Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Samuel Ebenezer KAYODE.
The common waste management methods which include direct dumping into water bodies, open-air combustion, and as land fillers are obsolete and are the major causes of environmental pollution. Conversion of biowastes into valuable materials aids proper waste management, and helps to attain a cleaner environment, in addition to the fact that wastes are turned into wealth. Biowastes are rich in carbon and can serve as excellent precursors for the synthesis of important carbon materials such as activated carbon, graphene, carbon nanotubes etc.
- environmental pollution
- biowastes
- carbonization
- activated carbon
- specific surface area
- electrode performance
1. Introduction
Biowaste residues are materials that are often generally referred to as waste by individuals, families, and bio-processing industries. The reason is that these materials seem not to be directly useful to them, and therefore are disposed through the easiest available methods which include, direct dumping into water bodies, burning in open air, or as land-filling materials as shown in Figure 1. During the burning and decay of biowaste materials, methane, carbon dioxide, and other toxic gases are generated and released into the environment. These methods of waste management are obsolete and hazardous to the plants, animals, humans, and other non-living components that constitute the environment. Meanwhile, continuous gathering of waste in the environment over a long period of time also pose ecological challenge. Processing of biowastes into useful materials aids proper disposal of wastes which in turn help to attain cleaner environment. Other methods such as vitrification, in which the ash obtained from carbonization of biowastes is subjected to very high temperatures of about 1500 °C to obtain glass-ceramic materials, has been reported as a facile and important method for waste disposal [1,2,3][1][2][3]. These biowastes such as rice husks, ground nut shells, sugarcane bagasse, spent coffee ground, brewers’ spent grains, corncob, coconut shells, wheat husks, and palm kernel shells among others are generated globally in very large amount every year, they are therefore readily available and at no cost [4].
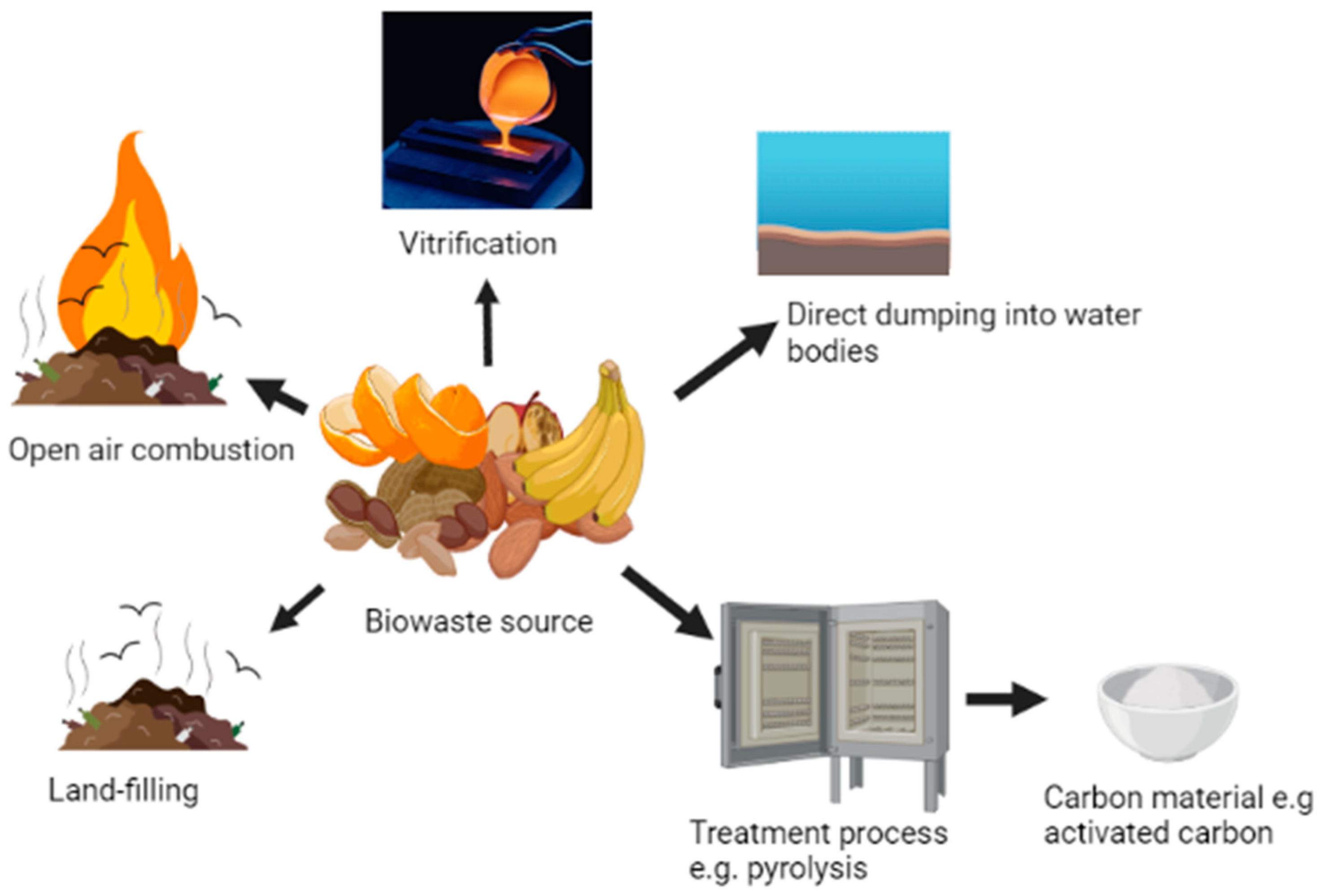
Figure 1. Different approach for managing biowaste materials.
2. Methods for Converting Biowastes into Value-Added Carbon Materials
2.1. Pyrolysis
Pyrolysis is a process that converts organic matter such as plants or animal residues into carbonaceous materials by subjecting them to high temperatures [35]. Dry biowaste material such as sugarcane bagasse, corncob, coffee shells, rice husk, etc., are exposed to high temperatures of about 700 °C in a furnace in an inert environment (usually nitrogen or argon atmosphere), to evaporate and remove the hydrocarbon contents in an oxygen-free environment. The product obtained from this process is called char or biochar [36]. Bai’s group reported the preparation of hierarchical porous carbon from pulp and paper wastes (lignosulphonates) for high-performance supercapacitor electrode via pyrolysis. The pretreated biowaste was pyrolyzed at 900 °C for 2 h in Ar atmosphere followed by ZnCl
