Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Neil Coville and Version 2 by Camila Xu.
Carbon allotropes are multifunctional materials, due to their unique physical and chemical properties. Carbon allotropes can be chemically modified by other elements via functionalization or doping, and they can also be used in combination with other materials to form carbon–carbon or metal–carbon composite materials.
- carbon dots
- thermal stability
- metal support
1. Introduction
Carbon allotropes are multifunctional materials, due to their unique physical and chemical properties. Carbon allotropes can be chemically modified by other elements via functionalization or doping, and they can also be used in combination with other materials to form carbon–carbon or metal–carbon composite materials [1]. The modification of carbon allotropes helps to enhance their properties, and also widens their spectrum of applications. Hence, carbon allotropes such as graphite, graphene, fullerene, carbon nanotubes (CNTs), carbon nanofibers (CNFs), carbon black (CB), carbon nano-onions (CNOs), carbon spheres (CSs) and carbon dots (CDs) have been successfully incorporated in fields such as nanomedicine, electronics, sensor fabrication and catalysis [1][2][3][4][1,2,3,4].
Carbon’s many allotropes have shown great potential when used as a catalyst support, and the carbon can even act as a catalyst in its own right [1][5][1,5]. The enormous interest in carbon-based support materials is due to their surface chemistry, variable surface area and the porosity of the carbon [1][5][1,5]. The use of carbon allotropes is also enhanced by their electronic properties, which are influenced by their structure and the carbon atom valence. The electronic effects can promote a high dispersion of the supported (metal) catalyst, and also of surface defects, and this can enhance their capability for gas storage, adsorption and/or separation processes [5][6][5,6].
Heterogeneous metal catalysts usually comprise a metallic catalyst that is anchored or supported on the surface of a material that serves to enhance the surface area of the metal while also improving the metal stability during chemical reactions [7]. Support materials are important for immobilizing and anchoring the active metal catalyst, improving its dispersion and durability, and aiding it in avoiding deactivation during a catalytic reaction [8]. Many studies have shown that a catalytic reaction rate is influenced by the catalyst dispersibility [9]. Metal–support interactions (MSI) are an important factor to consider when selecting a catalyst support, as the support directly influences the dispersibility, sintering, reducibility, and overall performance of the catalyst. MSIs are effects measured by the physical and chemical interactions between the metal catalyst and the support [10][11][12][10,11,12]. The stronger the MSI, the more resistant the catalyst is to deactivation via sintering during a reaction [12][13][12,13]. Carbon, in its various allotropes, generally forms a weak MSI with a metal catalyst. However, the MSI can be improved by means of functionalizing or doping of the carbon surfaces with heteroatoms. These effects create defects in the carbon nanostructure, and also alter the electronic properties of the carbon allotrope. Consequently, the dispersibility of the metal catalyst is increased and metal sintering/deactivation is reduced by doping/functionalization [12].
Most carbon allotropes are relatively ‘stable’ under harsh reaction conditions; this makes them less susceptible to structural and/or chemical changes during catalysis [12]. This is important, because the structural disintegration of a catalyst support can lead to deactivation of a catalyst. The properties of carbon supports have been studied under different conditions using varying pH, solvents, temperatures, time, etc. For example, carbon allotropes have successfully been used as supports for titania in photocatalysis [14], platinum in electrocatalysis [15], and cobalt, iron and ruthenium in catalysis for fuel production under a wide range of reaction conditions [12].
Carbon dots (CDs) are a fairly new type of carbon nanomaterial. CDs are defined as zero-dimensional quasi-spherical carbon nanomaterials with particle sizes below 10 nm [16]. Their general structure consists of sp2- and sp3-type carbons, with a large number of functional groups or polymer chains attached to their surfaces [17][18][19][17,18,19]. Numerous reviews have summarized the potential of CDs in many traditional and emerging areas, such as photoluminescence (PL) and photoelectrochemical-driven sensing, catalysis, imaging, and biomedicine applications, where they have been shown to be superior to other carbon allotropes [16][20][16,20]. Further, CDs, unlike metal-based quantum dots, have a high water dispersibility, low toxicity, and good biocompatibility [21]. Thousands of papers have been written about CDs in terms of their synthesis, characterization and uses.
