Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Leonardo A. Sechi.
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs), including alpha-linolenic acid (ALA) and its derivatives eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are “essential” fatty acids mainly obtained from diet sources comprising plant oils, marine blue fish, and commercially available fish oil supplements.
- omega-3 polyunsaturated fatty acids (ω-3 PUFAs)
- hypertension
- linoleic acid (LA)
- alpha-linoleic acid (ALA)
- eicosapentaenoic acid (EPA)
1. Introduction
Arterial hypertension is the most frequent chronic disease worldwide [1,2][1][2], with a prevalence that reaches 35% of the adult population accounting for 9.4 million yearly deaths [3]. Hypertension is a major modifiable cardiovascular risk factor, increasing the risk of cardiovascular death and morbidity related to chronic invalidating disorders, including coronary, cerebrovascular, and peripheral vascular disease, heart failure, and chronic kidney disease [4]. Primary hypertension is a complex pathophysiological condition that is characterized at the peripheral vascular level by an imbalance between vasoconstriction and vasodilatation [5]. This imbalance arises from an intricate interplay of genetic and environmental factors that include lifestyle and dietary habits [6]. In this regard and due to their proven ability to reduce blood pressure (BP), lifestyle changes are highly recommended in all patients as the first step to treat hypertension [1,2][1][2]. Among lifestyle recommendations, dietary interventions play an essential role to the extent that caloric restriction contributes to weight loss and supplementation with specific food components affects BP regulation.
In the last three decades, omega-3 polyunsaturated fatty acids (ω-3 PUFAs) have gained great interest within the research community, since seminal studies [7,8,9,10][7][8][9][10] reported a surprisingly low incidence of cardiovascular disease in populations, such as Eskimos and Alaskan Natives, traditionally eating high amounts of ω-3 PUFA-rich fatty fish. Later, epidemiological, observational, interventional studies, comprehensive reviews, and meta-analyses were performed with the aim to define the true potential of ω-3 PUFAs for cardiovascular prevention. Moreover, in vitro and in vivo animal and human studies have paved the way for a better understanding of the cellular and molecular mechanisms of ω-3 PUFA-mediated vascular protection. These studies have clearly demonstrated that ω-3 PUFAs possess antioxidant, anti-inflammatory, antithrombotic, and endothelium protective properties [11]. In addition, dietary supplementation with high doses of ω-3 PUFAs modifies plasma lipids concentrations decreasing serum triglyceride and increasing HDL-cholesterol levels [12,13,14,15][12][13][14][15]. Evidence suggests that triglyceride-rich lipoproteins contribute to the “residual lipoprotein attributable risk” that was reported in high-risk patients treated with high-dose statins, despite reaching very low LDL-cholesterol levels [16,17,18,19][16][17][18][19]. Despite inconsistent results of early randomized controlled trials with triglyceride-lowering drugs, subgroup analyses of more recent studies [20,21,22][20][21][22] and the results of the REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl Intervention Trial) [23] showed a significant cardiovascular benefit of triglyceride-lowering, thus expanding the potential role of ω-3 PUFAs for clinical practice. As a result, national and international guidelines for cardiovascular prevention currently recommend regular consumption of ω-3 PUFAs as part of a healthy diet [24,25,26,27][24][25][26][27]. Moreover, ω-3 PUFA-deficient diets have been classified as the sixth most relevant dietary risk factor, accounting for 1.5 million deaths and 33 million disability-adjusted life years worldwide [28].
