Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Julio José Jiménez-Alonso.
Cancer cells cannot proliferate and survive unless they obtain sufficient levels of the 20 proteinogenic amino acids (AAs). Unlike normal cells, cancer cells have genetic and metabolic alterations that may limit their capacity to obtain adequate levels of the 20 AAs in challenging metabolic environments. However, since normal diets provide all AAs at relatively constant levels and ratios, these potentially lethal genetic and metabolic defects are eventually harmless to cancer cells. If the normal diet of cancer patients is temporarily replaced with artificial diets in which the levels of specific AAs are manipulated, cancer cells may be unable to proliferate and survive.
- cancer metabolism
- anticancer activity
- artificial diets
1. Introduction
The first metabolic alteration of cancer cells was discovered almost one century ago by the German biochemist Otto Warburg. He observed that, unlike normal cells, cancer cells convert high amounts of glucose into lactate in the presence of normal oxygen levels [1]. This alteration in glucose metabolism, known as aerobic glycolysis or the Warburg effect, is now widely used in diagnostic imaging to trace cancers and evaluate cancer treatment responses [2,3][2][3]. The clinical use of FDG-based PET imaging has continually shown that most primary and metastatic cancers have a significant increase in glucose uptake compared to normal tissues [2,3][2][3].
Over many decades, the Warburg effect was considered to be an irrelevant oddity of cancer cells, probably because it was unknown why cancer cells used this primitive form of energy production when the availability of oxygen allows for a much more effective way of producing energy: oxidative phosphorylation. The explanation of the Warburg effect is simple when one realizes that glycolysis not only serves to produce energy, but also to produce building blocks to generate new cells [4]. Cancer cells have high glycolytic rates because the breakdown of glucose molecules generates the building blocks needed to produce many cellular components for the new cancer cells created during cell division. One cell cannot divide to produce two cells unless glucose is broken down into these building blocks. Since both glucose and oxygen are supplied together through the blood, cancer cells have no choice but to activate glycolysis in the presence of oxygen in order to proliferate. Since oxygen inhibits glycolysis (Pasteur Effect) indirectly via ATP generation, cancer cells partially uncouple oxygen utilization from ATP production to activate glycolysis in the presence of normal oxygen levels. By deviating oxygen metabolism from the route that generates ATP to the route that produces reactive oxygen species (ROS), cancer cells manage to keep sustained glycolytic rates under aerobic conditions [4,5,6][4][5][6]. The subsequent increased production of ROS, such as superoxide anion and hydrogen peroxide, leads to a state of increased basal oxidative stress, which represents another metabolic hallmark of cancer cells [5,7,8,9,10][5][7][8][9][10].
Targeting the Warburg effect for cancer therapy is difficult because cells from different normal tissues also need glucose for their survival and proliferation. However, understanding the Warburg effect is important to realize that the genetic alterations in cancer cells are insufficient for cancer cell proliferation and survival. Cancer cells also need to take glucose and other nutrients, such as amino acids (AAs), from the extracellular environment to proliferate and survive under conditions of elevated oxidative stress. Although the metabolic changes in cancer cells play an important role in carcinogenesis and cancer progression, these changes can also be exploited to develop new cancer therapies [10,11,12,13,14,15][10][11][12][13][14][15].
2. Protein Restriction
Dietary protein restriction can increase life expectancy [28][16] and reduce the incidence of age-related diseases such as cancer [29][17]. It is well known that proliferating cancer cells must produce new proteins for the new cells created during cell division. Since dietary proteins provide the AAs needed to generate the proteins of the new cancer cells, it is not surprising that low-protein diets can restrict tumor growth in animal models [30][18]. A reduction in IGF-1 levels has been proposed as a key mechanism by which low-protein diets induce anticancer activity. Murine models of melanoma and breast cancer have revealed that mice fed with a low-protein diet (4% kcal protein) had reduced IFG-1 levels and a reduced tumor progression compared to those fed with a high-protein diet (18% kcal protein) [31][19]. Weight loss was observed in elderly mice but not in young mice. Similarly, a low-protein diet reduced the IGF-1 levels in patients aged 50–65 years and reduced the risk of cancer death, while a low-protein diet increased the mortality among elderly patients (+65 years old) [31][19]. This suggests that low-protein diets might induce anticancer activity in middle-aged adults but not in elderly patients. Low-protein diets are not active in all cancer types. Enhanced cancer immunosurveillance is another possible mechanism by which low-protein diets induce anticancer activity. A reduction in dietary proteins (17–15% protein vs. 19% protein) induced IRE1α-dependent endoplasmic reticulum (ER) stress in cancer cells, which resulted in cytokine production and improved the anticancer immune response [33][20]. A lower protein intake (12%) reversed this anticancer effect, which suggested that a certain level of protein intake was needed for activity [33][20]. However, other studies have shown that diets with a lower protein intake (7% protein vs. 21% protein) inhibited cancer progression and induced a synergistic effect when combined to anti-PD-1 immunotherapy [34][21]. Low-protein diets also induced anticancer activity in immunosuppressed mice, therefore suggesting that the anticancer activity of protein restriction is not necessarily mediated by the immune system [35][22]. The type of protein can also modulate this cancer progression. Mice fed with a 20% plant-based protein diet showed a reduced tumor growth in xenograft and syngeneic cancer models when compared to mice fed with a 20% animal-based protein diet [35,36][22][23]. The anticancer activity of diets based on plant proteins was explained by declines in the plasma levels of IFG-1 and insulin, which decreased the activity of the IGF/AKT/mTOR pathway and led to epigenetic modifications that restricted tumor growth [35,36][22][23].3. Essential Amino Acids
3.1. Leucine
Leucine (Leu) is one of the nine EAAs for humans; this means that we cannot biosynthesize it from other nutrients and we must take it from the diet. Like all 20 proteinogenic AAs, Leu is necessary for protein synthesis. Leu is also important for other cellular functions. For example, Leu is a key intracellular sensor of AAs under starvation conditions and it regulates protein turnover through mTORC1 signaling [37][24]. Like isoleucine (Ile) and valine (Val), Leu is a branched-chain amino acid (BCAA); these AAs can regulate the lipid metabolism in cancer cells by providing carbon skeletons for fatty-acid biosynthesis [38][25]. The dietary restriction of Leu can induce in vivo anticancer effects. In 1956, Sugimura et al. [39][26] found that dietary deprivation of Leu for 5 days reduced the growth rate of Walker tumors in rats by 24%; however, it also induced body weight loss. In 1971, reducing the dietary levels of Leu from 0.8% to 0.1% for 3 weeks significantly reduced tumor growth in mice with breast adenocarcinomas; the tumor weights were 32 ± 10 g in mice fed with a standard AA-based diet (0.80% Leu), 38 ± 4 g for those on a 0.50% Leu diet, 32 ± 4 g for those on a 0.25% Leu diet, and 16 ± 6 g for those on a 0.10% Leu diet [40][27]. Body weight loss was observed in the mice fed with the 0.10% Leu diet [40][27]. Mechanistically, Leu limitation restricts protein synthesis, cell division, and tumor growth. In addition, Leu restriction can reduce Leu catabolism and limit the fatty acid biosynthesis and lipogenesis in cancer cells. BCAAs catabolism plays an important role in pancreatic cancer growth by regulating lipogenesis [38][25]. BCAT2 and BCKDHA knockdown impaired pancreatic cancer cell proliferation in vitro and in vivo by inhibiting fatty acid synthesis [38][25]. Since Leu restriction can reduce tumor growth, it makes sense to think that Leu supplementation may facilitate cancer progression. A study showed that a 5% Leu supplementation increased cancer growth in a syngeneic model of pancreatic cancer [49][28]. Maintaining high Leu levels may be important for preventing proteolysis, which could be beneficial in certain circumstances. Leu is a critical intracellular sensor of AAs under starvation conditions. This AA activates mTORC1 signaling and inhibits autophagy and proteasome-mediated proteolysis. Supplementing Leu may therefore prevent intracellular and extracellular proteolysis [50,51,52,53][29][30][31][32]. If muscle and liver proteolysis is not prevented, the lysis of proteins in these organs would supply any AA restricted in the diet [26,27,54][33][34][35]. The inhibition of proteolysis is also important in avoiding weight loss and cachexia. Cachexia is a syndrome of progressive body weight loss with reductions in skeletal muscle and fat mass [55][36]. Ultimately, cachexia reduces the tolerability of anticancer treatments and leads to a reduced life expectancy and quality of life [55,56][36][37]. Leu supplementation can alleviate cancer cachexia by activating mTORC1 and decreasing protein degradation [51,55,57,58][30][36][38][39].3.2. Isoleucine
