The term “liver disease” refers to any hepatic condition that leads to tissue damage or altered hepatic function and can be induced by virus infections, autoimmunity, inherited genetic mutations, high consumption of alcohol or drugs, fat accumulation, and cancer. Some types of liver diseases are becoming more frequent worldwide. This can be related to increasing rates of obesity in developed countries, diet changes, higher alcohol intake, and even the coronavirus disease 2019 (COVID-19) pandemic was associated with increased liver disease-related deaths. Although the liver can regenerate, in cases of chronic damage or extensive fibrosis, the recovery of tissue mass is impossible, and a liver transplant is indicated.
- liver diseases
- magnetic nanoparticles
- regenerative medicine
- biomaterials
1. Stem Cells as an Alternative Treatment to Liver Diseases
| Identifier at ClinicalTrials.gov |
Liver Condition | Stem Cell Type * | Study Phase | Enrolment/ Estimated Enrolment |
Status | Administration Route and Cell Dose |
|---|---|---|---|---|---|---|
| NCT03109236 | Cirrhosis | Autologous EPC CD133+ from BM | Phase 3 | 66 participants | Recruiting | 5–10 × 106 CD133 cells through the transhepatic route into the portal venous circulation. |
| NCT05331872 | Cirrhosis | UC-MSCs | Phase 1 | 20 participants | Recruiting | Route not informed. Cell dosage not informed. |
| NCT05227846 | Cirrhosis | UC-MSCs | Phase 1 | 9 participants | Recruiting | Cell dosage not informed. |
| NCT03945487 | Cirrhosis | UC-MSCs | Phase 2 | 200 participants | Recruiting | Intravenous administration of 1.0 × 106 cell/kg three times at three-week intervals. |
| NCT05121870 | Cirrhosis | UC-MSCs | Phase 2 | 240 participants | Recruiting | Intravenous administration of three doses (6.0 × 107 cells per event) at weeks 0, 4, and 8. |
| NCT03626090 | Cirrhosis | Autologous BM-MSCs | Phase 1/2 | 20 participants | Recruiting | A single dose of 0.5 to 1 × 106 cells/kg via peripheral venous access. |
| NCT03254758 | Cirrhosis | AD-MSCs | Phase 1/2 | 27 participants | Recruiting | Intravenous infusion; for phase 1, the cell dose escalated from low to mid and high; for phase 2, the recommended amount of cells was administered once a week for four weeks in the same route and time as in phase 1. Cell dosage not informed. |
| NCT05155657 | Alcoholic cirrhosis | UC-MSCs | Phase 1 | 36 participants | Recruiting | 0.5 × 106 cells/kg, 1.0 × 106 cells/kg, or 2.0 × 106 cells/kg via intravenous infusion. |
| NCT04689152 | Alcoholic cirrhosis | Autologous BM MSC | Phase 3 | 200 participants | Recruiting | 7 × 107 cells via the hepatic artery. |
| NCT03826433 | Cirrhosis due to hepatitis B | UC-MSCs | Phase 1 | 20 participants | Recruiting | 6 × 107 cells via peripheral intravenous injection. |
| NCT05507762 | Cirrhosis due to hepatitis B (compensation stage) |
UC-MSCs | Phase 1/2 | 20 participants | Recruiting | 1 × 106/kg/time per injection via intravenous infusion in the elbow. |
| NCT05106972 | Cirrhosis due to hepatitis B | UC-MSCs | NT | 30 participants | Recruiting | 1 × 108 cells/dose via intravenous infusion. |
| NCT00655707 | Liver disease | Autologous expanded CD34+ HCSs | Phase 1/2 | 5 participants | Completed | 1 × 109,1 × 1010, 2 × 1010, or 5 × 1010 cells via either the hepatic artery or the portal vein. |
| NCT00420134 | Liver failure /cirrhosis |
Autologous MSCs | Phase 1 Phase 2 |
30 participants | Completed | The cells were administered via the portal vein. Cell dosage not informed. |
| NCT00147043 | Cirrhosis | Autologous adult stem cells | Not Applicable | 5 participants | Completed | The cells were administered via the hepatic artery or portal vein. Cell dosage not informed. |
| NCT04243681 | Cirrhosis | Autologous CD34+ HSCs and MSCs | Phase 4 | 5 participants | Completed | The cells were administered via the hepatic artery. Cell dosage not informed. |
| NCT00713934 | Cirrhosis | Autologous BM-MNCs and enriched CD133+ HSCs | Phase 1/2 | 7 participants | Completed | The cells were administered via the portal vein. Cell dosage not informed. |
| NCT02297867 | Cirrhosis | ADSCs | Phase 1 | 6 participants | Completed | One milliliter of cell suspension via intrahepatic injection. |
| NCT03632148 | Cirrhosis | MSCs | Not applicable | 9 participants | Completed | Route not informed. Cell dosage not informed. |
| NCT01342250 | Cirrhosis | UC-MSCs | Phase 1 Phase 2 |