Different approach for managing biowaste materials.
2. Methods for Converting Biowastes into Value-Added Carbon Materials
2.1. Pyrolysis
Pyrolysis is a process that converts organic matter such as plants or animal residues into carbonaceous materials by subjecting them to high temperatures [5]. Dry biowaste material such as sugarcane bagasse, corncob, coffee shells, rice husk, etc., are exposed to high temperatures of about 700 °C in a furnace in an inert environment (usually nitrogen or argon atmosphere), to evaporate and remove the hydrocarbon contents in an oxygen-free environment. The product obtained from this process is called char or biochar [6]. Bai’s group reported the preparation of hierarchical porous carbon from pulp and paper wastes (lignosulphonates) for high-performance supercapacitor electrode via pyrolysis. The pretreated biowaste was pyrolyzed at 900 °C for 2 h in Ar atmosphere followed by ZnCl
2.2. Hydrothermal Method
This method is also referred to as high-pressure or high-temperature aqueous carbonization. During hydrothermal treatment, the biowaste is heated at a temperature of about 200 °C in an airtight reactor, usually an autoclave, in the presence of superheated water and self-generated pressure [38]. This process is used to prepare materials with controlled structures [39]. In an experiment, Liu’s group synthesized porous carbon from waste coffee grounds for supercapacitor application following hydrothermal synthesis method. They combined catalytic carbonization using FeCl
2.2. Hydrothermal Method
This method is also referred to as high-pressure or high-temperature aqueous carbonization. During hydrothermal treatment, the biowaste is heated at a temperature of about 200 °C in an airtight reactor, usually an autoclave, in the presence of superheated water and self-generated pressure [8]. This process is used to prepare materials with controlled structures [9]. In an experiment, Liu’s group synthesized porous carbon from waste coffee grounds for supercapacitor application following hydrothermal synthesis method. They combined catalytic carbonization using FeCl
3
as catalyst with KOH activation at 700 °C, followed by hydrothermal treatment of the resulting carbon material at 120 °C for 6 h to obtain an activated carbon with well-defined porosity (1.64 cm
3
g
−1
pore volume) and ultrahigh specific surface area of 3549 m
2
g
2.3. Activation
Activation of char produced from the pyrolysis process are classified into physical activation and chemical activation. In the physical one, the char is heated at temperature between 800 and 1100 °C in steam, CO
2.3. Activation
Activation of char produced from the pyrolysis process are classified into physical activation and chemical activation. In the physical one, the char is heated at temperature between 800 and 1100 °C in steam, CO
2
, N
2, or air mixture environment [41]. While in chemical activation, the char produced from pyrolysis is combined and mixed with activating agents such as KOH, NaOH, ZnCl