Like all proteinogenic AAs, the BCAA Ile is necessary for protein synthesis. Ile also participates in other biological processes, including lipogenesis and immune function regulation [37,38,69][24][25][40]. Experiments conducted several decades ago revealed that a complete dietary Ile restriction for 5 days inhibited tumor growth by 40% in Walker tumor-bearing rats; however, this force-fed intervention caused the animals to lose 1–2 g per day [39][26]. Dietary Ile restriction (from 0.5% to 0.05%) also resulted in tumor growth inhibition in C57BL/6 mice with BW10232 mammary carcinomas [40][27].3.3. Valine
Like Leu and Ile, Val is an essential and proteinogenic BCAA. It is also involved in other cellular functions, including the regulation of lipid and glucose metabolism [37,38,70][24][25][41]. A dietary depletion of Val for 5 days reduced tumor growth by 41% in Walker tumor-bearing rats [39][26]. However, all the animals on this Val-free diet rapidly sickened and failed to survive beyond 9 days on this diet. A dietary limitation of Val (from 0.7% to 0.1%) significantly decreased tumor growth in mice with breast adenocarcinomas, but also induced body weight loss [40][27].3.4. Threonine
Threonine (Thr) is an essential and proteinogenic AA. Like other AAs, Thr catabolism can also provide amino groups for the synthesis of NEAAs and carbon skeletons for biosynthesis and energy production [37][24]. The force feeding of a diet lacking in Thr for 5 days reduced tumor growth by 28% in Walker tumor-bearing rats [39][26]. This diet caused the animals to lose between 0.2 and 1.0 g/day over an 11-day period [39][26].3.5. Lysine
Lys is an essential and proteinogenic AA, whose deficiency can trigger severe malnutrition [37,71][24][42]. Lys is also used for carnitine production and participates in protein methylation, acetylation, ubiquitination, and glycosylation [37][24]. The anticancer activity of Lys restriction was evaluated 80 years ago in mice with spontaneous breast cancer [72][43]. The author of this research first devised a Lys-deficient diet suitable for human consumption (palatable, adequate in calories, minerals, and vitamins, and sufficient for keeping nitrogen balance). After observing in two healthy humans that nitrogen equilibrium could be maintained with this diet, he obtained and reproduced a strain of mice characterized by a high incidence of spontaneous mammary carcinomas. The mice that developed tumors were fed with the Lys-deficient diet. The diet inhibited the growth rate of the tumors, but also the rate of normal growth in the mice. These inhibitory effects were abolished upon the addition of Lys, therefore indicating that Lys was essential for both normal and malignant growth. When the Lys-deficient diet was fed to them for several weeks, the antitumor effect wore off and the tumors resumed rapid growth.3.6. Phenylalanine
Phenylalanine (Phe) is an essential and proteinogenic AA with an aromatic group in its structure. Phe can be used to synthesize tyrosine (Tyr), a proteinogenic NEAA that produces important molecules such as catecholamines (dopamine, epinephrine, and norepinephrine) and melanin [37][24]. Dietary Phe limitation is used in people with phenylketonuria, an inborn disease caused by the inactivity of the enzyme phenylalanine hydroxylase, which converts Phe into Tyr; the accumulation of Phe can lead to seizures and intellectual disability [73][44].3.7. Histidine
Histidine (His) is an aromatic EAA required for protein synthesis. This AA is involved in other cellular functions, including the synthesis of histamine and carnitine [37,92][24][45]. The force feeding of a diet lacking in His for 5 days reduced tumor growth by 19% in the Walker rat model [39][26]. More recently, the dietary limitation of His was found to selectively limit the growth of MYC-dependent neural tumors in a Drosophila model [93][46].3.8. Tryptophan
Although tryptophan (Trp) is the least abundant EAA in the diet, it is necessary for protein synthesis and the production of a variety of biologically active compounds, including serotonin, melatonin, and niacin (a component of NAD and NADP) [37,95][24][47]. In addition, Trp and its catabolic derivatives modulate the immune function and play key roles in autoimmune diseases and antitumor immunity [95,96][47][48]. In 1959, a total Trp restriction for 5 days inhibited tumor growth by 19% in Walker tumor-bearing rats, with moderate weight loss in the animals [39][26]. In 1971, a Trp limitation (from 0.10% to 0.02%) reduced tumor growth in C57BL/6 mice with BW10232 mammary carcinomas [40][27]. The tumor weights were 33 ± 6 g in mice fed with a standard AA-based diet (0.15% Trp), 33 ± 13 g for those on a 0.10% Trp diet, 31 ± 10 g for those on a 0.05% Trp diet, and 16 ± 8 g for those on a 0.02% Trp diet [40][27]. The mice fed with the 0.02% Trp diet lost 28% of their weight in 3 weeks [40][27]. A moderate dietary limitation of Trp (0.05%) did not show anticancer activity in C3H mice bearing mammary adenocarcinomas [97][49].3.9. Methionine
Methionine (Met) is an essential and proteinogenic AA that contains a sulfur atom in its structure. Met is the precursor of S-adenosyl methionine (SAM), which is a methyl donor involved in DNA methylation and epigenetics. Met also produces Cys through the irreversible transsulfuration pathway, which, in turn, produces several sulfur-containing molecules with important cellular roles, including glutathione (GSH), hydrogen sulfide (H2S), and taurine (Tau) [24,37,103,104][24][50][51][52]. Dietary Met restriction has shown anticancer activity in numerous preclinical studies [26,27,34,39,40,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125][21][26][27][33][34][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73]. A dietary Met depletion (0%) induced anticancer activity in rats [39,121,122,123,124][26][69][70][71][72] and mice [105,106,107,108,109,110,111,125][53][54][55][56][57][58][59][73] with different types of cancer. The force feeding of a diet lacking in Met for 5 days reduced tumor growth by 39% in the Walker rat model, but this diet caused the animals to lose l–2 g weight/day [39][26]. Met restriction (from 0.60% to 0.10%) reduced tumor growth in C57BL mice with BW10232 mammary carcinomas [40][27]. The tumor weights were 36 ± 13 g in mice fed with a standard AA-based diet (0.90% Met; 0.2% Cys), 33 ± 11 g for those on a 0.60% Met diet, 30 ± 6 g for those on a 0.40% Met diet, 29 ± 7 g for those on a 0.20% Met diet, and 16 ± 7 g for those on a 0.10% Met diet [40][27]. The mice fed with the 0.10% Met diet lost 10% of their initial weight in 3 weeks [40][27].4. Non-Essential Amino Acids
4.1. Cysteine
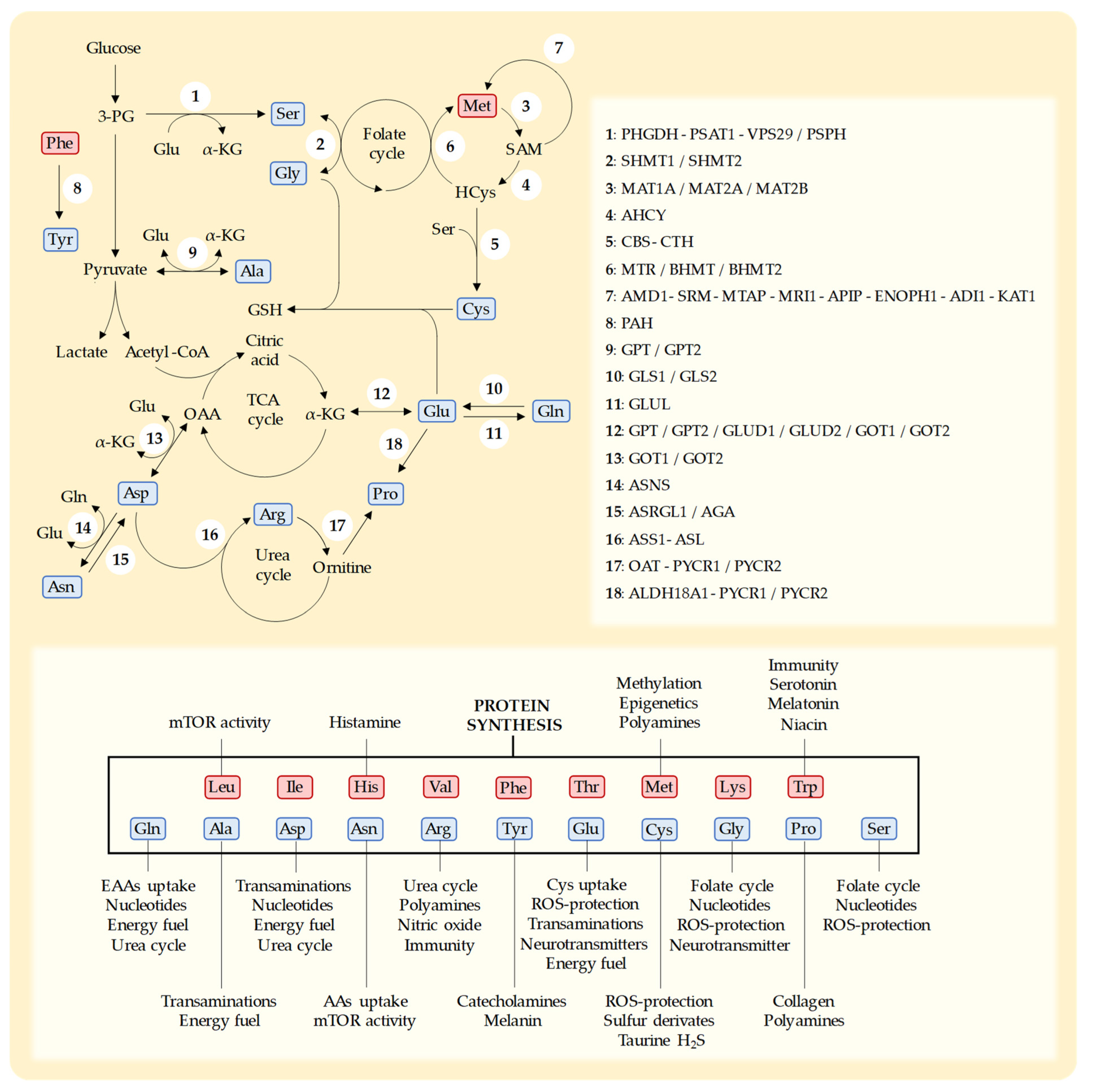
Cys is a sulfur-containing NEAA with multiple cellular roles [169][74]. Humans can biosynthesize Cys from the EAA Met through the transsulfuration pathway [24,169,170][50][74][75] (Figure 21). In addition to being necessary for protein synthesis, Cys is essential for the production of a variety of sulfur-containing molecules with important biological roles [170][75]. These include the iron–sulfur clusters found in enzymes of the electron transport chain (ETC) [171][76], coenzyme A, and thioredoxins [172][77]. Cys also produces taurine [37][24] and hydrogen sulfide (H2S) [37][24] and is the rate-limiting AA for the production of the tripeptide GSH (Glu-Cys-Gly) [169,170][74][75]. GSH is essential for protecting cells against the toxic effects ROS [169][74].
Figure 21. Schematic representation of key metabolic pathways for the biosynthesis of NEAAs, the enzymes in each pathway, and the main functions of each AA [54,173][35][78]. NEAAs are represented in blue and EAAs in red. Leucine (Leu), isoleucine (Ile), histidine (His), valine (Val), phenylalanine (Phe), threonine (Thr), methionine (Met), lysine (Lys), tryptophan (Trp), glutamine (Gln), alanine (Ala), aspartate (Asp), asparagine (Asn), arginine (Arg), tyrosine (Tyr), glutamate (Glu), cysteine (Cys), glycine (Gly), proline (Pro), and serine (Ser). 3-phospho-D-glycerate (3-PG), S-adenosylmethionine (SAM), homocysteine (HCys), glutathione (GSH), α-ketoglutarate (α-KGlu), tricarboxylic acid cycle (TCA), oxaloacetate (OAA), reactive oxygen species (ROS). D-3-phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase-1 (PSAT1), vacuolar protein sorting-associated protein-29 (VPS29), phosphoserine phosphatase (PSPH), serine hydroxymethyltransferase-1 (SHMT1), serine hydroxymethyltransferase-2 (SHMT2), S-adenosylmethionine synthase isoform type-1 (MAT1A), S-adenosylmethionine synthetase isoform type-2 (MAT2A), methionine adenosyltransferase 2 subunit beta (MAT2B), adenosylhomocysteinase (AHCY), cystathionine β-synthase (CBS), cystathionine γ-lyase (CTH), methionine synthase (MTR), betaine-homocysteine methyltransferase (BHMT), betaine-homocysteine methyltransferase-2 (BHMT2), S-adenosylmethionine decarboxylase (AMD1), spermidine synthase (SRM), 5′-methylthioadenosine phosphorylase (MTAP), methylthioribose-1-phosphate isomerase (MRI1), methylthioribulose 1-phosphate dehydratase (APIP), enolase-phosphatase (ENOPH1), 1,2-dihydroxy-3-keto-5-methylthiopentene dioxygenase (ADI1), 2-oxo-4-methylthiobutanoate aminotransferase (KYAT1), phenylalanine hydroxylase (PAH), alanine aminotransferase-1 (GPT), alanine aminotransferase-2 (GPT2), glutaminase-1 (GLS1), glutaminase-2 (GLS2), glutamine synthetase (GLUL), glutamate dehydrogenase-1 (GLUD1), glutamate dehydrogenase-2 (GLUD2), aspartate aminotransferase-1 (GOT1), aspartate aminotransferase-2 (GOT2), asparagine synthetase (ASNS), asparaginase (ASRGL1), aspartylglucosaminidase (AGA), argininosuccinate synthase (ASS1), argininosuccinate lyase (ASL), ornithine aminotransferase (OAT), pyrroline-5-carboxylate reductase-1 (PYCR1), pyrroline-5-carboxylate reductase-2 (PYCR2), and δ-1-pyrroline-5-carboxylate synthase (ALDH18A1). Enzymes that participate in consecutive steps in a metabolic pathway are separated by “-” and enzymes that catalyze the same step in a metabolic pathway are separated by “/”.