2. Biochemistry and Cellular Mechanisms of Polyunsaturated Fatty Acids (PUFAs)
2.1. General Biochemistry and Biologic Sources of PUFAs
The nomenclature of PUFAs is based on the number of carbons in the molecule (C) and the position of the first double bond relative to the terminal methyl carbon. Omega (ω) indicates the last methyl carbon as opposed to the carboxyl group of the acyl chain, and -6 or -3 indicates the position of the first double bond from the last methyl group [30][29]. PUFAs are categorized into essential and nonessential according to the ability of the human body to synthesize them de novo. Nonessential PUFAs are those of the ω-7 and ω-9 families since they can be synthesized directly from endogenous saturated fatty acids. Essential PUFAs include the ω-6 and ω-3 families, which cannot be synthesized de novo because mammals lack enzymes to build up double bonds in the fatty acid chain and must necessarily be obtained from the diet. The two parents essential PUFAs are linoleic acid (C18:2, LA) that is particularly abundant in vegetable oils derived from soybean, corn, sunflowers, and rapeseed and alpha-linoleic acid (C18:3, ALA) that is predominantly found in flaxseed, soybean, canola oils, pumpkin seeds and pumpkin seed oil, perilla seed oil, tofu, walnuts and walnut oil, camelina, hempseed, and some algae. Linoleic acid is the precursor of ω-6 PUFAs and is converted into arachidonic acid (C20:4, AA) through the action of elongases (which add carbons to the hydrocarbon chain of the fatty acid) and desaturases (which replace single bonds with double bonds). Alpha-linoleic acid (C18:3, ALA) is the precursor of long-chain ω-3 PUFAs through elongation and desaturation of its acyl chain, leading to the formation of eicosapentaenoic acid (C20:5, EPA) which in turn is converted into docosahexaenoic acid (C22:6, DHA) [31,32][30][31] (Figure 1).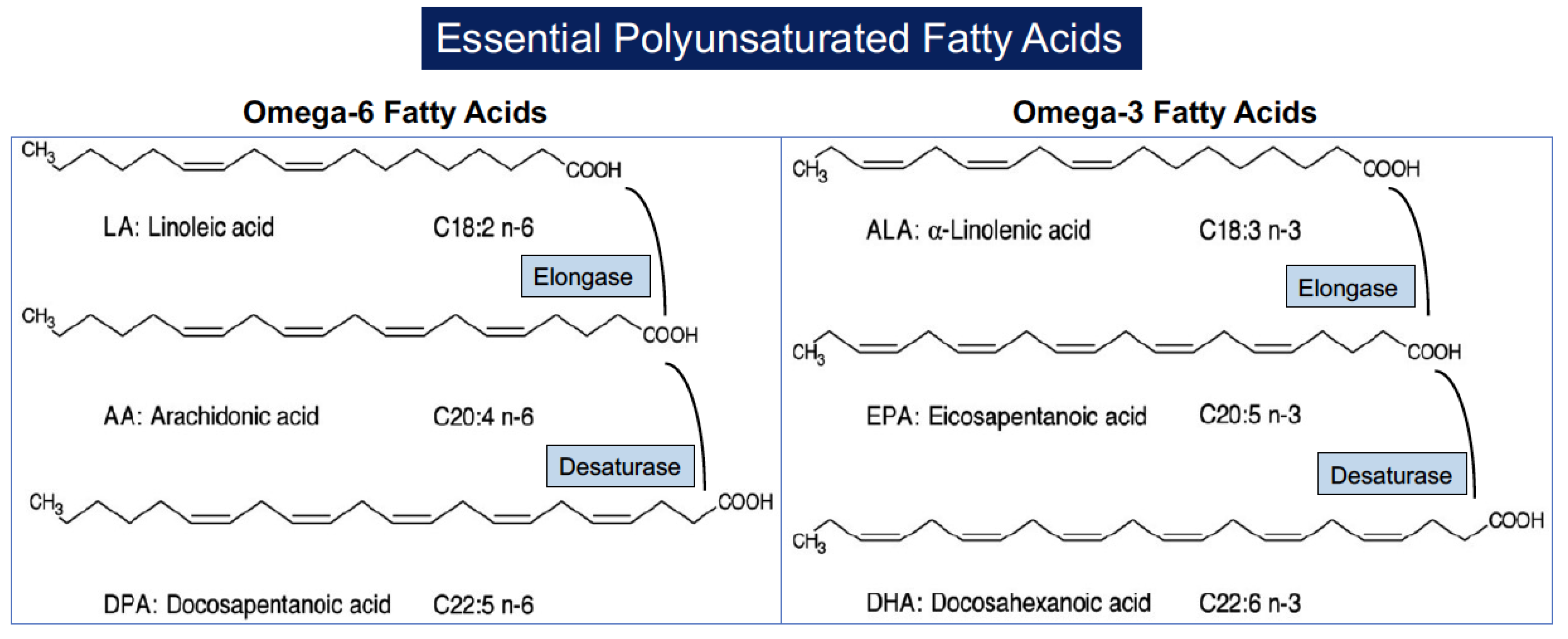
Figure 1. Structure of polyunsaturated fatty acids and their formation from parental molecules.