20 participants | Completed | The cells were administered at low, medium, or high doses. Route not informed. Cell dosage not informed. |
| NCT01333228 | Cirrhosis | Autologous BM-derived endothelial progenitor cells | Phase 1/2 | 14 participants | Completed | The cells were administered via the hepatic artery. Cell dosage not informed. |
| NCT01013194 | Cirrhosis | Human fetal liver cell | Phase 1/2 | 25 participants | Completed | 5 or 10 × 108 cells via the splenic artery infusion |
| NCT01454336 | Liver cirrhosis /fibrosis |
Autologous MSCs | Phase 1 | 3 participants | Completed | The cells were administered via the portal vein. Cell dosage not informed. |
| NCT01220492 | Cirrhosis | UC-MSCs | Phase 1/2 | 266 participants | Completed | The cells were administered once a week for four weeks at a dose of 0.5 × 106 /kg body and intravenously for eight weeks. |
| NCT01120925 | Cirrhosis | BM-MNCs and enriched CD133+ HSCs | Phase 1/2 | 30 participants | Completed | BM-MNC were administered at a dose of 2–3 × 109 cells and CD133 at a dose of 5–15 × 106 cells, both via the portal vein. |
| NCT03963921 | NASH—non-alcoholic steatohepatitis | Liver-derived MSCs | Phase 1/2 | 23 participants | Completed | Route not informed. Cell dosage not informed. |
| NCT01591200 | Alcoholic liver cirrhosis |
Allogeneic BM-MSCs | Phase 2 | 40 participants | Completed | The cells were administered at a low, medium, or high dose via the hepatic artery. Cell dosage not informed. |
| NCT01875081 | Alcoholic cirrhosis | Autologous BM-MSCs | Phase 2 | 72 participants | Completed | 5 × 107 cells via the hepatic artery. |
| NCT01378182 | Wilson’s cirrhosis | Allogeneic BM-MSCs | Not applicable | 10 participants | Completed | 1 × 106 cells/kg in total, with 1/2 of the dose in the peripheral vein and 1/2 of the dose in the right hepatic artery. |
| NCT01062750 | Cirrhosis | Autologous adipose tissue-derived stromal cells | Not applicable | 4 participants | Completed | The cells were administered via the hepatic artery. Cell dosage not informed. |
| NCT00956891 | Liver failure due to hepatitis B | Autologous BM-MSCs | Not applicable | 158 participants | Completed | The cells were administered via the hepatic artery. Cell dosage not informed. |
| NCT05517317 | Liver cirrhosis due to biliary atresia | Autologous BM-MNCs | Phase 1 | 12 participants | Completed | The cells were administered via the hepatic artery. Cell dosage not informed. |
2. Mechanisms for Nanoparticles Tissue Targeting
It is possible to analyze if administered nanoparticles reached the intended target tissue. In the case of tumors, the accumulation may occur due to a vascular phenomenon named enhanced permeability and retention effect (EPR) [17][93]. As most solid tumors receive a greater blood supply due to neovascularization, the nanostructures, macromolecules, and nutrients, tend to be better retained [18][94]. Moreover, some requisites should be observed, considering that intravenous injection may lead to blood clot formation and immune activation. The aggregation capacity, half-life in blood, size less than 400 nm, hydrophobicity, and surface charge should be determined to avoid plasma proteins adsorption and other potential side effects [19][95]. This approach’s efficiency also depends on the stage and type of tumor and local vascularization [20][96]. The conjugation of nanoparticles with selected molecules can increase therapeutic results; these include antibodies, bioactive peptides, growth factors, and several others. The nanostructures can also be combined with components that target the particles to specific tissues, improve the solubility and availability, minimize surface energy, and more [21][22][80,81]. An inherent advantage of associating multiple ligands and therapeutic components is producing specific nanoparticles for certain diseases [23][82]. Another mechanism for nanoparticle tissue targeting is based on magnetic forces, and this approach can also be used to reduce drug doses and side effects [24][97]. The main magnetic materials in biomedicine are metal oxides such as magnetite (Fe3O4) and ferrites, including CoFe2O4 NiFe2O4. Since iron is vulnerable to corrosion and rust in water, a non-porous coating is essential, and iron alloys such as FePt and FeAu are frequently used [25][98]. It was published that synthesized magnetite