Los estudios iIn vitro eand in vivo han demostrado lastudies have demonstrated the bioactividad de laty of rutina, un, a dietary flavonol dietético que se encuentra naturalmente en varias especies de plantas. A pesar del conocimiento generalizado de sunaturally found in several plant species. Despite widespread knowledge of its numerososus health beneficios para la salud, tales como efectos antiinflamatoriosts, such as anti-inflammatory, antidiabéticosetic, hepatoprotectores yive and cardiovasculares, el uso effects, industrial de lause of rutina aún es is still limitado debido a su bajaed due to its low solubilidad en medios acuosos, el sabor amargo y ty in aqueous media, the characteristic bitter and astringente característico de los compuestos fenólicos y su sus taste of phenolic compounds and its susceptibilidad a la ty to degradación durante el procesamiento. Paration during processing. To expandir sus aplicaciones y its applications and preservar su actividad biológica, se han desarrollado nuevos sistemas de encapsulacióne its biological activity, novel encapsulation systems have been developed.
- antioxidants
- extraction
- green methodologies
- biological activity
- nanoencapsulation
1. Introducción
2. Structure and Physicochemical Properties of Rutin Flavonol
Physicochemically, rutin has a molecular weight of 610.518 g/mol, pKa: 4.3 and log p value: −1.97 measured in acetonitrile at 50 °C. Rutin has poor water solubility in acidic and neutral environments but has greater solubility in an alkaline environment. This condition is due to the change in its electric charge and hydrophobicity (log D) with the change in pH [21] (Figure 1). Rutin has a strong negative charge and is highly hydrophilic under alkaline conditions, and is uncharged and slightly hydrophilic under acidic conditions [9]. The low solubility of rutin has limited its industrial applications. This condition is related to the ring structures, which are too large to be absorbed via a simple diffusion process [22].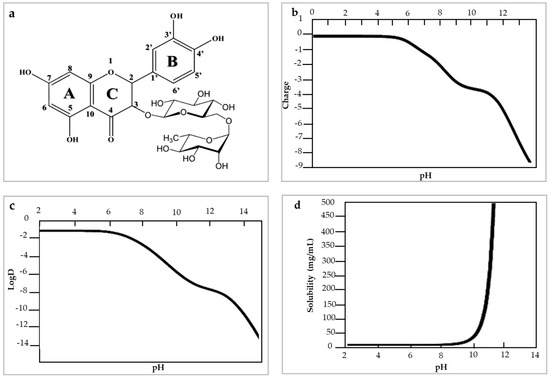
3. Sources of Rutin Obtained in Family Farming Products
Fruits and vegetables are a fundamental component of the regular diet and are among the most commonly consumed products worldwide. They are generally affordable and represent traditional agricultural practices within communities and families [25]. Although global initiatives such as the declaration of 2013 as the international year of Quinoa have highlighted the health benefits associated with the consumption of certain crops, many other fruit and vegetable crops have yet to receive comparable attention in terms of global consciousness [26]. Many consumers are unaware of the presence of bioactive compounds in fruits and vegetables. While nutritional components such as proteins and carbohydrates often receive the most attention, the added value of phytochemicals and other bioactive compounds in promoting health is frequently overlooked. It is crucial to disseminate information about the beneficial properties of these compounds to raise awareness and promote the consumption of fruits and vegetables as staples of a balanced diet. By means of identifying rich sources of phytonutrients and exploring appropriate extraction methods for these compounds, the production, marketing and consumption of horticultural products can be improved to benefit all sectors of the production chain. Antioxidants such as rutin flavonol are found in fruit and vegetable products of plant species including: Polygonaceae, Solanaceae, Capparaceae, Amaranthaceae, Asteraceae, Celastraceae, Asparagaceae, Chenopodiaceae, Lamiaceae and Rosaceae [27,28,29,30][27][28][29][30]. In addition, the search for sustainable sources of rutin extraction allowed the study of raw materials generated from agro-industrial waste. Rutin has been found in stems and calyx of fruits; these have been found after the harvest of varieties such as Physalis peruviana, some of the genus Fagopyrum and in banana leaves [31,32,33][31][32][33]. Leafy vegetables like lettuce are regularly consumed in salads and are a source of rutin. A rutin content of 750.82 µg/g has been reported in lettuce leaves, which varied in phenolic compounds depending on the season of cultivation, with winter-grown lettuce showing the highest rutin content [34]. Different parts of the lettuce plant have been studied to quantify rutin. For instance, it was found that the roots of lettuce had a higher rutin content (172.09 μg/g) than the leaves in the vegetative stage [35]. Meanwhile, a rutin content of 128 µg/g was reported during the harvest stage [36]. The content of rutin in species of the Brassicaceae family, such as broccoli, has been investigated to promote their consumption. In this case, rutin was quantified in broccoli stored in bulk and a concentration of 102.14 µg/g was found [37]. The authors linked the presence of antioxidant compounds in broccoli with health benefits, such as the prevention of degenerative diseases. Within the same Brassicaceae family, cauliflower sprouts were studied to quantify rutin, and the authors reported concentrations of 300 µg/g, a concentration close to the daily recommended intake of this type of compound [38]. Fruits and vegetables that belong to the Cucurbitaceae family, namely watermelon (Citrullus lanatus L.), pumpkin (Cucurbita maxima L.), cucumber (Cucumis sativus L.) and melon (Cucumis melo L.), are globally significant crops often utilized as salad ingredients, juice bases, desserts and in other culinary preparations. Additionally, melon has been traditionally used to treat liver inflammation, coughs and kidney disorders, such as urinary tract ulcers; it has also been indicated as a source of rutin [39]. The Chenopodiaceae family includes several significant tubers among fruit and vegetable crops, with beets being an example. In the culinary industry, beets are recognized for their striking purple–red hue and are incorporated into salads, entrees such as chips and purees and others. Additionally, beets are a rich source of nutrients that include complex B and C vitamins, minerals, fiber, protein and bioactive phenolic compounds such as betalains. Flavonoids such as rutin, kaempferol, rhamnetin and astragalin are among the most significant found in beets [40]. Pharmacologically, beetroot has been found to exhibit antioxidant, antimicrobial, anticancer, hypocholesterolemic and anti-inflammatory properties [41]. On the one hand, aromatic plants are often used in the culinary industry as seasonings and condiments to enhance the sensory properties of food. Additionally, these plants have been cultivated by humans since ancient times and are widely used in the pharmaceutical and agricultural industries as a source for treating a range of disorders. Aromatic plants contain active compounds that make them useful for treating physical and mental ailments, such as having anti-inflammatory, anti-infectious and sedative properties, among many others. They are effective against influenza, gastrointestinal disorders, anxiety, seizures, rheumatic pain, muscle spasms, ulcerations and hemorrhoids, and function as antiseptics, disinfectants, bactericides and fungicides [42]. Las plantas aromáticas son conocidas por su capacidad de prosperar en una amplia gama de condiciones de crecimiento y son relativamente fáciles de cultivar. Además, desempeñan un papel natural en la protección contra plagas de los cultivos cercanos, lo que los convierte en una opción ideal para los enfoques de agricultura familiar. Por lo tanto, los huertos que cuentan con una amplia gama de plantas aromáticas son comúnmente promovidos [ 43 ][43]. Varios estudios han reportado la extracción y cuantificación de rutina en varias especies aromáticas. Por ejemplo, los investigadores extrajeron la rutina de la caléndula utilizando una técnica asistida por ultrasonido y reportaron un porcentaje de rendimiento del 2,28 % ( p / p ) [ 44 ][44]. En un estudio separado, se demostró un contenido de rutina del 8,9 % en el orégano mediante extracción hidroetanólica [ 45 ][45]. Además, se encontró que la albahaca contenía una concentración de rutina de 15 mg/100 g, mientras que el cilantro exhibió una concentración significativamente mayor de 115 mg/100 g [46 ] [46]. En consecuencia, los métodos eficientes de extracción de rutina involucran el modelado y la optimización de variables operativas, además del uso de enzimas y solventes seguros que permitan la extracción selectiva. Debido a la estructura esquelética del flavonoide de rutina y los numerosos grupos hidroxilo, los compuestos próticos como el etanol, el glicerol o el 1,3 butanodiol se utilizan a menudo para extraer la rutina. El proceso de extracción se maneja a temperaturas que oscilan entre 30 y 70 °C, como se informó [ 47 ][47]. Las tecnologías alternativas como alta presión (fluidos supercríticos, líquido presurizado), ultrasonido, microondas y metodologías emergentes como NADES se informan en la investigación actual sobre la extracción de rutina.3. Encapsulación de rutina en sistemas coloidales y heterodispersos