, or air mixture environment [11]. While in chemical activation, the char produced from pyrolysis is combined and mixed with activating agents such as KOH, NaOH, ZnCl
2
, H
3
PO
4
, K
2
CO
3
, and FeCl
3 in suitable ratios and then heated at a temperature of 800 °C or above to obtain activated carbons with desired properties [42]. Over the past few decades, activation of carbonaceous materials to obtain activated carbon has gained tremendous recognition because of the simplicity, time saving, and cost effectiveness. Activation can be either a one single step or a two-step process which is often preceded by low temperature carbonization or hydrothermal treatment, which helps to control the porosity and surface area of the activated carbon obtained.
in suitable ratios and then heated at a temperature of 800 °C or above to obtain activated carbons with desired properties [12]. Over the past few decades, activation of carbonaceous materials to obtain activated carbon has gained tremendous recognition because of the simplicity, time saving, and cost effectiveness. Activation can be either a one single step or a two-step process which is often preceded by low temperature carbonization or hydrothermal treatment, which helps to control the porosity and surface area of the activated carbon obtained.
3. Features of Carbon-Based Electrodes
3.1. Electrocatalysis
Electrocatalysis is a process which leads to increasing the speed (rate) of half-cell reactions at the surfaces of electrodes.Electrocatalysis is a process which leads to increasing the speed (rate) of half-cell reactions at the surfaces of electrodes. An electrocatalyst is a catalyst that takes part in electrochemical reactions. They are substances which particularly cause the increase in the speed of half-cell reactions. Electrocatalysts function at electrode surfaces and most commonly may be that electrode surface itself. All-round importance of electrocatalysis in scientific research cannot be overemphasized. Fields such as wastewater treatment, energy devices, corrosion science, electro-organic synthesis, and development of electro-analysis sensors actively engage the applications of electro-catalysis [13]. The properties of a good electrocatalyst include excellent catalytic activity, high surface area, stability toward anodic corrosion, low energy consumption, and outstanding mechanical and chemical resistance [14].
An electrocatalyst is a catalyst that takes part in electrochemical reactions. They are substances which particularly cause the increase in the speed of half-cell reactions. Electrocatalysts function at electrode surfaces and most commonly may be that electrode surface itself. All-round importance of electrocatalysis in scientific research cannot be overemphasized. Fields such as wastewater treatment, energy devices, corrosion science, electro-organic synthesis, and development of electro-analysis sensors actively engage the applications of electro-catalysis [44]. The propertsorption process ies of a good electrocatalyst include excellent catalytic activity, high surface area, stability toward anodic corrosion, low energy consumption, and outstanding mechanical and chemical resistance [45].