nanoparticles of about 80 nm are good vehicles for biomedical applications. Their therapeutic potential was tested in vitro using the hepatocellular carcinoma cell line HepG2 [26][99] at different concentrations, and they induced cellular proliferation and production of ROS in a dose- and time-dependent matter. Although magnetite nanoparticles have no therapeutic potential themselves, they can be subjected to a magnetic field for biological effects. When a magnetic force was applied at twenty-four and seventy-two hours, the mitochondrial activity increased, a phenomenon not observed without the magnetic field. According to the authors, this can be explained by the ability of cancer cells to regulate the gene expression of proteins involved in iron absorption and the regulation of tumor signaling pathways such as a hypoxia-inducible factor (HIF) [26][99]. SPIO magnetic nanoparticles are increasingly being used in biotechnology and biomedicine, mainly for being a contrast agent in MRI. Moreover, due to its biocompatibility, when SPIO interacts with cells, it provides a magnetic field with oscillations, consequently inducing a phase shift of protons, which facilitates their detection by MRI [27][101]. Despite its biocompatibility, SPIO has an aggregatory nature, requiring modifications on its surface to minimize, for example, interactions with plasma proteins and elimination by resident tissue macrophages, which can lead to inflammatory processes, thrombosis, and anaphylaxis [28][102]. In the literature, it was reported that coating SPIO with poly(ethylene glycol) gives the nanoparticle a greater hydrophilicity and minimizes nonspecific interactions with other biomolecules. However, factors such as the hydrodynamic size of the coated nanoparticles and the nature of the crosslinking agent will directly affect the half-life in blood and iron absorption by macrophages [29][103]. Another class of polymers used was monosaccharides, which aim to provide the nanoparticle with nonspecific adsorption prevention, specific targeting, and cell internalization. This is possible because carbohydrates attached to the surface can connect to cell membrane receptors and direct nanoparticles to subcellular compartments [30][104]. However, many studies must be directed to understand the interaction between SPIO and the extracellular and intracellular environments. Regarding the mechanisms that lead to magnetic nanoparticle degradation, it is known that insoluble ferric ions (Fe3+) bind to free transferrin in the blood. Then, this protein transports the ion and mediates the binding to the cell membrane transferrin receptor (TFRC). Intracellularly, Fe3+ is converted to a ferrous ion (Fe2+) under acidic conditions by the enzyme metalloreductase (STEAP3). Once converted, Fe2+ can be stored in ferritin or oxidized and exported to the extracellular environment by ferroportin (FPN) [31][108]. With an excess of Fe2+ in the intracellular medium, it can be used as a catalyst to convert hydrogen peroxide (H2O2) into hydroxyl radicals (OH), a redox process known as the Fenton reaction [32][109]. In turn, free OH can interact with polyunsaturated fatty acids of the lipid membrane and induce lipid peroxidation [33][110]. Under normal conditions, formed lipid hydroperoxides can be neutralized by the enzyme glutathione peroxidase 4 (GPX4), a selenoprotein that uses free glutathione (GSH) in the cell to reduce lipid hydroperoxides into their respective alcohols. Thus, this mechanism protects the cells against oxidative stress [34][111]. However, under stress conditions, excess of intracellular free iron can increase phospholipid oxidation, leading to the degradation of plasma membrane phospholipids and consequently favoring oxidative cell death, a process known as ferroptosis. Some factors contribute to ferroptosis-mediated cell death, such as the inhibition of the cystine-glutamate (Xc-), an anti transport system by erastin, directly affecting intracellular GSH replacement [35][112]. Another factor is the binding of the RSL3 molecule to GPX4, inhibiting its catalytic activity. The enzyme inactivation causes the accumulation of lipid peroxides, resulting in increased ROS [36][113]. Thus, ferroptosis induction by free iron excess is a major concern in applying magnetic nanoparticles such as magnetite and maghemite (Fe2O4). It was seen that