Si bien las técnicas de encapsulación de rutina han arrojado resultados prometedores para preservar la actividad biológica de varios compuestos, las propiedades ligeramente lipófilas de la rutina la hacen adecuada para la encapsulación mediante técnicas a nanoescala basadas en sistemas coloidales y heterodispersos que incorporan lípidos o polímeros. Entre estas técnicas se encuentran los complejos de fosfolípidos, los fitosomas, los sistemas liposomales, las nanoemulsiones y las nanopartículas lipídicas y biopoliméricas, que se examinan en esta revisión [ 109 , 110 , 111 ][48][49][50]. Las estructuras de diseño con partículas de tamaño nanométrico asociadas a sistemas coloidales representan inherentemente un proceso de formación no espontáneo donde el cambio en la energía libre de Gibbs es positivo y, por lo tanto, se requiere la aplicación de energía externa para ajustar el tamaño de partícula y prolongar la estabilidad del sistema [ 112 ][51]. Además, la reducción de tamaño genera un aumento del área superficial que favorece la solubilidad de los compuestos lipofílicos sin descuidar los fenómenos de fuerza que se originan, como el movimiento browniano, que es más significativo que la gravedad y, en consecuencia, tendría una mayor estabilidad cinética debido a la una fuerza gravitacional más débil [ 112 ][51]. La estabilidad de los sistemas coloidales se define por el equilibrio de las fuerzas de atracción y repulsión y el impedimento estérico. De acuerdo con la teoría de Deryagin-Landau-Verwey-Overbeek (DLVO), la carga superficial debe mantenerse a una distancia de la neutralidad para mantener estable un sistema coloidal [ 113 ][52]. Los parámetros calculables como el índice de polidispersidad y el potencial Z se pueden utilizar para estimar la estabilidad en sistemas coloidales nanoestructurados mediante métodos electroforéticos y electroacústicos. Finalmente, el cálculo del potencial Z ayuda a estimar la carga superficial en función del movimiento electroforético de las partículas [ 114 ][53]. Es esencial seleccionar cuidadosamente los componentes del sistema para modular los valores del potencial Z. Esto implica identificar el tipo de surfactante a utilizar, incorporarlo a la interfaz de fase continua y seleccionar compuestos para adsorberse en la superficie estructural, como biopolímeros o compuestos químicos de interés para ser encapsulados [ 115 ] [54] .References
- FAO. Agricultura Familiar en América Latina y el Caribe 2015. Available online: www.fao.org/publications (accessed on 5 April 2023).
- FAO. Frutas y Hortalizas Oportunidades y Desafios Para la Agricultura Sostenible a Pequeña Escala. Frutas y Hortalizas; FAO-CIRAD: Rome, Italy, 2021.
- OMS. OMS 2008 Microbiological Hazards in Fresh Leafy Vegetables and Herbs Meeting Report; World Health Organization: Geneva, Switzerland, 2008.
- FAO (Food and Agriculture Organisation). Urban and Peri-Urban Agriculture Sourcebook; FAO: Rome, Italy, 2020.
- Gawlik-Dziki, U. Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. J. Funct. Foods 2012, 4, 872–882.
- Li, X.; Park, N.I.; Xu, H.; Woo, S.-H.; Park, C.H.; Park, S.U. Differential Expression of Flavonoid Biosynthesis Genes and Accumulation of Phenolic Compounds in Common Buckwheat (Fagopyrum esculentum). J. Agric. Food Chem. 2010, 58, 12176–12181.
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2016, 25, 149–164.
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312.
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079.
- Rauf, A.; Imran, M.; Patel, S.; Muzaffar, R.; Bawazeer, S.S. Rutin: Exploitation of the flavonol for health and homeostasis. Biomed. Pharmacother. 2017, 96, 1559–1561.
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Roles of rutin in cardiac remodeling. J. Funct. Foods 2020, 64, 103606.
- Gullón, B.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235.
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817.
- Chang, C.; Song, M.; Ma, M.; Song, J.; Cao, F.; Qin, Q. Preparation, Characterization and Molecular Dynamics Simulation of Rutin–Cyclodextrin Inclusion Complexes. Molecules 2023, 28, 955.
- Li, C.; Chen, L.; McClements, D.J.; Peng, X.; Qiu, C.; Long, J.; Ji, H.; Zhao, J.; Zhou, X.; Jin, Z. Preparation and Characterization of Rutin–Loaded Zein–Carboxymethyl Starch Nanoparticles. Foods 2022, 11, 2827.