The importance of Cys in tumor growth was first reported in 1936 [133][79]. In this study, Voegtlin et al. observed that a diet deficient in Cys/Met reduced tumor growth in mice with spontaneous breast cancer, and the addition of Cys abruptly stimulated tumor growth [133][79]. Since Met can produce Cys, Met has usually been restricted in many studies evaluating the anticancer activity of Cys depletion/restriction [26,27,34,40,111,113,120,121,122,133][21][27][33][34][59][61][68][69][70][79]. Intravenous parenteral nutrition with double Cys/Met restriction showed anticancer activity in rats with sarcoma [121,122][69][70] and inhibited the cancer proliferation in mice xenografted with human glioma cells [111][59].
Mechanistically, Cys restriction may induce anticancer activity by reducing the capacity of cancer cells to eliminate ROS. Cancer cells produce high levels of ROS, which may accumulate and produce cell death [8]. Cancer cells rely on GSH to reduce these ROS levels [24][50]. A dietary Cys restriction can decrease Cys plasma levels [176][80], reduce GSH biosynthesis [175,176][80][81], and increase the ROS levels in cancer cells [174,175,176][80][81][82].
Since Cys is necessary for immune cells, Cys restriction may reduce the ability of the immune system to eliminate cancer cells. Cys is essential for T-cell activation and function [177][83]. High CysS plasma levels have been associated with a higher probability of response to immune checkpoint inhibitors in patients with lung cancer [178,179][84][85]. However, the negative effect of Cys restriction on the immune antitumor response is controversial, because other studies have suggested that Cys restriction can increase the antitumor immune response [34,180][21][86].
Pharmacological approaches based on an enzymatic depletion of Cys and inhibition of Cys transporters support the idea that Cys restriction has potential for cancer therapy. These pharmacological interventions have been useful for understanding the possible mechanisms by which Cys restriction induces in vivo anticancer effects. In 2017, an optimized human cyst(e)inase enzyme was able to reduce the Cys and CysS plasma levels in mice and primates without causing toxicity [181][87]. Cyst(e)inase has shown anticancer activity in mouse models of a variety of cancers, including prostate, breast, chronic lymphocytic leukemia, pancreas, lung, renal, melanoma, and ovarian cancer [180,181,182,183,184,185,186][86][87][88][89][90][91][92]. Cyst(e)inase administration increases ROS levels, depletes the intracellular levels of GSH, and triggers ferroptosis in cancer cells [181,182,183,184,185,186][87][88][89][90][91][92].
4.2. Serine
Serine (Ser) is synthesized from 3-phosphoglycerate (glucose metabolite) and Glu (nitrogen donor) through the de novo Ser synthesis pathway [205][93]. In addition to being a proteinogenic AA, Ser plays an important role in one-carbon metabolism [205,206,207][93][94][95]. Ser is the main source of carbon units in the folate cycle, which is mainly used for the synthesis of purines and pyrimidines and the conversion of HCys into Met. Ser is also used to produce Gly and provides the carbon skeleton for the synthesis of Cys through the transsulfuration pathway. It also has other important functions, such as the production of certain lipids, including ceramide and phosphatidylserine [205,206,207][93][94][95]. Ser and Gly are easily interconverted by SHMT1/2 enzymes [207][95]. Therefore, Gly is usually restricted in most dietary studies evaluating the anticancer activity of Ser limitation. Dietary Ser/Gly can reduce the Ser and Gly levels in plasma [208][96] and tumors [209][97]. Although both AAs can be synthesized by human cells, cancer cells may depend on an external supply of these AAs to keep their high proliferative demands. Mechanistically, dietary Ser/Gly restriction can induce anticancer activity by restricting two important building blocks in biosynthesis. The new cancer cells created during tumor growth need new proteins, nucleic acids, and specific lipids; these processes require the synthesis or acquisition of sufficient levels of these two AAs.4.3. Glycine
Gly is an NEAA that can be synthetized from Ser. Gly is essential for protein synthesis. Collagen, which is the most abundant protein in the human body (30–40% of total body protein), contains approximately 33% of Gly [229][98]. This AA also acts as an inhibitory neurotransmitter [37][24]. Gly can also be used for the synthesis of the antioxidant tripeptide GSH, Ser, purines, creatine, and heme group [37][24]. Evidence has suggested that rapidly growing cancer cells have a high Gly dependency [230][99].4.4. Arginine
Arginine (Arg) is an NEAA used for protein synthesis. It also participates in many other biological processes, including the synthesis of nitric oxide, creatinine, ornithine, agmatine, and polyamines [37,231][24][100]. It also plays a key role in the urea cycle [37][24]. Normal cells can synthesize Arg from citrulline and aspartate (Asp) through ASS1 (argininosuccinate synthase 1) and ASL (argininosuccinate lyase) in the urea cycle. Arg-free diets can decrease the plasma levels of Arg in healthy volunteers. An Arg-free diet taken for 6 days reduced Arg plasma levels by approximately 20–40% [243][101]. In another study, 4 weeks of a dietary restriction of Arg and other precursors of Arg (Asp, Pro, and Glu) significantly decreased Arg plasma levels without causing side effects [244][102]. Mechanistically, dietary Arg deprivation may induce selective anticancer activity because many cancer cells express low levels of ASS1, which is involved in the synthesis of Arg. The downregulation of ASS1 facilitates cancer cell proliferation by increasing the aspartate availability for pyrimidine biosynthesis [245][103]. The importance of Arg for cancer cell proliferation and survival has been supported by numerous studies that have shown that a pharmacological depletion of Arg levels with Arg-depleting enzymes induces anticancer activity. Two different enzymes are currently under clinical development: ADI-PEG20 (pegylated arginine deiminase) and PEG-BCT-100 (pegylated recombinant human arginase 1). These enzymes have shown anticancer activity in a wide variety of cancers, including melanoma, hepatocarcinoma, and glioblastoma [231,234,247,248,249][100][104][105][106][107]. As occurs with other AAs, Arg restriction may have a negative impact on immunogenic cancers. Some cancer cells create an immunosuppressive microenvironment by converting myeloid cells into M2 macrophages or myeloid-derived suppressive cells [274][108]. These immunosuppressive cells express arginase, which hydrolyzes Arg to ornithine and urea, therefore reducing the Arg levels in the tumor microenvironment [274][108]. Arg is essential for T-cell proliferation and the expression of arginase can disrupt antitumor immunity [241,274,275][108][109][110].4.5. Glutamine
Gln is a non-essential proteinogenic AA that can be considered as essential under certain conditions [283][111]. It is the most abundant AA in human plasma and tissues and is involved in many biological processes [284][112]. It participates in the transport and detoxification of ammonia in the urea cycle, helping to maintain the pH balance [285,286][113][114]. Gln is the main source of nitrogen atoms for the biosynthesis of nucleotides (pyrimidines and purines) and NEAAs (Glu, Asn, Ala, Asp, Ser, Pro, and citrulline) [285][113]. Gln also mediates the cellular uptake of certain EAAs; for example, LAT1 imports the EAA Leu while simultaneously exporting Gln [287][115]. Proliferating cancer cells have a high Gln demand. Cancer cells obtain high Gln levels by increasing their biosynthesis or by obtaining it from the extracellular environment [285][113]. The increased Gln uptake of cancer cells has been associated with lower plasma levels of Gln in patients with several types of cancer [290,291][116][117]. The increased Gln uptake by tumors is actually being studied for diagnostic purposes with PET imaging using 18F-(2S,4R)-4-fluoroglutamine [292,293,294,295][118][119][120][121]. The increased Gln uptake of cancer cells is related to their high expression of ASCT2 (SLC1A5) [296,297,298,299,300][122][123][124][125][126]; this chief Gln transporter is upregulated by the oncogenes MYC and KRAS [301,302][127][128]. Limiting Gln levels and targeting Gln acquisition and utilization have been studied as possible anticancer strategies. Few studies have evaluated the in vivo anticancer activity of diets deficient in Gln. In 2017, the dietary restriction of Gln was found to induce anticancer activity in vitro and in vivo in a p73-expressing medulloblastoma xenograft model [313][129]. The Gln-restricted diet increased mice survival and also showed a synergistic effect with cisplatin. Although the only difference between the control and experimental diets was the presence/restriction of Gln, both diets also lacked Glu, Ala, Asn, Asp, and Pro. This diet reduced the Gln and Glu levels in the cerebellum and cerebrospinal fluid of the mice [313][129]. Most anticancer strategies targeting the altered Gln metabolism of cancer cells have focused on the pharmacological inhibition of Gln acquisition and utilization [285][113]. These include the inhibition of GLS1 with inhibitors such as CB-839 (telaglenastat) [315][130], BPTES [316][131], and C.968 [317,318][132][133]. CB-839, which is orally bioavailable, has been tested in clinical trials. There are at least 21 completed or ongoing phase I-II clinical trials, 8 of which have been completed [319][134]. In general, CB-839 was safe and well tolerated by cancer patients [320,321,322,323,324,325,326][135][136][137][138][139][140][141]. In most of the completed clinical trials, CB-839 was combined with other anticancer drugs [285][113]. Its benefit for cancer progression has been modest so far [285][113]. There are other experimental anticancer drugs targeting Gln metabolism. The inhibition of Gln uptake by V-9302, an inhibitor of the ASCT2 transporter, induced anticancer activity in murine cancer models [327][142]. JHU083, which is a prodrug of the Gln antagonist DON [328][143], is selectively activated in the tumor microenvironment and disrupts cancer cell metabolism while improving T-cell anticancer responses. This compound induced marked anticancer activity alone and in combination with immunotherapies in several murine cancer models [328,329,330,331,332,333][143][144][145][146][147][148].4.6. Glutamate