2.2. PUFAs and Cellular Membranes
As structural components of membrane phospholipids, PUFAs influence membrane properties and modulate cellular function. Fatty acids are quickly incorporated in phospholipids of plasma, platelets, neutrophils, and red blood cells, whereas enrichment of other tissues takes a longer time. EPA and DHA interact differently with cellular membranes [46,47,48][45][46][47]. Preclinical data show that EPA has a more stable interaction with surrounding saturated fatty acids, contributing to membrane stability and inhibiting lipid oxidation and cholesterol domain formation. DHA is in a curved shape causing conformational changes that increase membrane fluidity and form cholesterol domains with reduced antioxidant activity. These peculiarities may at least in part account for the differences in their clinical effects, with EPA seemingly more efficient in the mitigation of the atherosclerotic process. ω-3 PUFAs accumulate preferentially in the cerebral cortex, retina, testes, muscle, and liver, while ῳ-6 PUFAs are ubiquitous in all tissues. ω-3 PUFAs are incorporated into membrane phospholipids, where they usually represent less than 10% of the total amount of fatty acids (in a typical western diet: 10–20% AA, 0.5–1% EPA, and 2–4% DHA). Of note, the dietary intake of ω-3 PUFAs can modify the composition of cell membranes in a relatively short time (from days to weeks) [49,50,51][48][49][50]. Plasma membranes consist of a mosaic of functional microdomains that facilitate interactions between resident proteins and lipids [52,53][51][52]. The lipid content of cell membranes affects the function of cells and intracellular organelles. Incorporation of ω-3 PUFAs into these membranes modifies membrane fluidity and biophysics, size, and composition of these microdomains, namely, lipid rafts and caveolae, that modulate protein function and signaling events. Lipid rafts are small heterogeneous membrane microdomains rich in cholesterol, sphingolipids, and saturated acyl chain [53,54][52][53] that influence membrane fluidity, protein–protein interaction, ion channel kinetics, signaling processes, and membrane protein and receptor trafficking. Caveolae represent a specific subtype of lipid raft macrodomain that forms flask-shaped membrane invaginations rich in proteins that play a role in endocytosis and signal transduction, including the structural protein caveolin-1 and many signal transduction proteins [55][54]. ω-3 PUFAs have been shown to increase the size and to change the content of cholesterol and sphingolipids of lipid rafts because of their low polar affinity with these types of lipids. These changes by modulating signaling events such as activation of G-protein, endothelial nitric oxide synthase (eNOS), tumor necrosis factor alpha (TNF-alpha), adhesion molecules, and Toll-like receptors (TLR) can contribute to the anti-inflammatory and antiatherosclerotic properties of ω-3 PUFAs [56,57,58][55][56][57]. For example, enrichment of cellular membranes with ω-3 PUFAs disrupts dimerization and recruitment of toll-like receptor-4, which might contribute to anti-inflammatory effects by down-regulation of nuclear factor-kappa B (NF-ⱪB) activation. On the other hand, the incorporation of ω-3 PUFAs into lipid membranes might modulate a variety of ion channels [59][58]. These include certain voltage-gated (Kv) and inwardly rectifying (Kir) K+ channels, voltage-gated (Nav) and epithelial (ENaC) Na+ channels, L-type Ca2+ channels, hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, transient receptor potential (TRP) channels, various connexins, chloride channels, and P2X receptors [59][58].2.3. PUFA and Intracellular Signaling