3.2. Adsorption
3.2. Adsorption
Adsorption process involvolves the movement and gathering of dissolved particles on the surface of an adsorbent material. This process can occur because of physical forces (physisorption) or chemical bonds (chemisorption). It is a reversible process; the reversed process is called desorption [48][15]. A good adsorbent possesses the following characteristics: excellent capacity, high sorption rate, good selectivity, fast kinetics, and low-cost. The texture, surface morphology, chemical composition of material and chemical activation (functionalization) are important factors which determine the sorption capacity of an adsorbent [48,49][15][16]. Physisorption occurs due to weak Van der Waals’ attraction forces and because the adsorbent generally has low specific surface area and is non-porous in nature.4. Biowaste-Based Activated-Carbon and Their Applications in Energy Storage Systems
4.1. Batteries
Electrochemical cells can either produce electrical energy from chemical reactions or utilize electrical energy to generate chemical reactions. Batteries are electrochemical cells that utilize reversible redox reactions to produce and store electrical energy. The energy stored in a battery can be used to power electrical devices such as portable electronics (e.g., cell phones) and electric vehicles. Conventional batteries include lithium-ion batteries (LiIBs), lithium-sulfur batteries (LiSBs), sodium-ion batteries (NaIBs), and metal-air (metal-oxygen) batteries [51][17]. The four basic parts of batteries include cathode, anode, electrolyte, and separator (membrane). Biowastes has been reported to be good precursors for the preparation of electrode materials for battery applications.4.2. Lithium-Ion Batteries (LiIBs)
LiIBs are rechargeable battery composed of cells containing lithium ions. When a LiIB is in use (i.e., during discharge), lithium ions (Li+) move from the anode to the cathode, while during charging, the reverse reaction occurs. Carbon materials are normally used as anode in the conventional lithium-ion cells, the cathode is made of typical metal oxide while the electrolyte composed of lithium salt is dissolved in organic solvent [57][18]. In a typical LiIB, the following reactions occur during charging and discharging processes [58][19].4.3. Sodium-Ion Batteries (NaIBs)
NaIBs are rechargeable batteries analogous to the LiIBs, but charges are carried by sodium ions (Na+) instead of the Li+. The working principle and the cell construction of NaIBs are like those of the LiIB types that have gained commercial recognition. A major advantage of NaIBs over LiIBs is the abundance of sodium in nature. Therefore, commercial production of NaIBs is expected to be cheap compared to LiIBs [61][20]. NaIB cells are made up of cathodes which are based on materials that contain sodium, and the anode is made of inert materials and electrolyte which can be an aqueous or non-aqueous solution of sodium salt. The aqueous electrolytes have the disadvantage of producing low voltage due to the limited electrochemical stability window of water. Therefore, non-aqueous electrolytes such as sodium hexafluorophosphate are often preferred because of their ability to extend the window range [62,63,64][21][22][23]. During the charging process, sodium ions are removed from the cathode and migrate into the anode where at the same time, electrons travel through the external circuit from the cathode into the anode. The reverse process occurs during discharging. Due to the difference in the physical and electrochemical properties of sodium and lithium, materials generally used for LiIBs are not always suitable for NaIBs [65][24].4.4. Other Batteries
In metal-air electrochemical cells, the anode is usually a pure metal with an external cathode of ambient air and an aqueous electrolyte. When the battery is in use, reduction reaction normally takes place at the air cathode and the anode undergo oxidation [70][25]. Metal-air batteries possess higher energy density and specific capacity compared to LiIBs. Because of this, MABs are becoming important potential candidate for applications in electric vehicles. The commercial application of MABs is faced with complications that are associated with challenges such as slow oxygen-reduction reactions at the cathode which has slow-down its development and commercialization [71][26]. Introducing electron-rich atoms into the carbon matrix to serve as catalyst for the acceleration of oxygen reduction reactions in air cathodes is found to be an encouraging solution to the problem of slow kinetics of the oxygen reduction reactions in MABs. Ma’s group co-doped nitrogen and sulfur in porous carbon derived from the carbonization of garlic stems. The material obtained was used as cathode electrocatalyst with promising faster oxygen reduction reaction capability in a zinc-air cell [46][27]. Another promising technology is the aqueous Zn-ion battery (ZnIB). In this type of battery, the working principle is the charge transfer between the Zn metal anode and the cathode [73][28]. They present lower cost and higher safety compared to Li-ion batteries because aqueous electrolytes are much safer than organic electrolytes commonly used for lithium salts. However, their narrow electrochemical window limits their applications and hinders their competence with Li-ion and other technologies.4.5. Supercapacitors