Glu is an NEAA closely related to Gln. This AA is used in protein synthesis and has many other cellular functions. Glu is as a nitrogen donor for transaminases [37][24]. It is used in the synthesis of many NEAAs, including Ala, Asp, Ser, Pro, and Gln [54][35]. Transaminases and glutamic dehydrogenase (GDH) can convert Glu into αKG, which can be used to fuel the TCA cycle for energy production. In the brain, Glu is an excitatory neurotransmitter and can also be used for the synthesis of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) [37][24]. Glu participates in ROS protection by allowing CysS uptake by the xCT antiporter, and by directly participating in the synthesis of the tripeptide GSH [288][149]. Although Glu supports cancer cell proliferation and survival, the anticancer activity of dietary Glu restriction has not been extensively studied, probably because Glu can be easily obtained from Gln, Asp, and Ala, and is also produced in the degradation pathways of many AAs, including Leu, Ile, Val, Lys, Phe, His, Tyr, and Pro.4.7. Asparagine
Asn is an NEAA that can be synthesized from Asp by the enzyme ASNase. Asn is needed for protein synthesis, but the importance of Asn in other cellular processes is less understood [20][150]. Asn can modulate mTORC1 activity and serve as an exchange molecule for the uptake of other AAs (e.g., Ser, Arg, and His), and the maintenance of intracellular Asn levels seems to be critical for cancer cell growth [346][151]. Asn is commonly used to exemplify the relevance of NEAA restriction in cancer therapy, because the Asn-depleting enzyme ASNase is a useful drug for patients with acute lymphoblastic leukemia (ALL) and acute lymphoblastic lymphoma (ALLy). ASNase is an enzyme from E. coli that deaminates Asn to Asp and ammonium; its intravenous administration quickly depletes the Asn from serum and cells [347][152]. ALL cells usually rely on external Asn for their survival, and the depletion of Asn with ASNase leads to apoptosis in leukemia cells [348][153]. ASNase is pegylated (PEG-ASNase) to extend its half-life and reduce the immunogenicity of the enzyme. Nowadays, ASNase is included in most chemotherapy regimens for pediatric ALL and ALLy, achieving high survival rates [23][154]. The efficacy of ASNase is generally correlated with the expression of ASNS in leukemia cells [349,350][155][156]; this enzyme allows the synthesis of Asn from Asp. However, in some cases, ASNS expression after ASNase has not been associated with resistance to treatment [351][157]. ASNase also has Gln-depleting activity, which may participate in the anticancer activity of this enzyme [334,335][158][159].4.8. Aspartate
In addition to its role in protein synthesis, Asp participates in the synthesis of purines, pyrimidines, Asn, and Arg [37][24]. It also plays a role in the urea cycle, the malate-Asp shuttle, and transamination reactions [37][24]. Due to its role in the synthesis of nucleotides, Asp is crucial for proliferating cancer cells. Although Asp can become a limiting factor for tumor growth, the antitumor activity of dietary Asp deprivation has not been evaluated individually, probably because this AA can be easily obtained from Glu and OAA through GOT1/2 (AST) transaminases (Figure 21). Since these enzymes are expressed in many tissues, including the liver, a dietary Asp restriction would not result in a systemic Asp restriction.4.9. Tyrosine
Tyr is an aromatic NEAA that can be obtained from the EAA Phe. In addition to its role in protein synthesis, Tyr is necessary for producing catecholamines (dopamine, epinephrine, and norepinephrine) and melanin [37][24]. Since Phe is a precursor of Tyr, both AAs are usually restricted simultaneously in most cancer studies. A dual restriction of Phe and Tyr has been evaluated in animal studies and cancer patients with several positive results.4.10. Alanine
Ala is a proteinogenic NEAA with other important metabolic functions. It is involved in transamination reactions and the glucose-alanine cycle (Cahill cycle). Ala can be easily converted into pyruvate by GPT1/2 transaminases [37][24]; pyruvate is a carbon source for energy production, fatty acid biosynthesis, and gluconeogenesis [37,366][24][160]. The antitumor activity of dietary Ala deprivation has not been evaluated independently of other AAs, probably because Ala can be easily obtained from Glu and pyruvate through GPT1/2 transaminases (Figure 21). Since these enzymes are expressed in many tissues, including the liver, a dietary Ala restriction would not result in a systemic Ala restriction.4.11. Proline
Pro is a proteinogenic NEAA that can be synthesized from Glu or ornithine [369][161] (Figure 21). Pro can be used for the synthesis of Arg, Glu, and polyamines, and participates in wound healing and the immune response [37,370][24][162]. Like Gly, Pro is a major building block for the synthesis of collagen [37][24]. Collagen is the main Pro storage in the human body [369][161], and some cancer cells, such as pancreatic cancer cells, can use extracellular collagen to obtain Pro under conditions of nutrient deprivation [371][163]. Dietary Pro restriction inhibited tumor growth in mice xenografted with PC-9 lung cancer cells, but not in mice with PaTu-8902 pancreatic cancer cells [372][164]. Mechanistically, Pro starvation induced endoplasmic reticulum stress in cancer cells with a hyperactivation of mTORC1-mediated 4EBP1 signaling [372][164].5. Manipulation of Multiple Amino Acids Simultaneously
Since the metabolic routes of many AAs are interconnected (Figure 2), the cellular requirements of specific AAs are probably influenced by the levels of other AAs. Manipulating several AAs simultaneously may therefore be more therapeutically useful than restricting AAs individually. As discussed in the previous sections, several pairs of AAs have usually been restricted together. Phe is the precursor of Tyr, and several studies have shown in vivo anticancer activity when both AAs were restricted together [40,75,76,77,78][27][165][166][167][168]. Similarly, since Met can be used to synthesize Cys, a dietary restriction of both AAs has induced in vivo anticancer activity in different cancer types [26,27,34,40,111,113,120,121,122,133][21][27][33][34][59][61][68][69][70][79]. The NEAAs Ser and Gly can be easily interconverted by SHMT1/2 enzymes (Figure 21), and the simultaneous restriction of both AAs has induced anticancer activity in several murine cancer models [208,209,210,211,212,213,214,215,216,217,218,219,220,221,222][96][97][169][170][171][172][173][174][175][176][177][178][179][180][181].6. Conclusions
Cancer cells reprogram their metabolism to produce the large amounts of building blocks required for biosynthesis and proliferation, fulfill their high energy demands, and survive under conditions of elevated oxidative stress. The altered AA metabolism of cancer cells is one of most therapeutically relevant metabolic features of cancer.References
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314.
- Kostakoglu, L.; Agress, H.; Goldsmith, S.J. Clinical Role of FDG PET in Evaluation of Cancer Patients. Radiographics 2003, 23, 315–340.
- Ben-Haim, S.; Ell, P. 18F-FDG PET and PET/CT in the Evaluation of Cancer Treatment Response. J. Nucl. Med. 2009, 50, 88–99.
- Lopez-Lazaro, M. The Warburg Effect: Why and How Do Cancer Cells Activate Glycolysis in the Presence of Oxygen? Anticancer Agents Med. Chem. 2008, 8, 305–312.
- López-Lázaro, M. Does Hypoxia Really Control Tumor Growth? Anal. Cell. Pathol. 2006, 28, 327–329.
- Lopez-Lazaro, M. A New View of Carcinogenesis and an Alternative Approach to Cancer Therapy. Mol. Med. 2010, 16, 144–153.
- López-Lázaro, M. Excessive Superoxide Anion Generation Plays a Key Role in Carcinogenesis. Int. J. Cancer 2007, 120, 1378–1380.
- López-Lázaro, M. Dual Role of Hydrogen Peroxide in Cancer: Possible Relevance to Cancer Chemoprevention and Therapy. Cancer Lett. 2007, 252, 1–8.
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197.
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab. 2022, 34, 355–377.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674.
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47.
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162.
- Pranzini, E.; Pardella, E.; Paoli, P.; Fendt, S.-M.; Taddei, M.L. Metabolic Reprogramming in Anticancer Drug Resistance: A Focus on Amino Acids. Trends Cancer 2021, 7, 682–699.
- Bergers, G.; Fendt, S.-M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180.
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The Impact of Dietary Protein Intake on Longevity and Metabolic Health. EBioMedicine 2019, 43, 632–640.
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and Amino Acid Restriction, Aging and Disease: From Yeast to Humans. Trends Endocrinol. Metab. 2014, 25, 558–566.
- Yin, J.; Ren, W.; Huang, X.; Li, T.; Yin, Y. Protein Restriction and Cancer. Biochim. Biophys. Acta—Rev. Cancer 2018, 1869, 256–262.
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.-W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014, 19, 407–417.
- Rubio-Patiño, C.; Bossowski, J.P.; De Donatis, G.M.; Mondragón, L.; Villa, E.; Aira, L.E.; Chiche, J.; Mhaidly, R.; Lebeaupin, C.; Marchetti, S.; et al. Low-Protein Diet Induces IRE1α-Dependent Anticancer Immunosurveillance. Cell Metab. 2018, 27, 828–842.e7.
- Orillion, A.; Damayanti, N.P.; Shen, L.; Adelaiye-Ogala, R.; Affronti, H.; Elbanna, M.; Chintala, S.; Ciesielski, M.; Fontana, L.; Kao, C.; et al. Dietary Protein Restriction Reprograms Tumor-Associated Macrophages and Enhances Immunotherapy. Clin. Cancer Res. 2018, 24, 6383–6395.
- Fontana, L.; Adelaiye, R.M.; Rastelli, A.L.; Miles, K.M.; Ciamporcero, E.; Longo, V.D.; Nguyen, H.; Vessella, R.; Pili, R. Dietary Protein Restriction Inhibits Tumor Growth in Human Xenograft Models of Prostate and Breast Cancer. Oncotarget 2013, 4, 2451–2461.
- Taha, A.A.A.; Koshiyama, M.; Matsumura, N.; Abiko, K.; Yamaguchi, K.; Hamanishi, J.; Baba, T.; Kharma, B.; Mohamed, I.H.; Ameen, M.M.; et al. The Effect of the Type of Dietary Protein on the Development of Ovarian Cancer. Oncotarget 2018, 9, 23987–23999.
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17.