magnetic nanoparticles loaded with sulfasalazine (SAS) were “camouflaged” in the platelet membrane (PLT), forming a Fe3O4-SAS@PLT complex with a hydrodynamic size of 268 nm. When used in in vitro experiments, an increase in the levels of ROS and lipid peroxides, depletion of GSH and the XcT system was observed, factors that indicated that the ferroptosis pathway was activated. In in vivo models, the authors observed that GPX4 expression in mice was reduced after treatment with Fe3O4-SAS@PLT, indicating that ferroptosis may be involved in controlling metastatic tumors. The strategy, in this case, was to combine ferroptosis with immunotherapy to eliminate metastatic cells and increase treated animals’ survival [37][114]. A recent study produced gelatin microspheres loaded with magnetic nanoparticles and the drug adriamycin (ADM) to treat hepatocellular carcinoma in a combined therapy, using radiofrequency hyperthermia and chemotherapy. When analyzing the participation of ferroptosis, the data indicate that in the presence of ferroptosis inhibitors with ADM/Fe3O4-MS, the viability was greater when compared to the group without inhibitors. In in vivo models, a combined therapy also reduced liver tumor mass, demonstrating antitumor efficiency compared to untreated groups [38][115]. Magnetic nanoparticles were used not only to induce cell death by ferroptosis, but also to detect tumor cells [39][117]. This is particularly relevant because, currently, the most common method used to detect tumor cells is based on biopsies, invasive procedures for histopathological analysis [40][118]. By using magnetic nanoparticles, it is possible to detect tumor cells less invasively by MRI. Thus, nanoparticle formulations made of Fe3O4 were modified with dimercaptosuccinic (DMSA) and coated with gold, forming Fe3O4-Au with a particle size of 28 nm. When tested on gastric carcinoma cells, the nanoparticles demonstrated low cytotoxicity. The attenuation capacity of the analyzed X-rays showed an increase in intensity depending on the concentration of nanoparticles. Another factor that contributed to high radiographic attenuation was the ultrafine structure of the nanoparticles, enabling increased contrast by computed tomography (CT). When tested in an in vivo model, these nanoparticles predominantly accumulated in the liver after 45 min of injection, which was demonstrated by the darkening of the liver tissue when analyzed by CT and confirmed by MRI. These results were validated by applying the methodology to mice affected by non-alcoholic fatty liver disease. In this case, the CT image better showed the accumulation of nanoparticles in the entire liver tissue, different from what was observed in the untreated group, indicating a high uptake of magnetic nanoparticles. Dual modality contrast CT/MRI performed to detect orthotopic liver cancer was facilitated after nanoparticles administration, showing the border of liver lesions. These lesions were confirmed by pathological analysis that showed nuclear pleomorphism and hypercholemasia, multinucleation of hepatocytes, and infiltration of tumor cells. Although the authors were concerned with in vivo cytotoxicity, histological studies confirmed nanoparticles’ presence in the liver and spleen after 24 h of treatment, with low infiltration of immune cells. In the long term, tissues collected for analysis did not show acute injuries or post-administration chronic inflammation [41][119]. Another study aimed at treating liver cancer using formulated magnetic nanoparticles loaded with doxorubicin (DOX) and functionalized with poly(ethylene oxide)-trimellitic chloride-folate anhydride (PEO-TMA-FA). This antitumor nanoplatform treatment was performed in rabbits with xenografts of VX2 liver tumors, a metastatic tumor, and a tumor reduction was morphologically and macroscopically observed. Moreover, fluorescence analyses indicated that this high potency inhibited tumor proliferation and angiogenesis [42][120]. In conclusion, most studies using magnetic nanoparticles direct efforts for application in oncology due to their extrinsic characteristics, such as magnetic field targeting [43][121] and elimination of tumor cells by hyperthermic therapy [44][45][122,123] or by ferroptosis [46][124]. However, for the regeneration of injured tissues, one possibility would be to produce magnetic microcapsules