Supercapacitor is another type of energy storage device that stores and gives-out energy at a very fast rate, given the high current within a short time duration [75][29]. Activated carbon is possibly the most used material in supercapacitors due to its low cost, simple processing method, and high surface area [76,77][30][31]. The components and design of the supercapacitors are like those of the batteries. Supercapacitors are made up of electrode, electrolyte, current collector, binder, and separator/selective ion membrane. Although all the components have contributions to the storage performance of a supercapacitor, the electrode and electrolyte both play a major role. In supercapacitors, energy is stored as charges at the electrode–electrolyte interface, the extent of charge storage by the electrode is known as the capacitance. An important parameter to achieve high capacitance is the surface area. Electrodes that possess large surface area have capacity to store more charges and thereby have higher capacitance compared to those with small surface area. Based on the electrode materials, the supercapacitors can be classified into three categories which are: electric double-layer capacitors (EDLC), in which the working principle is based on the Helmholtz layer; pseudocapacitors based on faradaic reactions; and hybrid capacitors which combine both behaviors [78][32]. The carbon materials such as activated carbon, carbon aerogels, carbon nanotubes (CNTs), graphene etc., show the EDLC behavior. Nevertheless, other alternative materials and structures such as metal organic frameworks derivatives (MOF-D) have demonstrated promising characteristics toward this application [79][33]. These electrode materials are electrochemically passive, the charge storage takes place only due to the physical buildup of charges/ions on the electrode surface [80][34].5. Biowaste-Based Composites
Composite materials are prepared to: (i) improve on the performance (such as conductivity and cycle stability) of different metal oxides and conducting polymers such as polyaniline or polythiophenes (Figure 32), (ii) reduce cost, and (iii) reduce environmental hazards. Activated carbon, graphene, carbon nanotubes, and carbon aerogels are the often-used carbon nanomaterials for the preparation of nanocomposites. Carbon nanocomposites offer a high conductivity and specific surface area which is essential for charge adsorption and storage [91][35]. These carbon nanomaterials can easily be obtained from cheap, abundant, and eco-friendly biowaste sources as discussed in the previous sections.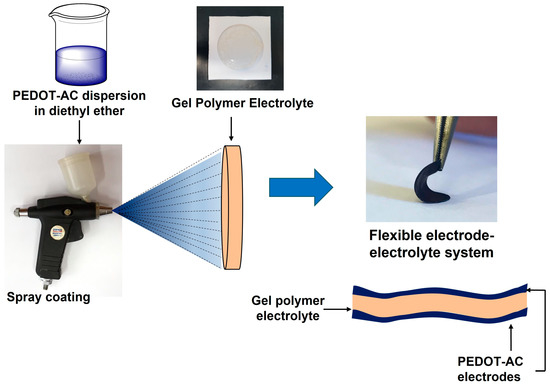
Figure 32. Scheme of preparation of poly(3,4-ethylenedioxythiophene) (PEDOT) and activated carbon derived from brewer’s spent grain. Reproduced from MDPI [15][36].
Continuous effort to improve the electrochemical performance, cost, and environmental hazards is on-going in the materials research community. Very recently, Bigdeloo et al., prepared activated carbon from fennel flower waste as efficient and sustainable source of carbon, the activated carbon was further combined with functionalized graphene oxide to form a binary nanocomposite. A very thin layer of poly-orthoaminophenol was coated on the binary nanocomposite to obtain a ternary nanocomposite of poly-orthoaminophenol/functionalized graphene oxide/activated carbon from fennel flower. Electrochemical measurements were conducted on the ternary nanocomposite material formed using cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS) to evaluate the energy storage behavior of the electrode material. The ternary nanocomposite shows a specific capacitance of 1400.2 F/g at a current density of 2.0 A g−1, capacity retention of 94.4% after 5000 cycles. Brunauer–Emmett–Teller (BET) analysis also reveal that the nanocomposite possesses a specific surface area of 2199.2 m2 g−1 [92][37].
References
- Guo, L.; Hu, Y.; Lei, Y.; Wu, H.; Yang, G.; Wang, Y.; Wei, G. Vitrification of petrochemical sludge for rapid, facile, and sustainable fixation of heavy metals. J. Environ. Chem. Eng 2022, 10, 108812.
- Sharifikolouei, E.; Ferraris, M. Potential role of vitrification and waste vitrification in the circular economy. In Ceramics, Glass and Glass-Ceramics; Baino, F., Tomalino, M., Tulyaganov, D., Eds.; PoliTO Springer Series; Springer: Cham, Switzerland, 2021.