CDs, like many carbon allotropes, have been used as both catalysts and catalyst ‘supports,’ and reviews on the use of CDs both as carbocatalysts and as composites with metal and metal oxide supports have been reported [22][23][24][22,23,24]. As is known, there are many advantages to using CDs and CD/metal complexes in catalysis, but there are also limitations on the use of these carbons. For example, in many of the studies it is not clear if the integrity of the carbon has been retained, and the degree to which metal sintering has taken place during their synthesis or in their catalytic reactions. Indeed, some studies have revealed that CDs and CD/metal materials, lose their shape/size during reaction, especially at high temperatures (>100 °C) and in the presence of easy-to-reduce metals. This has been observed in carbon–carbon coupling reactions [25], hydrogen evolution reactions [26] and hydrogenation reactions, where the CDs either decomposed or were transformed into other carbon structures [27][28][27,28].
2. The Structure of Carbon Dots (CDs)
2.1. Types of CDs
CDs are usually divided into four distinct subgroups. These are graphene quantum dots (GQDs), carbon quantum dots (CQDs), carbon nanodots (CNDs) and carbonized polymer dots (CPDs) [29][30][31][32][29,30,31,32]. In the literature, these four types are usually called carbon dots (CDs), and generally they are all regarded as being similar in their catalytic (and many other) properties. However, this may not always be true. To date, this issue has not been explored, and there is evidence to suggest that the different CDs will react differently as a function of temperature and when in the presence of metals that can aid their decomposition [27]. CDs must be differentiated from carbon nano-onions (CNOs) and onion-like nanocarbons (OLNCs), which are also in the nanometer range [33][34]. CNOs and OLNCs have structures with carbon layers similar to those found in a concentric-like onion structure (see Figure 12), while CDs tend to have the carbon layers arranged as parallel graphene sheets. The CNOs/OLNCs are prepared at high temperatures (>500 °C), while CDs are typically prepared at T < 200 °C. This results in differences in their thermal stabilities that impact on their chemical properties.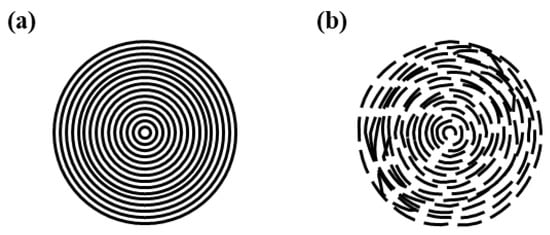
Figure 12. Graphical representation showing the differences between (a) a carbon nano-onion (CNOs), and (b) an onion-like nanocarbon (OLCN).
2.2. Surface Properties of CDs
CDs have many useful properties associated with their small size and functional groups. While the surface of the CD is important in correlating with their physical and chemical properties, the role of the core is still poorly understood [35][36]. Thus, most studies on the use of CDs that have been performed have related to their surface chemistry [36][37]. The surface of a CD contains many functional groups, and these groups are responsible for the catalytic activity of the CD. CDs are typically synthesized with both oxidizing and reducing groups that are used to bring about organic redox transformation reactions. These functional groups are also used to modify their PL spectra. Their surface chemistry can be modified by classical procedures associated with modifying any carbon surface. The surface modification can be achieved by covalent and non-covalent bonding of reactants with the CD surface, as reported in work by Yan et al. [37][38]. This type of modification is carried out to improve the properties of the CDs for a specific application such as biosensing, or metal and molecule detection [37][38]. The surface charge on the CD can be modified by changing the functional groups. The starting precursors can be manipulated in order to obtain CDs that are either hydrophilic or hydrophobic [38][39]. Typically, CDs are synthesized using multifunctional organic chemicals, and this produces hydrophilic CDs. Similarly, hydrophobic CDs can be produced using aliphatic chemicals such as dodecylamine [38][39][39,40]. It is further noted that the surface charge on the CD can be modified by changing the functional groups, and this can be done without post modification of the CDs [40][41]. Thus, CDs with different surface charges [40][41][41,42] and polarities [39][42][40,43] have been reported. For example, most CDs are made with a negative charge (associated with COO− groups). An important approach to generate positively charged CDs is by modifying CD surfaces with ionic liquids (ILs) and then annealing the material at ca. 240 °C. The IL-covered CDs were then used to detect metal ions in solution [40][41] or to make inks [41][42]. CDs can also react with themselves. For example, CD–CD linkages have been achieved from CDs that were made from C60 fullerene (by a base reaction). The CDs self-assembled when they were freeze dried [43][44]. After annealing (600–800 °C), the materials were used in capacitor studies. The self-assembly was proposed to be achieved with ice crystals acting as a template [43][44]. Studies have also been carried out in which a chemical reaction between two different CDs produced CD assemblies with surface