- Lee, J.H.; Cho, Y.R.; Kim, J.H.; Kim, J.; Nam, H.Y.; Kim, S.W.; Son, J. Branched-Chain Amino Acids Sustain Pancreatic Cancer Growth by Regulating Lipid Metabolism. Exp. Mol. Med. 2019, 51, 1–11.
- Sugimura, T.; Birnbaum, S.M.; Winitz, M.; Greenstein, J.P. Quantitative Nutritional Studies with Water-Soluble, Chemically Defined Diets. VIII. The Forced Feeding of Diets Each Lacking in One Essential Amino Acid. Arch. Biochem. Biophys. 1959, 81, 448–455.
- Theuer, R.C. Effect of Essential Amino Acid Restriction on the Growth of Female C57BL Mice and Their Implanted BW10232 Adenocarcinomas. J. Nutr. 1971, 101, 223–232.
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine Supplementation Differentially Enhances Pancreatic Cancer Growth in Lean and Overweight Mice. Cancer Metab. 2014, 2, 6.
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct Anabolic Signalling Responses to Amino Acids in C2C12 Skeletal Muscle Cells. Amino Acids 2010, 38, 1533–1539.
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-TRNA Synthetase Is an Intracellular Leucine Sensor for the MTORC1-Signaling Pathway. Cell 2012, 149, 410–424.
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino Acid-Dependent Control of MTORC1 Signaling: A Variety of Regulatory Modes. J. Biomed. Sci. 2020, 27, 87.
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261.
- Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer. Nutrients 2022, 14, 3378.
- Calderón-Montaño, J.M.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Jiménez-González, V.; Burgos-Morón, E.; Mate, A.; Pérez-Guerrero, M.C.; López-Lázaro, M. Manipulation of Amino Acid Levels with Artificial Diets Induces a Marked Anticancer Activity in Mice with Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 16132.
- López-Lázaro, M. Selective Amino Acid Restriction Therapy (SAART): A Non-Pharmacological Strategy against All Types of Cancer Cells. Oncoscience 2015, 2, 857.
- Beaudry, A.G.; Law, M.L. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients 2022, 14, 2824.
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105.
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976.
- Osburn, S.C.; Vann, C.G.; Church, D.D.; Ferrando, A.A.; Roberts, M.D. Proteasome- and Calpain-Mediated Proteolysis, but Not Autophagy, Is Required for Leucine-Induced Protein Synthesis in C2C12 Myotubes. Physiologia 2021, 1, 22–33.
- Gu, C.; Mao, X.; Chen, D.; Yu, B.; Yang, Q. Isoleucine Plays an Important Role for Maintaining Immune Function. Curr. Protein Pept. Sci. 2019, 20, 644–651.
- Xiao, F.; Yu, J.; Guo, Y.; Deng, J.; Li, K.; Du, Y.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of Individual Branched-Chain Amino Acids Deprivation on Insulin Sensitivity and Glucose Metabolism in Mice. Metabolism 2014, 63, 841–850.
- Williams, C.D. A Nutritional Disease of Childhood Associated with a Maize Diet. Arch. Dis. Child. 1983, 58, 550–560.
- Kocher, R.A. Effects of a Low Lysine Diet on the Growth of Spontaneous Mammary Tumors in Mice and on the N2 Balance in Man. Cancer Res. 1944, 4, 251–256.
- Van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Prim. 2021, 7, 36.
- Brosnan, M.E.; Brosnan, J.T. Histidine Metabolism and Function. J. Nutr. 2020, 150, 2570S–2575S.
- Froldi, F.; Pachnis, P.; Szuperák, M.; Costas, O.; Fernando, T.; Gould, A.P.; Cheng, L.Y. Histidine Is Selectively Required for the Growth of Myc-dependent Dedifferentiation Tumours in the Drosophila CNS. EMBO J. 2019, 38, e99895.
- Opitz, C.A.; Somarribas Patterson, L.F.; Mohapatra, S.R.; Dewi, D.L.; Sadik, A.; Platten, M.; Trump, S. The Therapeutic Potential of Targeting Tryptophan Catabolism in Cancer. Br. J. Cancer 2020, 122, 30–44.
- Kim, M.; Tomek, P. Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO. Front. Immunol. 2021, 12, 636081.
- Kamath, S.K.; Conrad, N.C.; Olson, R.E.; Kohrs, M.B.; Ghosh, L. Amino Acid-Restricted Diets in the Treatment of Mammary Adenocarcinoma in Mice. J. Nutr. 1988, 118, 1137–1142.
- Tajan, M.; Vousden, K.H. Dietary Approaches to Cancer Therapy. Cancer Cell 2020, 37, 767–785.
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-Adenosylmethionine Metabolism and Liver Disease. Ann. Hepatol. 2013, 12, 183–189.
- Weber, R.; Birsoy, K. The Transsulfuration Pathway Makes, the Tumor Takes. Cell Metab. 2019, 30, 845–846.
- Hoshiya, Y.; Kubota, T.; Matsuzaki, S.W.; Kitajima, M.; Hoffman, R.M. Methionine Starvation Modulates the Efficacy of Cisplatin on Human Breast Cancer in Nude Mice. Anticancer Res. 1996, 16, 3515–3518.
- Hoshiya, Y.; Kubota, T.; Inada, T.; Kitajima, M.; Hoffman, R.M. Methionine-Depletion Modulates the Efficacy of 5-Fluorouracil in Human Gastric Cancer in Nude Mice. Anticancer Res. 1997, 17, 4371–4375.
- Guo, H.; Lishko, V.K.; Herrera, H.; Groce, A.; Kubota, T.; Hoffman, R.M. Therapeutic Tumor-Specific Cell Cycle Block Induced by Methionine Starvation in vivo. Cancer Res. 1993, 53, 5676–5679.
- Jeon, H.; Kim, J.H.; Lee, E.; Jang, Y.J.; Son, J.E.; Kwon, J.Y.; Lim, T.; Kim, S.; Park, J.H.Y.; Kim, J.-E.; et al. Methionine Deprivation Suppresses Triple-Negative Breast Cancer Metastasis in vitro and in vivo. Oncotarget 2016, 7, 67223–67234.
- Strekalova, E.; Malin, D.; Good, D.M.; Cryns, V.L. Methionine Deprivation Induces a Targetable Vulnerability in Triple-Negative Breast Cancer Cells by Enhancing TRAIL Receptor-2 Expression. Clin. Cancer Res. 2015, 21, 2780–2791.
- Malin, D.; Lee, Y.; Chepikova, O.; Strekalova, E.; Carlson, A.; Cryns, V.L. Methionine Restriction Exposes a Targetable Redox Vulnerability of Triple-Negative Breast Cancer Cells by Inducing Thioredoxin Reductase. Breast Cancer Res. Treat. 2021, 190, 373–387.
- Liu, H.; Zhang, W.; Wang, K.; Wang, X.; Yin, F.; Li, C.; Wang, C.; Zhao, B.; Zhong, C.; Zhang, J.; et al. Methionine and Cystine Double Deprivation Stress Suppresses Glioma Proliferation via Inducing ROS/Autophagy. Toxicol. Lett. 2015, 232, 349–355.
- Sinha, R.; Cooper, T.K.; Rogers, C.J.; Sinha, I.; Turbitt, W.J.; Calcagnotto, A.; Perrone, C.E.; Richie, J.P. Dietary Methionine Restriction Inhibits Prostatic Intraepithelial Neoplasia in TRAMP Mice. Prostate 2014, 74, 1663–1673.
- Xu, Q.; Li, Y.; Gao, X.; Kang, K.; Williams, J.G.; Tong, L.; Liu, J.; Ji, M.; Deterding, L.J.; Tong, X.; et al. HNF4α Regulates Sulfur Amino Acid Metabolism and Confers Sensitivity to Methionine Restriction in Liver Cancer. Nat. Commun. 2020, 11, 3978.
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary Methionine Influences Therapy in Mouse Cancer Models and Alters Human Metabolism. Nature 2019, 572, 397–401.
- Hens, J.R.; Sinha, I.; Perodin, F.; Cooper, T.; Sinha, R.; Plummer, J.; Perrone, C.E.; Orentreich, D. Methionine-Restricted Diet Inhibits Growth of MCF10AT1-Derived Mammary Tumors by Increasing Cell Cycle Inhibitors in Athymic Nude Mice. BMC Cancer 2016, 16, 349.
- Komninou, D.; Leutzinger, Y.; Reddy, B.S.; Richie, J.P. Methionine Restriction Inhibits Colon Carcinogenesis. Nutr. Cancer 2006, 54, 202–208.
- Liu, C.; Wang, J.L.; Wu, D.Z.; Yuan, Y.W.; Xin, L. Methionine Restriction Enhances the Chemotherapeutic Sensitivity of Colorectal Cancer Stem Cells by MiR-320d/c-Myc Axis. Mol. Cell. Biochem. 2022, 477, 2001–2013.
- Li, T.; Tan, Y.-T.; Chen, Y.-X.; Zheng, X.-J.; Wang, W.; Liao, K.; Mo, H.-Y.; Lin, J.; Yang, W.; Piao, H.-L.; et al. Methionine Deficiency Facilitates Antitumour Immunity by Altering m 6 A Methylation of Immune Checkpoint Transcripts. Gut 2023, 72, 501–511.
- Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets with Altered Levels of Sulfur Amino Acids Induce Anticancer Activity in Mice with Metastatic Colon Cancer, Ovarian Cancer and Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 4587.
- Upadhyayula, P.S.; Higgins, D.M.; Mela, A.; Banu, M.; Dovas, A.; Zandkarimi, F.; Patel, P.; Mahajan, A.; Humala, N.; Nguyen, T.T.T.; et al. Dietary Restriction of Cysteine and Methionine Sensitizes Gliomas to Ferroptosis and Induces Alterations in Energetic Metabolism. Nat. Commun. 2023, 14, 1187.
- Goseki, N.; Endo, M.; Onodera, T.; Kosaki, G. Anti-Tumor Effect of L-Methionine-Deprived Total Parenteral Nutrition with 5-Fluorouracil Administration on Yoshida Sarcoma-Bearing Rats. Ann. Surg. 1991, 214, 83–88.
- Goseki, N.; Nagahama, T.; Maruyama, M.; Endo, M. Enhanced Anticancer Effect of Vincristine with Methionine Infusion after Methionine-Depleting Total Parenteral Nutrition in Tumor-Bearing Rats. Jpn. J. Cancer Res. 1996, 87, 194–199.
- Guo, H.; Tan, Y.; Kubota, T.; Moossa, A.R.; Hoffman, R.M. Methionine Depletion Modulates the Antitumor and Antimetastatic Efficacy of Ethionine. Anticancer Res. 1996, 16, 2719–2723.