- Dávalos, J.; Bonilla, A.; Villaquirán-Caicedo, M.A.; Gutiérrez, R.M.; Rincón, J.M. Preparation of glass-ceramic materials from coal ash and rice husk ash: Microstructural, physical and mechanical properties. Céram. Vidr. 2021, 60, 183–193.
- Singh, P.; Bhargava, C.; Kumar, M.; Singh, A.; Singh, P.K.; Kumar, P.; Sachdeva, A. Extraction and comparative study of green energy using different types of biowaste material. Mater. Today Proc. 2021, 49, 3474–3481.
- Al, A.; Meltem, R. High-performance nanostructured bio-based carbon electrodes for energy storage applications. Cellulose 2021, 28, 5169–5218.
- Bian, R.; Shi, W.; Luo, J.; Li, W.; Wang, Y.; Joseph, S.; Gould, H.; Zheng, J.; Zhang, X.; Liu, X.; et al. Copyrolysis of food waste and rice husk to biochar to create a sustainable resource for soil amendment: A pilot-scale case study in Jinhua, China. J. Clean. Prod. 2022, 347, 131269.
- Bai, X.; Wang, Z.; Luo, J.; Wu, W.; Liang, Y.; Tong, X.; Zhao, Z. Hierarchical Porous Carbon with Interconnected Ordered Pores from Biowaste for High-Performance Supercapacitor Electrodes. Nanoscale Res. Lett. 2020, 15, 88.
- Jung, S.; Myung, Y.; Kim, B.N.; Kim, I.G.; You, I.; Kim, T. Activated Biomass-derived Graphene-based Carbons for Supercapacitors with High Energy and Power Density. Sci. Rep. 2018, 8, 1915.
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94.
- Liu, X.; Zhang, S.; Wen, X.; Chen, X.; Wen, Y.; Shi, X.; Mijowska, E. High yield conversion of biowaste coffee grounds into hierarchical porous carbon for superior capacitive energy storage. Sci. Rep. 2020, 10, 3518.
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. J. Environ. Chem. Lett. 2020, 18, 393–415.
- Tobi, A.R.; Dennis, J.O. Activated carbon from composite of palm bio-waste as electrode material for solid-state electric double layer capacitor. J. Energy Storage 2021, 42, 103087.
- Patel, P.S.; Bandre, N.; Saraf, A.; Ruparelia, J.P. Electro-catalytic materials (electrode materials) in electrochemical wastewater treatment. Procedia Eng. 2013, 51, 430–435.
- An, H.; Cui, H.; Zhang, W.; Zhai, J.; Qian, Y.; Xie, X.; Li, Q. Fabrication and electrochemical treatment application of a microstructured TiO2-NTs/Sb-SnO2/PbO2 anode in the degradation of C.I. Reactive Blue 194 (RB 194). Chem. Eng. J. 2012, 209, 86–93.
- Ţurcanu, A.A.; Matei, E.; Râpă, M.; Predescu, A.M.; Coman, G.; Predescu, C. Biowaste Valorization Using Hydrothermal Carbonization for Potential Wastewater Treatment Applications. Water 2022, 14, 2344.
- Gan, L.; Wu, Y.; Song, H.; Zhang, S.; Lu, C.; Yang, S.; Wang, Z.; Jiang, B.; Wang, C.; Li, A. Selective removal of nitrate ion using a novel activated carbon composite carbon electrode in capacitive deionization. Sep. Purif. Technol. 2019, 212, 728–736.
- Lu, Y.; Zhang, Q.; Chen, J. Recent progress on lithium-ion batteries with high electrochemical performance. Sci. China Chem. 2019, 62, 533–548.
- Mauger, A.; Julien, C.M. Critical review on lithium-ion batteries: Are they safe? Sustainable? Ionics 2017, 23, 1933–1947.
- Amatucci, G.G.; Tarascon, J.M.; Klein, L.C. CoO2, the end member of the LixCoO2 Solid Solution. J. Electrochem. Soc. 1996, 143, 1114–1123.
- Wang, Q.; Zhu, X.; Liu, Y.; Fang, Y.; Zhou, X.; Bao, J. Rice husk-derived hard carbons as high-performance anode materials for sodium-ion batteries. Carbon. 2018, 127, 658–666.