properties associated with the different CDs used. Zhou et al. made three different types of CDs, and under room temperature conditions and with different CD combinations, they were linked together via the functional groups on the CDs. In one instance, the CDs, after reaction with each other, gave nanostructures that were used as drug nanocarriers [44][45][45,46]. Many other examples have also been reported [27]. Self-assembly of CDs has been achieved by the use of tannic acid. Modification of the functional groups on the CDs with ionic liquids provided for a good interaction of the CDs, with the negative charges on the tannic acid allowing for formation of assembled species (in some cases, dCD > 100 nm), which were readily detected by Tyndall cone measurements and transmission electron microscopy (TEM) measurements [46][47]. Supramolecular organization of CDs after alkylation of amine-functionalized CDs [38][39] has led to a series of alkyl-functionalized CDs, which could be separated by chromatography and that formed an organized structure in the solid state. The new assemblies were used in nonlinear optical studies [39][40]. A recent extension of this work described the self-assembly of CDs made from chiral cholesteryl to make thermotropic liquid crystals with a range of architectures [42][43]. These self-assembled CDs could provide an entry into novel structures for use as both carbocatalysts and to make CD-metal catalysts. A key property associated with a CD surface is its hydrophilicity, which has led to the extensive use of CDs in medicinal chemistry [47][48]. Another important property is their photoluminescence (PL), which has allowed them to be used in sensing devices [48][49]. Typically, addition of a metal reactant to the CD functional groups results in a decrease in their PL spectrum, and hence these types of experiments are usually conducted to detect metal ions within various media [49][50]. The changes in the PL spectra of CDs will be influenced, in a catalytic reaction, by the varying concentrations of products and reactants that could bind to the CD surface, thus allowing for the exploration of a reaction mechanism. The addition of polymers to a CD surface has been found to lead to improved PL properties [29]. Due to their small size (<10 nm), the separation and purification of CDs is not simple. Further, yields of the purified CDs are not always reported, and it is thus difficult to assess the usefulness of many synthetic strategies. While CDs have a small size, their surface area tends to vary, and can be lower than expected. The surface area can vary, for example, between 16.4 m2 g−1 [50][51] and 1690 m2 g−1 [51][52].Thus, interaction with a metal ion or particle will be limited by this property. However, the many surface groups can be used to reduce metal ions to metal particles and in so doing lead to the formation of small metal particles, by limiting the site of the reduction. As expected, when CD surfaces, as in all carbons, are doped with N atoms, metal particle agglomeration is reduced [52][53]. The role of carbons as supports is limited by their reactions under oxygen, hydrogen or inert gases. In the presence of oxygen, most carbons will oxidize (to CO, CO2) below 600 °C [53][54][54,55], while under H2, the carbon can react to form CH4, typically at temperatures above 500 °C [55][56]. Under an inert atmosphere, surface groups on the carbon can be removed at temperatures dependent on the carbon-to-element bond. In the absence of a catalyst, and under an inert atmosphere, the carbon core can be stable to temperatures above 600 °C. CDs, because of their size, have a high surface-to-bulk (core) carbon atom ratio [56][57]. Thus, all the reactions listed above can be expected to be modified when CDs are used, in relation to reactions with larger carbon molecules.2.3. Synthesis of Carbon Dots and Their Application as Reducing Agents
CDs can be synthesized by “top-down” procedures, by cutting down larger carbon allotropes such as graphene, fullerene and CNTs using strong oxidizing agents like sulphuric and nitric acid [57][58]. The CDs can also be prepared by the “bottom-up” process, and are generally made from precursors that contain functional groups that are typically retained from their synthesis precursors [29]. For example, CDs can be prepared from highly oxygenated starting materials such as ascorbic acid, sucrose, and citric acid [58][59][59,60], using a “bottom-up” synthesis approach (Figure 24). Further functionalization using a variety of methods can be carried out to advance the surface chemistry and other properties of the CDs [29][37][29,38]. The CD surface groups affect their overall chemical behavior, such as their electronic properties. These electronic properties have been exploited for oxidizing and reducing metals, and in this way generate CD/metal catalysts [24][60][24,61].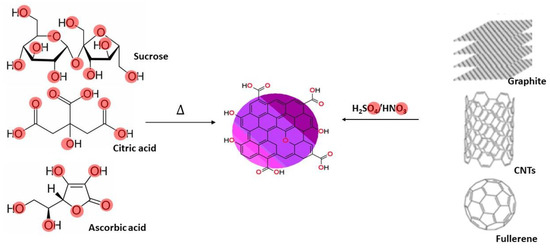
Figure 24. Schematic presentation of the “bottom-up” and “top-down” CDs synthesis procedures from oxygen-rich starting materials (highlighted in red) or by reacting ‘large’ carbon allotropes with oxidizing acids.