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2020, 592, 120028.
- Remanan, M.K.; Zhu, F. Encapsulation of rutin in Pickering emulsions stabilized using octenyl succinic anhydride (OSA) modified quinoa, maize, and potato starch nanoparticles. Food Chem. 2023, 405, 134790.
- Amjadi, S.; Shahnaz, F.; Shokouhi, B.; Azarmi, Y.; Siahi-Shadbad, M.; Ghanbarzadeh, S.; Kouhsoltani, M.; Ebrahimi, A.; Hamishehkar, H. Nanophytosomes for enhancement of rutin efficacy in oral administration for diabetes treatment in streptozotocin-induced diabetic rats. Int. J. Pharm. 2021, 610, 121208.
- Sengupta, P.; Das, D.; Bhattacharya, S.; Sur, R.; Bose, A.; Sen, K. A pH-driven method for liposomal encapsulation of dietary flavonoid rutin: Sustained release and enhanced bioefficacy. Food Biosci. 2023, 52, 102392.
- Safarbalou, A.; Haghipanah, M.; Moradi-Kor, N.; Ramezani, E.; Hosseini, S.M.F.; Roudsari, S.S.T.; Sadat Afraz, E. Physicochemical properties of rutin loaded into nanoliposomes and its uses for the treatment of oral ulcers. Eurasian Chem. Commun. 2022, 4, 202–208.
- Huang, A.; McClements, D.J.; Luo, S.; Chen, T.; Ye, J.; Liu, C. Fabrication of rutin-protein complexes to form and stabilize bilayer emulsions: Impact of concentration and pretreatment. Food Hydrocoll. 2021, 122, 107056.
- Ravi, G.S.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-lipid Complex of Rutin: Development, Characterisation and In Vivo Investigation of Hepatoprotective, Antioxidant Activity and Bioavailability Study in Rats. AAPS PharmSciTech 2018, 19, 3631–3649.
- Dong, R.; Yu, B.; Yan, S.; Qiu, Z.; Lei, J.; Chen, C.; Li, Y.; Cao, B. Analysis of Vitamin P Content and Inheritance Models in Eggplant. Hortic. Plant J. 2020, 6, 240–246.
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT -Food Sci. Technol. 2008, 41, 1060–1066.
- Martin, C. The interface between plant metabolic engineering and human health. Curr. Opin. Biotechnol. 2013, 24, 344–353.
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622.
- Kim, D.S.; Lim, S.B. Subcritical water extraction of rutin from the aerial parts of common buckwheat. J. Supercrit. Fluids 2019, 152, 104561.
- Liao, J.; Qu, B.; Liu, D.; Zheng, N. New method to enhance the extraction yield of rutin from Sophora japonica using a novel ultrasonic extraction system by determining optimum ultrasonic frequency. Ultrason. Sonochemistry 2015, 27, 110–116.
- Moreira, G.C.; Dias, F.D.S. Mixture design and Doehlert matrix for optimization of the ultrasonic assisted extraction of caffeic acid, rutin, catechin and trans-cinnamic acid in Physalis angulata L. and determination by HPLC DAD. Microchem. J. 2018, 141, 247–252.
- Habtemariam, S.; Varghese, G.K. Extractability of Rutin in Herbal Tea Preparations of Moringa stenopetala Leaves. Beverages 2015, 1, 169–182.
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. A multi-analytical platform based on pressurized-liquid extraction, in vitro assays and liquid chromatography/gas chromatography coupled to high resolution mass spectrometry for food by-products valorisation. Part 2: Characterization of bioactive compound. J. Chromatogr. A 2019, 1584, 144–154.
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.-M.; Gil-Izquierdo, Á. Potential of Physalis peruviana calyces as a low-cost valuable resource of phytoprostanes and phenolic compounds. J. Sci. Food Agric. 2017, 99, 2194–2204.
- Toro, R.M.; Aragón, D.M.; Ospina, L.F.; Ramos, F.A.; Castellanos, L. Phytochemical analysis, antioxidant and anti-inflammatory activity of calyces from physalis peruviana. Nat. Prod. Commun. 2014, 9, 1573–1575.