or nanocapsules loaded with molecules that could help in the recovery of damaged tissue, such as: targeting EGFs and HGFs to be released locally and help with remodelling the hepatic matrix [47][48][25,125]. Another possibility would be the use of liver cells or stem cells as nanoparticle carriers. However, a delicate point to be analyzed would be the ideal concentration of magnetic nanoparticles in the polymeric matrix that would respond to the magnetic field, since the concentration of ferrous ions could lead to cell death or even impair tissue recovery. Thus, there are countless possibilities to be studied and many doors to be opened in this field.3. Applications of Stem Cells in Nanomedicine
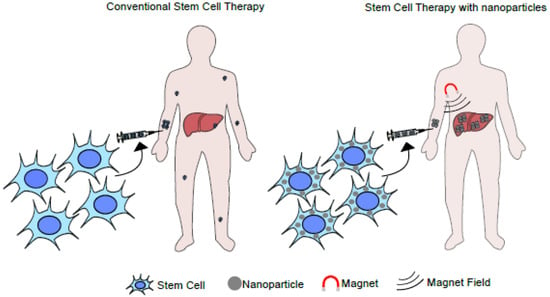
Given promising results, a new area of study is emerging in regenerative medicine and tissue engineering, known as “stem cell nanotechnology”. In this field, nanotechnology improves the efficiency of stem cells’ therapeutic action, promoting healing and tissue repair [49][126]. A study published in 2016 used bioactive glasses and polymers as scaffold nanomaterials to deliver stem cells in the bone tissue for regeneration. This strategy was used because bioactive glasses have high osteoconductivity and biocompatibility and can interconnect with bone tissue. Moreover, gelatin was used as a porous material since it is commonly used to structure the ECM. The study aimed to compare the effect of different stem cells (BM-MSCs, UC-MSCs, and AD-MSCs) on bone regeneration using nanocomposite scaffolds made of bioactive glass and gelatin. The study showed that the scaffolds used were not cytotoxic and suitable for cell development and expansion. To analyze the regenerative capacity of this bioconstruct, an injury was made in the calvaria area of the skull in male Wistar rats after four to twelve weeks of implantation. Bone regeneration depended on the implantation time, and tissue healing was better in rats that received the implant. They also observed that BM-MSCs cells had more potential for differentiation and bone consolidation when compared to the other cell types. On the other hand, UC-MSCs had a greater capacity for angiogenesis [50][127]. MRI showed that rat MSCs labeled with Fe3O4 migrated to the hepatic fibrotic tissue after one hour in a liver fibrosis model [51][132]. Another study reported that stem cells labeled with a magnetic nanomaterial were retained in the liver after intrahepatic administration. However, both studies reported the loss of MRI signal after seven days, indicating that the cells died, migrated to other tissues, or were endocytosed by Kupffer cells [52][133]. Another therapeutic possibility is the application of stem cell membranes to coat nanoparticles. This study used membrane-coated nanoparticles containing the DOX drug for colon cancer treatment. The nanostructures had preserved membrane proteins and good stability over fourteen days in vitro, and the diameter was compatible with renal excretion. The drug release kinetic was evaluated at different pHs, and it was observed that 60% of the drug was fully released within 36 h at pH 5, unlike pH 7.5, when only 20% of the drug was released. Apparently, the membrane helped to control the drug release, and the cytotoxicity of the nanostructure-associated drug was lower than the drug diluted in a medium. Finally, the in vivo experiments using mice showed a significant reduction in tumor growth [53][134]. Multiple advantages are associated with this technology, such as phenotype conservation, ease of in vitro expansion to obtain stem cell membranes, and a high potential for intrinsic migration to regions of high inflammation, which facilitates specific drug delivery [54][135]. Moreover, it has low immunogenicity, increased time for blood clearance due to its “camouflage”, low neurotoxicity, and the capacity to interact with receptors and molecules on other cells in the tissues [55][136]. Another promising possibility is using stem cells loaded with magnetic nanoparticles to treat acute or chronic liver diseases, such as cirrhosis, ALF, hepatitis, alcoholic or non-alcoholic liver diseases, and hereditary liver diseases. The central idea would be to administer modified stem cells and subject the individual to a magnetic field to concentrate the loaded cells in a specific anatomic location (Figure 13).