- Xiao, H.B.; Cao, W.X.; Yin, H.R.; Lin, Y.Z.; Ye, S.H. Influence of L-Methionine-Deprived Total Parenteral Nutrition with 5-Fluorouracil on Gastric Cancer and Host Metabolism. World J. Gastroenterol. 2001, 7, 698–701.
- Hoshiya, Y.; Guo, H.; Kubota, T.; Inada, T.; Asanuma, F.; Yamada, Y.; Koh, J.I.; Kitajima, M.; Hoffman, R.M. Human Tumors Are Methionine Dependent in vivo. Anticancer Res. 1995, 15, 717–718.
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678.
- Zhang, H.-F.; Klein Geltink, R.I.; Parker, S.J.; Sorensen, P.H. Transsulfuration, Minor Player or Crucial for Cysteine Homeostasis in Cancer. Trends Cell Biol. 2022, 32, 800–814.
- Poltorack, C.D.; Dixon, S.J. Understanding the Role of Cysteine in Ferroptosis: Progress & Paradoxes. FEBS J. 2022, 289, 374–385.
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer Cell 2015, 27, 211–222.
- HumanCyc: Encyclopedia of Human Genes and Metabolism. Available online: https://humancyc.org/ (accessed on 29 May 2023).
- Voegtlin, C.; Johnson, J.M.; Thompson, J.W. Glutathione and Malignant Growth. Public Health Rep. 1936, 51, 1689.
- Ruiz-Rodado, V.; Dowdy, T.; Lita, A.; Kramp, T.; Zhang, M.; Jung, J.; Dios-Esponera, A.; Zhang, L.; Herold-Mende, C.C.; Camphausen, K.; et al. Cysteine Is a Limiting Factor for Glioma Proliferation and Survival. Mol. Oncol. 2022, 16, 1777–1794.
- Wu, J.; Yeung, S.-C.J.; Liu, S.; Qdaisat, A.; Jiang, D.; Liu, W.; Cheng, Z.; Liu, W.; Wang, H.; Li, L.; et al. Cyst(e)Ine in Nutrition Formulation Promotes Colon Cancer Growth and Chemoresistance by Activating MTORC1 and Scavenging ROS. Signal Transduct. Target. Ther. 2021, 6, 188.
- Zhang, T.; Bauer, C.; Newman, A.C.; Uribe, A.H.; Athineos, D.; Blyth, K.; Maddocks, O.D.K. Polyamine Pathway Activity Promotes Cysteine Essentiality in Cancer Cells. Nat. Metab. 2020, 2, 1062–1076.
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Inhibit T Cell Activation by Depleting Cystine and Cysteine. Cancer Res. 2010, 70, 68.
- Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine Metabolic Circuitries: Druggable Targets in Cancer. Br. J. Cancer 2021, 124, 862–879.
- Hatae, R.; Chamoto, K.; Kim, Y.H.; Sonomura, K.; Taneishi, K.; Kawaguchi, S.; Yoshida, H.; Ozasa, H.; Sakamori, Y.; Akrami, M.; et al. Combination of Host Immune Metabolic Biomarkers for the PD-1 Blockade Cancer Immunotherapy. JCI Insight 2020, 5, e133501.
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 2019, 569, 270–274.
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic Depletion of L-Cyst(e)Ine with Cyst(e)Inase Increases Reactive Oxygen Species and Suppresses Tumor Growth. Nat. Med. 2017, 23, 120–127.
- Kshattry, S.; Saha, A.; Gries, P.; Tiziani, S.; Stone, E.; Georgiou, G.; DiGiovanni, J. Enzyme-Mediated Depletion of l-Cyst(e)Ine Synergizes with Thioredoxin Reductase Inhibition for Suppression of Pancreatic Tumor Growth. Npj Precis. Oncol. 2019, 3, 16.
- Saha, A.; Zhao, S.; Chen, Z.; Georgiou, G.; Stone, E.; Kidane, D.; DiGiovanni, J. Combinatorial Approaches to Enhance DNA Damage Following Enzyme-Mediated Depletion of L-Cys for Treatment of Pancreatic Cancer. Mol. Ther. 2021, 29, 775–787.
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.-J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine Depletion Induces Pancreatic Tumor Ferroptosis in Mice. Science 2020, 368, 85–89.
- Poursaitidis, I.; Wang, X.; Crighton, T.; Labuschagne, C.; Mason, D.; Cramer, S.L.; Triplett, K.; Roy, R.; Pardo, O.E.; Seckl, M.J.; et al. Oncogene-Selective Sensitivity to Synchronous Cell Death Following Modulation of the Amino Acid Nutrient Cystine. Cell Rep. 2017, 18, 2547–2556.
- Kerimoglu, B.; Lamb, C.; McPherson, R.D.; Ergen, E.; Stone, E.M.; Ooi, A. Cyst(e)Inase-Rapamycin Combination Induces Ferroptosis in Both In Vitro and In Vivo Models of Hereditary Leiomyomatosis and Renal Cell Cancer. Mol. Cancer Ther. 2022, 21, 419–426.
- Yang, M.; Vousden, K.H. Serine and One-Carbon Metabolism in Cancer. Nat. Rev. Cancer 2016, 16, 650–662.
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.K.; Sethumadhavan, S.; Woo, H.-K.K.; Jang, H.G.; Jha, A.K.; et al. Functional Genomics Reveal That the Serine Synthesis Pathway Is Essential in Breast Cancer. Nature 2011, 476, 346–350.
- Labuschagne, C.F.; van den Broek, N.J.F.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D.K. Serine, but Not Glycine, Supports One-Carbon Metabolism and Proliferation of Cancer Cells. Cell Rep. 2014, 7, 1248–1258.
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; Van Den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Modulating the Therapeutic Response of Tumours to Dietary Serine and Glycine Starvation. Nature 2017, 544, 372–376.
- Gravel, S.-P.; Hulea, L.; Toban, N.; Birman, E.; Blouin, M.-J.; Zakikhani, M.; Zhao, Y.; Topisirovic, I.; St-Pierre, J.; Pollak, M. Serine Deprivation Enhances Antineoplastic Activity of Biguanides. Cancer Res. 2014, 74, 7521–7533.
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2018, 50, 29–38.
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science 2012, 336, 1040–1044.
- Chen, C.-L.; Hsu, S.-C.; Ann, D.K.; Yen, Y.; Kung, H.-J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541.
- Castillo, L.; Chapman, T.E.; Sanchez, M.; Yu, Y.M.; Burke, J.F.; Ajami, A.M.; Vogt, J.; Young, V.R. Plasma Arginine and Citrulline Kinetics in Adults given Adequate and Arginine-Free Diets. Proc. Natl. Acad. Sci. USA 1993, 90, 7749–7753.
- Tharakan, J.F.; Yu, Y.M.; Zurakowski, D.; Roth, R.M.; Young, V.R.; Castillo, L. Adaptation to a Long Term (4 Weeks) Arginine- and Precursor (Glutamate, Proline and Aspartate)-Free Diet. Clin. Nutr. 2008, 27, 513–522.
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis, A.; Helbling, D.; et al. Diversion of Aspartate in ASS1-Deficient Tumours Fosters de Novo Pyrimidine Synthesis. Nature 2015, 527, 379–383.
- Cheng, C.-T.; Qi, Y.; Wang, Y.-C.; Chi, K.K.; Chung, Y.; Ouyang, C.; Chen, Y.-R.; Oh, M.E.; Sheng, X.; Tang, Y.; et al. Arginine Starvation Kills Tumor Cells through Aspartate Exhaustion and Mitochondrial Dysfunction. Commun. Biol. 2018, 1, 178.
- Agrawal, V.; Woo, J.H.; Mauldin, J.P.; Jo, C.; Stone, E.M.; Georgiou, G.; Frankel, A.E. Cytotoxicity of Human Recombinant Arginase I (Co)-PEG5000 in the Presence of Supplemental L-Citrulline Is Dependent on Decreased Argininosuccinate Synthetase Expression in Human Cells. Anticancer Drugs 2012, 23, 51–64.
- Bowles, T.L.; Kim, R.; Galante, J.; Parsons, C.M.; Virudachalam, S.; Kung, H.J.; Bold, R.J. Pancreatic Cancer Cell Lines Deficient in Argininosuccinate Synthetase Are Sensitive to Arginine Deprivation by Arginine Deiminase. Int. J. Cancer 2008, 123, 1950–1955.
- Hajji, N.; Garcia-Revilla, J.; Soto, M.S.; Perryman, R.; Symington, J.; Quarles, C.C.; Healey, D.R.; Guo, Y.; Orta-Vázquez, M.L.; Mateos-Cordero, S.; et al. Arginine Deprivation Alters Microglial Polarity and Synergizes with Radiation to Eradicate Non-Arginine-Auxotrophic Glioblastoma Tumors. J. Clin. Investig. 2022, 132, e142137.
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532.
- Satoh, Y.; Kotani, H.; Iida, Y.; Taniura, T.; Notsu, Y.; Harada, M. Supplementation of L-Arginine Boosts the Therapeutic Efficacy of Anticancer Chemoimmunotherapy. Cancer Sci. 2020, 111, 2248–2258.
- Fletcher, M.; Ramirez, M.E.; Sierra, R.A.; Raber, P.; Thevenot, P.; Al-Khami, A.A.; Sanchez-Pino, D.; Hernandez, C.; Wyczechowska, D.D.; Ochoa, A.C.; et al. L-Arginine Depletion Blunts Antitumor T-Cell Responses by Inducing Myeloid-Derived Suppressor Cells. Cancer Res. 2015, 75, 275–283.
- Lacey, J.M.; Wilmore, D.W. Is Glutamine a Conditionally Essential Amino Acid? Nutr. Rev. 1990, 48, 297–309.
- Soeters, P.B.; Grecu, I. Have We Enough Glutamine and How Does It Work? A Clinician’s View. Ann. Nutr. Metab. 2012, 60, 17–26.
- Halama, A.; Suhre, K. Advancing Cancer Treatment by Targeting Glutamine Metabolism—A Roadmap. Cancers 2022, 14, 553.
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564.
- Hodson, N.; Brown, T.; Joanisse, S.; Aguirre, N.; West, D.; Moore, D.; Baar, K.; Breen, L.; Philp, A. Characterisation of L-Type Amino Acid Transporter 1 (LAT1) Expression in Human Skeletal Muscle by Immunofluorescent Microscopy. Nutrients 2017, 10, 23.
- Kubota, A.; Meguid, M.M.; Hitch, D.C. Amino Acid Profiles Correlate Diagnostically with Organ Site in Three Kinds of Malignant Tumors. Cancer 1992, 69, 2343–2348.