- de Souza, A.S.N.; Schmidt, H.d.O.; Pagno, C.; Rodrigues, E.; da Silva, M.A.S.; Flôres, S.H.; Rios, A.d.O. Influence of cultivar and season on carotenoids and phenolic compounds from red lettuce influence of cultivar and season on lettuce. Food Res. Int. 2022, 155, 111110.
- Omer, H. Radiobiological effects and medical applications of non-ionizing radiation. Saudi J. Biol. Sci. 2021, 28, 5585–5592.
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red butterhead lettuce dictated by different developmental stages of harvest maturity. Antioxidants 2020, 9, 300.
- Upadhyay, R.; Sehwag, S.; Singh, S.P. Antioxidant Activity and Polyphenol Content of Brassica oleracea Varieties. Int. J. Veg. Sci. 2015, 22, 353–363.
- Gratacós-Cubarsí, M.; Ribas-Agustí, A.; García-Regueiro, J.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chem. 2010, 121, 257–263.
- Ezzat, S.M.; Raslan, M.; Salama, M.M.; Menze, E.T.; El Hawary, S.S. In vivo anti-inflammatory activity and UPLC-MS/MS profiling of the peels and pulps of Cucumis melo var. cantalupensis and Cucumis melo var. reticulatus. J. Ethnopharmacol. 2019, 237, 245–254.
- Lechner, J.F.; Stoner, G.D. Red beetroot and betalains as cancer chemopreventative agents. Molecules 2019, 24, 1602.
- Bangar, S.P.; Sharma, N.; Sanwal, N.; Lorenzo, J.M.; Sahu, J. Bioactive potential of beetroot (Beta vulgaris). Food Res. Int. 2022, 158, 111556.
- Tacherfiout, M.; Kherbachi, S.; Kheniche, M.; Mattonai, M.; Degano, I.; Ribechini, E.; Khettal, B. HPLC-DAD and HPLC-ESI-MS-MS profiles of hydroalcoholic extracts of Chamaemelum nobile and Mentha pulegium, and study of their antihemolytic activity against AAPH-induced hemolysis. S. Afr. J. Bot. 2022, 150, 678–690.
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–18.
- Martins, F.S.; da Conceição, E.C.; Bandeira, E.S.; Silva, J.; Costa, R.M.R. The effects of extraction method on recovery rutin from Calendula officinalis L. (Asteraceae). Pharmacogn. Mag. 2014, 10, S569–S573.
- Méabed, E.M.; El Sayed, N.M.; Abou-Sreea, A.I.; Roby, M.H. Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine 2018, 43, 158–163.
- Slimestad, R.; Fossen, T.; Brede, C. Flavonoids and other phenolics in herbs commonly used in Norwegian commercial kitchens. Food Chem. 2019, 309, 125678.
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2020, 1635, 461770.
- Bernardo, J.; Videira, R.A.; Valentão, P.; Veiga, F.; Andrade, P.B. Extraction of phospholipid-rich fractions from egg yolk and development of liposomes entrapping a dietary polyphenol with neuroactive potential. Food Chem. Toxicol. 2019, 133, 110749.
- Echeverry, S.M.; Valderrama, I.H.; Costa, G.M.; Ospina-Giraldo, L.F.; Aragón, D.M. Development and optimization of microparticles containing a hypoglycemic fraction of calyces from Physalis peruviana. J. Appl. Pharm. Sci. 2018, 8, 10–18.
- Dammak, I.; do Amaral Sobral, P.J. Formulation and Stability Characterization of Rutin-Loaded Oil-in-Water Emulsions. Food Bioprocess Technol. 2017, 10, 926–939.
- Kumar, H.; Kumar, V. Ultrasonication assisted formation and stability of water-in-oil nanoemulsions: Optimization and ternary diagram analysis. Ultrason. Sonochem. 2018, 49, 79–88.
- Cho, Y.-H.; McClements, D.J. Theoretical stability maps for guiding preparation of emulsions stabilized by protein−polysaccharide interfacial complexes. Langmuir 2009, 25, 6649–6657.
- Lane, L.A.; Qian, X.; Smith, A.M.; Nie, S. Physical chemistry of nanomedicine: Understanding the complex behaviors of nanoparticles in vivo. Annu. Rev. Phys. Chem. 2015, 66, 521–547.
- Zorzi, G.K.; Carvalho, E.L.S.; von Poser, G.L.; Teixeira, H.F. On the use of nanotechnology-based strategies for association of complex matrices from plant extracts. Rev. Bras. De Farm. 2015, 25, 426–436.