- Barnes, P.; Smith, K.; Parrish, R.; Jones, C.; Skinner, P.; Storch, E.; White, Q.; Deng, C.; Karsann, D.; Lau, M.L.; et al. A Non-Aqueous Sodium Hexafluorophosphate-Based Electrolyte Degradation Study: Formation and Mitigation of Hydrofluoric Acid. J. Power Sources 2020, 447, 227363.
- Ould, D.M.C.; Menkin, S.; O’Keefe, C.A.; Coowar, F.; Barker, J.; Grey, C.P.; Wright, D.S. New Route to Battery Grade NaPF6 for Na-Ion Batteries: Expanding the Accessible Concentration. Angew. Chem.-Int. Ed. 2021, 60, 24882–24887.
- Landesfeind, J.; Hosaka, T.; Graf, M.; Kubota, K.; Komaba, S.; Gasteiger, H.A. Comparison of Ionic Transport Properties of Non-Aqueous Lithium and Sodium Hexafluorophosphate Electrolytes. J. Electrochem. Soc. 2021, 168, 040538.
- Walter, M.; Kovalenko, M.V.; Kravchyk, K.V. Challenges and benefits of post-lithium-ion batteries. New J. Chem. 2020, 44, 1677–1683.
- Zhang, X.; Wang, X.; Xie, Z.; Zhou, Z. Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ. 2016, 1, 4–17.
- Li, Y.; Lu, J. Metal—air Batteries: Will They Be the Future Electrochemical Energy Storage Device of choice? Energy Lett. 2017, 2, 1370–1377.
- Ma, Z.; Wang, K.; Qiu, Y.; Liu, X.; Cao, C.; Feng, Y.; Hu, P. Nitrogen and sulfur co-doped porous carbon derived from biowaste as a promising electrocatalyst for zinc-air battery. Energy 2017, 143, 43–55.
- Li, Y.; Wang, Z.; Cai, Y.; Pam, M.E.; Yang, Y.; Zhang, D.; Wang, Y.; Huang, S. Designing Advanced Aqueous Zinc-Ion Batteries: Principles, Strategies, and Perspectives. Energy Environ. Mater. 2022, 5, 823–851.
- Mensah-darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy. Sustainability 2019, 11, 414.
- Mangisetti, S.R.; Kamaraj, M.; Sundara, R. Green Approach for Synthesizing Three Different Carbon Microstructures from a Single Biowaste Bombax malabaricum for Fully Biocompatible Flexible Supercapacitors and Their Performance in Various Electrolytes. ACS Omega 2019, 4, 6399–6410.
- Misnon, I.I.; Zain, N.K.M.; Lei, T.S.; Vijayan, B.L.; Jose, R. Activated carbon with graphitic content from stinky bean seedpod biowaste as supercapacitive electrode material. Ionics 2020, 26, 4081–4093.
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522.
- Al-Thabaiti, S.A.; Mostafa, M.M.M.; Ahmed, A.I.; Salama, R.S. Synthesis of Copper/Chromium Metal Organic Frameworks-Derivatives as an Advanced Electrode Material for High-Performance Supercapacitors. Ceram. Int. 2023, 49, 5119–5129.
- Samantara, A.K.; Ratha, S. Components of Supercapacitor BT. In Materials Development for Active/Passive Components of a Supercapacitor: Background, Present Status and Future Perspective; Briefs in Materials; Spinger: Berlin/Heidelberg, Germany, 2018; pp. 11–39.
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646.
- González, F.J.; Montesinos, A.; Araujo-Morera, J.; Verdejo, R.; Hoyos, M. ‘In-Situ’ Preparation of Carbonaceous Conductive Composite Materials Based on PEDOT and Biowaste for Flexible Pseudocapacitor Application. J. Compos. Sci. 2020, 4, 87.
- Bigdeloo, M.; Kowsari, E.; Ehsani, A.; Ramakrishna, S.; Chinnappan, A. Activated carbon derived from fennel flower waste as high-efficient sustainable materials for improving cycle stability and capacitance performance of electroactive nanocomposite of conductive polymer. J. Energy Storage 2022, 55, 105793.
More