- Miyagi, Y.; Higashiyama, M.; Gochi, A.; Akaike, M.; Ishikawa, T.; Miura, T.; Saruki, N.; Bando, E.; Kimura, H.; Imamura, F.; et al. Plasma Free Amino Acid Profiling of Five Types of Cancer Patients and Its Application for Early Detection. PLoS ONE 2011, 6, e24143.
- Pollard, A.C.; Paolillo, V.; Radaram, B.; Qureshy, S.; Li, L.; Maity, T.; Wang, L.; Uddin, M.N.; Wood, C.G.; Karam, J.A.; et al. PET/MR Imaging of a Lung Metastasis Model of Clear Cell Renal Cell Carcinoma with (2S,4R)-4-Fluoroglutamine. Mol. Imaging Biol. 2022, 24, 959–972.
- Dunphy, M.P.S.; Harding, J.J.; Venneti, S.; Zhang, H.; Burnazi, E.M.; Bromberg, J.; Omuro, A.M.; Hsieh, J.J.; Mellinghoff, I.K.; Staton, K.; et al. In Vivo PET Assay of Tumor Glutamine Flux and Metabolism: In-Human Trial of 18f-(2S,4R)-4-Fluoroglutamine. Radiology 2018, 287, 667–675.
- Xu, X.; Zhu, H.; Liu, F.; Zhang, Y.; Yang, J.; Zhang, L.; Zhu, L.; Li, N.; Kung, H.F.; Yang, Z. Imaging Brain Metastasis Patients with 18F-(2S,4 R)-4-Fluoroglutamine. Clin. Nucl. Med. 2018, 43, e392–e399.
- Grkovski, M.; Goel, R.; Krebs, S.; Staton, K.D.; Harding, J.J.; Mellinghoff, I.K.; Humm, J.L.; Dunphy, M.P.S. Pharmacokinetic Assessment of 18F-(2S,4R)-4-Fluoroglutamine in Patients with Cancer. J. Nucl. Med. 2020, 61, 357–366.
- Van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 Controls Glutamine Uptake and Tumour Growth in Triple-Negative Basal-like Breast Cancer. Oncogene 2016, 35, 3201–3208.
- Bhutia, Y.D.; Ganapathy, V. Glutamine Transporters in Mammalian Cells and Their Functions in Physiology and Cancer. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 2531–2539.
- Wang, R.; Xiang, W.; Xu, Y.; Han, L.; Li, Q.; Dai, W.; Cai, G. Enhanced Glutamine Utilization Mediated by SLC1A5 and GPT2 Is an Essential Metabolic Feature of Colorectal Signet Ring Cell Carcinoma with Therapeutic Potential. Ann. Transl. Med. 2020, 8, 302.
- Cormerais, Y.; Massard, P.A.; Vucetic, M.; Giuliano, S.; Tambutté, E.; Durivault, J.; Vial, V.; Endou, H.; Wempe, M.F.; Parks, S.K.; et al. The Glutamine Transporter ASCT2 (SLC1A5) Promotes Tumor Growth Independently of the Amino Acid Transporter LAT1 (SLC7A5). J. Biol. Chem. 2018, 293, 2877–2887.
- Zhang, Z.; Liu, R.; Shuai, Y.; Huang, Y.; Jin, R.; Wang, X.; Luo, J. ASCT2 (SLC1A5)-Dependent Glutamine Uptake Is Involved in the Progression of Head and Neck Squamous Cell Carcinoma. Br. J. Cancer 2020, 122, 82–93.
- Wise, D.R.; Deberardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc Regulates a Transcriptional Program That Stimulates Mitochondrial Glutaminolysis and Leads to Glutamine Addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787.
- Toda, K.; Nishikawa, G.; Iwamoto, M.; Itatani, Y.; Takahashi, R.; Sakai, Y.; Kawada, K. Clinical Role of ASCT2 (SLC1A5) in KRAS-Mutated Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 1632.
- Niklison-Chirou, M.V.; Erngren, I.; Engskog, M.; Haglöf, J.; Picard, D.; Remke, M.; McPolin, P.H.R.; Selby, M.; Williamson, D.; Clifford, S.C.; et al. TAp73 Is a Marker of Glutamine Addiction in Medulloblastoma. Genes Dev. 2017, 31, 1738–1753.
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; et al. Antitumor Activity of the Glutaminase Inhibitor CB-839 in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2014, 13, 890–901.
- Robinson, M.M.; McBryant, S.J.; Tsukamoto, T.; Rojas, C.; Ferraris, D.V.; Hamilton, S.K.; Hansen, J.C.; Curthoys, N.P. Novel Mechanism of Inhibition of Rat Kidney-Type Glutaminase by Bis-2-(5-Phenylacetamido-1,2,4-Thiadiazol-2-Yl)Ethyl Sulfide (BPTES). Biochem. J. 2007, 406, 407–414.
- Wang, J.B.; Erickson, J.W.; Fuji, R.; Ramachandran, S.; Gao, P.; Dinavahi, R.; Wilson, K.F.; Ambrosio, A.L.B.; Dias, S.M.G.; Dang, C.V.; et al. Targeting Mitochondrial Glutaminase Activity Inhibits Oncogenic Transformation. Cancer Cell 2010, 18, 207–219.
- Hoerner, C.R.; Chen, V.J.; Fan, A.C. The ‘Achilles Heel’ of Metabolism in Renal Cell Carcinoma: Glutaminase Inhibition as a Rational Treatment Strategy. Kidney Cancer 2019, 3, 15–29.
- Clinical Trials Using CB839 in Cancer|List Results. Available online: https://clinicaltrials.gov/ct2/results?cond=Cancer&term=CB839&cntry=&state=&city=&dist= (accessed on 29 May 2023).
- Kalinsky, K.; Harding, J.; DeMichele, A.; Infante, J.; Gogineni, K.; Owonikoko, T.; Isakoff, S.; Iliopoulos, O.; Patel, M.; Munster, P.; et al. Abstract PD3-13: Phase 1 Study of CB-839, a First-in-Class Oral Inhibitor of Glutaminase, in Combination with Paclitaxel in Patients with Advanced Triple Negative Breast Cancer. Cancer Res. 2018, 78, PD3-13.
- Calithera Biosciences, Inc. Initial Results from Phase 2 Study of CB-839 in Combination with Opdivo® (Nivolumab) to Be Presented at the Society for Immunotherapy of Cancer Meeting. Available online: https://www.globenewswire.com/en/news-release/2017/11/07/1176454/32478/en/Initial-Results-from-Phase-2-Study-of-CB-839-in-Combination-with-Opdivo-nivolumab-to-be-Presented-at-the-Society-for-Immunotherapy-of-Cancer-Meeting.html (accessed on 29 May 2023).
- Harding, J.J.; Telli, M.L.; Munster, P.N.; Le, M.H.; Molineaux, C.; Bennett, M.K.; Mittra, E.; Burris, H.A.; Clark, A.S.; Dunphy, M.; et al. Safety and Tolerability of Increasing Doses of CB-839, a First-in-Class, Orally Administered Small Molecule Inhibitor of Glutaminase, in Solid Tumors. J. Clin. Oncol. 2015, 33, 2512.
- Lee, C.H.; Motzer, R.; Emamekhoo, H.; Matrana, M.; Percent, I.; Hsieh, J.J.; Hussain, A.; Vaishampayan, U.; Liu, S.; McCune, S.; et al. Telaglenastat plus Everolimus in Advanced Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled, Phase II ENTRATA Trial. Clin. Cancer Res. 2022, 28, 3248–3255.
- Tannir, N.M.; Agarwal, N.; Porta, C.; Lawrence, N.J.; Motzer, R.; McGregor, B.; Lee, R.J.; Jain, R.K.; Davis, N.; Appleman, L.J.; et al. Efficacy and Safety of Telaglenastat Plus Cabozantinib vs. Placebo Plus Cabozantinib in Patients with Advanced Renal Cell Carcinoma. JAMA Oncol. 2022, 8, 1411–1418.
- Wang, E.S.; Frankfurt, O.; Orford, K.W.; Bennett, M.; Flinn, I.W.; Maris, M.; Konopleva, M. Phase 1 Study of CB-839, a First-in-Class, Orally Administered Small Molecule Inhibitor of Glutaminase in Patients with Relapsed/Refractory Leukemia. Blood 2015, 126, 2566.
- Motzer, R.J.; Lee, C.-H.; Emamekhoo, H.; Matrana, M.; Percent, I.; Hsieh, J.J.; Hussain, A.; Vaishampayan, U.N.; Graham, R.; Liu, S.; et al. ENTRATA: Randomized, Double-Blind, Phase II Study of Telaglenastat (Tela; CB-839) + Everolimus (E) vs. Placebo (Pbo) + E in Patients (Pts) with Advanced/Metastatic Renal Cell Carcinoma (MRCC). Ann. Oncol. 2019, 30, v889–v890.
- Schulte, M.L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S.T.; Kondo, J.; Coffey, R.J.; Johnson, M.O.; Rathmell, J.C.; et al. Pharmacological Blockade of ASCT2-Dependent Glutamine Transport Leads to Antitumor Efficacy in Preclinical Models. Nat. Med. 2018, 24, 194–202.
- Leone, R.D.; Zhao, L.; Englert, J.M.; Sun, I.-M.; Oh, M.-H.; Sun, I.-H.; Arwood, M.L.; Bettencourt, I.A.; Patel, C.H.; Wen, J.; et al. Glutamine Blockade Induces Divergent Metabolic Programs to Overcome Tumor Immune Evasion. Science 2019, 366, 1013–1021.
- Hanaford, A.R.; Alt, J.; Rais, R.; Wang, S.Z.; Kaur, H.; Thorek, D.L.J.; Eberhart, C.G.; Slusher, B.S.; Martin, A.M.; Raabe, E.H. Orally Bioavailable Glutamine Antagonist Prodrug JHU-083 Penetrates Mouse Brain and Suppresses the Growth of MYC-Driven Medulloblastoma. Transl. Oncol. 2019, 12, 1314–1322.
- Yamashita, A.S.; da Costa Rosa, M.; Stumpo, V.; Rais, R.; Slusher, B.S.; Riggins, G.J. The Glutamine Antagonist Prodrug JHU-083 Slows Malignant Glioma Growth and Disrupts MTOR Signaling. Neuro-Oncol. Adv. 2021, 3, vdaa149.
- Suru, A.; Islam, M.; Tam, A.; Gross, J.; Llosa, N. 580 Glutamine Blockade in Combination with Immune Checkpoint Blockade Remodels the Myeloid Landscape in Mouse Models of Soft Tissue Sarcomas. J. Immunother. Cancer 2021, 9, A609–A610.
