedance Spectroscopy Existing Technologies
Electrochemical impedance spectroscopy is a powerful tool for the time-dependent mechanism examination of biofilms. This method provides real-time and non-destructive measurements. Hence, it is well suited to analyze biofilm dynamics at various time frames. Thus, it can be used to understand biofilm formation, growth, and response to environmental changes.
3.1. Flexible Platform for In-Situ Impedimetric Detection
In another research, a real-time biofilm formation detection system and treatment on surfaces of 3D biomedical devices was developed [44][13]. They designed a flexible platform on polyimide substrates with gold-interdigitated electrodes. The characterization was carried out using a specially designed flow system, and the sensor was mounted within a urinary catheter. To monitor the formation of the biofilm a 50 mV signal amplitude impedance change was used at 100 Hz. Escherichia coli (E. coli) biofilm growth was related to a 30% reduction in impedance within 24 h. The platform allowed for biofilm removal via the bioelectric effect; a small amount of antibiotic in combination with the application of an AC voltage signal resulted in a synergistic decrease in biofilm, which caused a 12% rise in impedance. The results of the impedance detection matched with variations in the quantity of biofilm biomass on the sensor, according to biomass characterization performed with crystal violet staining. The reduction in size and allowing for on-the-go wireless implementation proved integration by using an impedance converter chip. The impedance converter decreased by 5% impedance, which simulated the potentiostat trend was connected to the development of biofilm.
3.2. Monitoring of Bacteria Biofilms by In-Situ Impedimetric Biosensor Chip
In another research, a chip of biosensor with interdigital microelectrodes was designed and used for observing the development of E. coli and Salmonella biofilms [36][5]. An interdigital microelectrode with a glass substrate and a PDMS layer with small cavities formed the chip of the biosensor. By applying 100 mV of alternating voltage and using a 1 Hz to 100 kHz range of frequency for 48 h, the biosensor chip monitored the EIS of E. coli and Salmonella biofilms. It was observed that the impedance spectroscopy of biofilms changed with culture time. Additionally, a model of an analogous circuit that considers the biofilm resistance (Rb) and capacitance (Cb) properties was used to fit the impedance spectroscopy of biofilm. The findings showed that the Cb first drops and then increases during the time of culture, but the Rb showed a vice-versa trend with respect to culture time. Additionally, it was shown that E. coli and Salmonella had very distinct Cb and Rb changing trends with culture time. Due to its distinct characteristics of continuity, in-situ monitoring, and non-invasion for bacteria biofilms identification and real-time, the chip of a biosensor offered a feasible platform for more research into biofilms. This biosensor chip can monitor the biofilm formation of Salmonella and E. coli in real-time.
3.3. Integrated Microsystem for Real-Time Detection of Bacterial Biofilms
Subramanian et al. 2017 designed an impedance monitoring device with threshold-activated feedback for simultaneous treatment and in-situ biofilm recognition [45][14]. They showed how to measure the fractional relative change (FRC) in absolute impedance to properly detect the development of biofilms of Escherichia coli in microfluidic flow cells. Additionally, they also showed that growth measurements can be used as a threshold-activated initiation tool to start effective biofilm handling by using the bioelectric effect (BE), conducted, and using the same array of sensing electrodes. It was achieved by a customized database that (a) allowed the threshold-based activation of BE treatment and (b) provided in-situ detection of the elimination and development of biofilms inside the microfluidic channels. This developed microsystem will enable real-time detection of the onset of biofilms and their in-situ treatment on the surfaces of medical implants.
References
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41.
- Bard, A.; Faulkner, L. Fundamentals and applications. Electrochem. Methods 2001, 2, 580–632.
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Paredes, J.; Becerro, S.; Arana, S. Label-free interdigitated microelectrode based biosensors for bacterial biofilm growth monitoring using Petri dishes. J. Microbiol. Methods 2014, 100, 77–83.
- Liu, L.; Xu, Y.; Cui, F.; Xia, Y.; Chen, L.; Mou, X.; Lv, J. Monitoring of bacteria biofilms forming process by in-situ impedimetric biosensor chip. Biosens. Bioelectron. 2018, 112, 86–92.
- Goikoetxea, E.; Routkevitch, D.; De Weerdt, A.; Green, J.J.; Steenackers, H.; Braeken, D. Impedimetric fingerprinting and structural analysis of isogenic E. coli biofilms using multielectrode arrays. Sens. Actuators B Chem. 2018, 263, 319–326.
- Yang, L.; Bashir, R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008, 26, 135–150.
- Bayoudh, S.; Othmane, A.; Ponsonnet, L.; Ouada, H.B. Electrical detection and characterization of bacterial adhesion using electrochemical impedance spectroscopy-based flow chamber. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 291–300.
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588.
- Becerro, S.; Paredes, J.; Mujika, M.; Lorenzo, E.P.; Arana, S. Electrochemical real-time analysis of bacterial biofilm adhesion and development by means of thin-film biosensors. IEEE Sens. J. 2015, 16, 1856–1864.
- Min, J.; Baeumner, A.J. Characterization and optimization of interdigitated ultramicroelectrode arrays as electrochemical biosensor transducers. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 724–729.
- Rahimi, M.; Mikkelsen, S.R. Cyclic biamperometry at micro-interdigitated electrodes. Anal. Chem. 2011, 83, 7555–7559.
- Huiszoon, R.C.; Subramanian, S.; Rajasekaran, P.R.; Beardslee, L.A.; Bentley, W.E.; Ghodssi, R. Flexible platform for in situ impedimetric detection and bioelectric effect treatment of Escherichia coli biofilms. IEEE Trans. Biomed. Eng. 2018, 66, 1337–1345.
- Subramanian, S.; Tolstaya, E.I.; Winkler, T.E.; Bentley, W.E.; Ghodssi, R. An integrated microsystem for real-time detection and threshold-activated treatment of bacterial biofilms. ACS Appl. Mater. Interfaces 2017, 9, 31362–31371.
More