- Oh, M.-H.; Travers, M.; Brown, S.; Zhao, L.; Sun, I.-M.; Sun, I.-H.; Arwood, M.; Xu, W.; Collins, S.; Leone, R.; et al. Abstract LB-022: Targeting Glutamine Metabolism as a Mean of Treating a Murine Model of Ovarian Cancer and Ascites Development. Cancer Res. 2019, 79, LB-022.
- Oh, M.H.; Sun, I.H.; Zhao, L.; Leone, R.D.; Sun, I.M.; Xu, W.; Collins, S.L.; Tam, A.J.; Blosser, R.L.; Patel, C.H.; et al. Targeting Glutamine Metabolism Enhances Tumor-Specific Immunity by Modulating Suppressive Myeloid Cells. J. Clin. Investig. 2020, 130, 3865–3884.
- Daemen, A.; Liu, B.; Song, K.; Kwong, M.; Gao, M.; Hong, R.; Nannini, M.; Peterson, D.; Liederer, B.M.; de la Cruz, C.; et al. Pan-Cancer Metabolic Signature Predicts Co-Dependency on Glutaminase and De Novo Glutathione Synthesis Linked to a High-Mesenchymal Cell State. Cell Metab. 2018, 28, 383–399.e9.
- Choi, B.-H.; Coloff, J.L. The Diverse Functions of Non-Essential Amino Acids in Cancer. Cancers 2019, 11, 675.
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine Promotes Cancer Cell Proliferation through Use as an Amino Acid Exchange Factor. Nat. Commun. 2016, 7, 11457.
- Appel, I.M.; Kazemier, K.M.; Boos, J.; Lanvers, C.; Huijmans, J.; Veerman, A.J.P.; van Wering, E.; den Boer, M.L.; Pieters, R. Pharmacokinetic, Pharmacodynamic and Intracellular Effects of PEG-Asparaginase in Newly Diagnosed Childhood Acute Lymphoblastic Leukemia: Results from a Single Agent Window Study. Leukemia 2008, 22, 1665–1679.
- Story, M.D.; Voehringer, D.W.; Stephens, L.C.; Meyn, R.E. L-Asparaginase Kills Lymphoma Cells by Apoptosis. Cancer Chemother. Pharmacol. 1993, 32, 129–133.
- Pui, C.H.; Mullighan, C.G.; Evans, W.E.; Relling, M.V. Pediatric Acute Lymphoblastic Leukemia: Where Are We Going and How Do We Get There? Blood 2012, 120, 1165–1174.
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The Growing Landscape of Lysine Acetylation Links Metabolism and Cell Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550.
- Aslanian, A.M.; Kilberg, M.S. Multiple Adaptive Mechanisms Affect Asparagine Synthetase Substrate Availability in Asparaginase-Resistant MOLT-4 Human Leukaemia Cells. Biochem. J. 2001, 358, 59–67.
- Fine, B.M.; Kaspers, G.J.L.; Ho, M.; Loonen, A.H.; Boxer, L.M. A Genome-Wide View of the in Vitro Response to L-Asparaginase in Acute Lymphoblastic Leukemia. Cancer Res. 2005, 65, 291–299.
- Chan, W.K.; Horvath, T.D.; Tan, L.; Link, T.; Harutyunyan, K.G.; Pontikos, M.A.; Anishkin, A.; Du, D.; Martin, L.A.; Yin, E.; et al. Glutaminase Activity of L-Asparaginase Contributes to Durable Preclinical Activity against Acute Lymphoblastic Leukemia. Mol. Cancer Ther. 2019, 18, 1587–1592.
- Offman, M.N.; Krol, M.; Patel, N.; Krishnan, S.; Liu, J.Z.; Saha, V.; Bates, P.A. Rational Engineering of L-Asparaginase Reveals Importance of Dual Activity for Cancer Cell Toxicity. Blood 2011, 117, 1614–1621.
- Sarabhai, T.; Roden, M. Hungry for Your Alanine: When Liver Depends on Muscle Proteolysis. J. Clin. Investig. 2019, 129, 4563–4566.
- Geng, P.; Qin, W.; Xu, G. Proline Metabolism in Cancer. Amino Acids 2021, 53, 1769–1777.
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and Hydroxyproline Metabolism: Implications for Animal and Human Nutrition. Amino Acids 2011, 40, 1053–1063.
- Olivares, O.; Mayers, J.R.; Gouirand, V.; Torrence, M.E.; Gicquel, T.; Borge, L.; Lac, S.; Roques, J.; Lavaut, M.N.; Berthezène, P.; et al. Collagen-Derived Proline Promotes Pancreatic Ductal Adenocarcinoma Cell Survival under Nutrient Limited Conditions. Nat. Commun. 2017, 8, 16031.
- Sahu, N.; Dela Cruz, D.; Gao, M.; Sandoval, W.; Haverty, P.M.; Liu, J.; Stephan, J.-P.; Haley, B.; Classon, M.; Hatzivassiliou, G.; et al. Proline Starvation Induces Unresolved ER Stress and Hinders MTORC1-Dependent Tumorigenesis. Cell Metab. 2016, 24, 753–761.
- Demopoulos, H.B. Effects of Low Phenylalanine-Tyrosine Diets on S91 Mouse Melanomas. J. Natl. Cancer Inst. 1966, 37, 185–190.
- Bounous, G.; Kongshavn, P.A.L. The Effect of Dietary Amino Acids on the Growth of Tumors. Experientia 1981, 37, 271–273.
- Abdallah, R.M.; Starkey, J.R.; Meadows, G.G. Dietary Restriction of Tyrosine and Phenylalanine: Inhibition of Metastasis of Three Rodent Tumors. J. Natl. Cancer Inst. 1987, 78, 759–769.
- Elstad, C.A.; Meadows, G.G.; Abdallah, R.M. Specificity of the Suppression of Metastatic Phenotype by Tyrosine and Phenylalanine Restriction. Clin. Exp. Metastasis 1990, 8, 393–416.
- Van Nyen, T.; Planque, M.; van Wagensveld, L.; Duarte, J.A.G.; Zaal, E.A.; Talebi, A.; Rossi, M.; Körner, P.R.; Rizzotto, L.; Moens, S.; et al. Serine Metabolism Remodeling after Platinum-Based Chemotherapy Identifies Vulnerabilities in a Subgroup of Resistant Ovarian Cancers. Nat. Commun. 2022, 13, 25.
- Sullivan, M.R.; Mattaini, K.R.; Dennstedt, E.A.; Nguyen, A.A.; Sivanand, S.; Reilly, M.F.; Meeth, K.; Muir, A.; Darnell, A.M.; Bosenberg, M.W.; et al. Increased Serine Synthesis Provides an Advantage for Tumors Arising in Tissues Where Serine Levels Are Limiting. Cell Metab. 2019, 29, 1410–1421.e4.
- Muthusamy, T.; Cordes, T.; Handzlik, M.K.; You, L.; Lim, E.W.; Gengatharan, J.; Pinto, A.F.M.; Badur, M.G.; Kolar, M.J.; Wallace, M.; et al. Serine Restriction Alters Sphingolipid Diversity to Constrain Tumour Growth. Nature 2020, 586, 790–795.
- Fujihara, K.M.; Zhang, B.Z.; Jackson, T.D.; Ogunkola, M.O.; Nijagal, B.; Milne, J.V.; Sallman, D.A.; Ang, C.-S.; Nikolic, I.; Kearney, C.J.; et al. Eprenetapopt Triggers Ferroptosis, Inhibits NFS1 Cysteine Desulfurase, and Synergizes with Serine and Glycine Dietary Restriction. Sci. Adv. 2022, 8, eabm9427.
- Maddocks, O.D.K.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine Starvation Induces Stress and P53-Dependent Metabolic Remodelling in Cancer Cells. Nature 2013, 493, 542–546.
- Humpton, T.J.; Hock, A.K.; Maddocks, O.D.K.; Vousden, K.H. P53-Mediated Adaptation to Serine Starvation Is Retained by a Common Tumour-Derived Mutant. Cancer Metab. 2018, 6, 18.
- LeBoeuf, S.E.; Wu, W.L.; Karakousi, T.R.; Karadal, B.; Jackson, S.R.E.; Davidson, S.M.; Wong, K.K.; Koralov, S.B.; Sayin, V.I.; Papagiannakopoulos, T. Activation of Oxidative Stress Response in Cancer Generates a Druggable Dependency on Exogenous Non-Essential Amino Acids. Cell Metab. 2020, 31, 339–350.e4.
- Tajan, M.; Hennequart, M.; Cheung, E.C.; Zani, F.; Hock, A.K.; Legrave, N.; Maddocks, O.D.K.; Ridgway, R.A.; Athineos, D.; Suárez-Bonnet, A.; et al. Serine Synthesis Pathway Inhibition Cooperates with Dietary Serine and Glycine Limitation for Cancer Therapy. Nat. Commun. 2021, 12, 366.
- Falcone, M.; Uribe, A.H.; Papalazarou, V.; Newman, A.C.; Athineos, D.; Stevenson, K.; Sauvé, C.E.G.; Gao, Y.; Kim, J.K.; Del Latto, M.; et al. Sensitisation of Cancer Cells to Radiotherapy by Serine and Glycine Starvation. Br. J. Cancer 2022, 127, 1773–1778.
- Pranzini, E.; Pardella, E.; Muccillo, L.; Leo, A.; Nesi, I.; Santi, A.; Parri, M.; Zhang, T.; Uribe, A.H.; Lottini, T.; et al. SHMT2-Mediated Mitochondrial Serine Metabolism Drives 5-FU Resistance by Fueling Nucleotide Biosynthesis. Cell Rep. 2022, 40, 111233.
- Polet, F.; Corbet, C.; Pinto, A.; Rubio, L.I.; Martherus, R.; Bol, V.; Drozak, X.; Grégoire, V.; Riant, O.; Feron, O. Reducing the Serine Availability Complements the Inhibition of the Glutamine Metabolism to Block Leukemia Cell Growth. Oncotarget 2016, 7, 1765–1776.
- Méndez-Lucas, A.; Lin, W.; Driscoll, P.C.; Legrave, N.; Novellasdemunt, L.; Xie, C.; Charles, M.; Wilson, Z.; Jones, N.P.; Rayport, S.; et al. Identifying Strategies to Target the Metabolic Flexibility of Tumours. Nat. Metab. 2020, 2, 335–350.
- Hamanaka, R.B.; Nigdelioglu, R.; Meliton, A.Y.; Tian, Y.; Witt, L.J.; O’Leary, E.; Sun, K.A.; Woods, P.S.; Wu, D.; Ansbro, B.; et al. Inhibition of Phosphoglycerate Dehydrogenase Attenuates Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 585–593.
More