Modulation of physicochemical properties of cellular and organelle membranes is not the only mechanism through which ω-3 PUFAs exert their physiological effects. ω-3 PUFAs directly interact with membrane channels and proteins. For example, direct modulation of ion channels or G-protein-coupled receptor 120 (GPR 120) might contribute to antiarrhythmic or anti-inflammatory effects, respectively [60,61,62][59][60][61]. GPR 120, highly expressed in human adipocytes and macrophages, has been identified in recent years as the receptor of ω-3 PUFAs [63,64][62][63] and has been consequently renamed “Free fatty acid receptor 4” (FFAR4). Binding and activation of FFAR4 by ω-3 PUFAs in macrophages and Kupffer cells trigger a downstream signaling cascade leading to the assembly of ῳ-3PUFA/FFAR4/β-arrestin-2 complex, which in turn dissociates the TAK1/TAB1 heterodimer by binding to and inactivating the TAB1 subunit, consequently reducing NF-κB-mediated cyclooxygenase expression and inflammation [62][61]. Interestingly, in a study of rodents and lipopolysaccharide-primed bone-marrow-derived macrophages, the ῳ-3PUFA/FFAR4/β-arrestin-2 complex has also demonstrated to inhibit NOD-like receptor protein 3 (NLRP3) inflammasome-dependent inflammation [65][64]. NLRP3 inflammasome has been identified in the last decade as a functional bridge between inflammation and atherosclerosis [66][65], and its downregulation results in decreased levels of interleukin-1β and decreased production of interleukin-6 release by macrophages and C-reactive protein by hepatocytes. Moreover, ω-3 PUFAs directly regulate gene expression via nuclear receptors and transcription factors, being the natural ligands of many key nuclear receptors in multiple tissues, including peroxisome proliferator-activated receptors (PPAR-alpha, -beta, -delta, and -gamma), hepatic nuclear factors (HNF-4; -alpha and -gamma), retinoid X receptors (RXR), and liver X receptors (alpha and beta) [67,68,69,70,71,72][66][67][68][69][70][71]. ω-3 PUFAs are transported into the nucleus by cytoplasmic lipid-binding proteins. Sterol regulatory element binding protein-1c (SREBP-1c) is an example of a transcription factor whose function is altered by ω-3 PUFAs, contributing to their effects on lipid metabolism and inflammatory pathways [73,74][72][73].2.4. PUFAs and Its Metabolites: Role of Oxylipins
A further mechanism of action of ω-3 PUFAs directly involves their metabolites (ω-3 Oxylipins) that in recent years have gathered great research interest. After being released from phospholipids by cytosolic phospholipase A2 (cPLA2), both ω-6 PUFAs and ω-3 PUFAs are oxygenated by different enzymes, such as cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) enzymes. This leads to the synthesis of a broad variety of bioactive lipid compounds, many of which take part in the regulation of vascular function and exert potent anti-inflammatory effects [75,76][74][75]. “Classic oxylipins” are eicosanoids (including prostaglandins, thromboxanes, and leukotrienes) derived from both ῳ-6 PUFAs and ω-3 PUFAs. ω-6 PUFA AA is the precursor of two types of prostaglandins, thromboxanes, and four types of leukotrienes with strong pro-inflammatory, pro-thrombotic, and vasoconstrictive properties [77,78][76][77]. EPA is the precursor of three types of prostaglandins and five types of leukotrienes [38][37] that are 10- to 100-fold less biologically active than their counterparts that are derived from AA and are antagonistic with their effects on vascular tone, platelet aggregation, and inflammation [77][76]. In this context, when balancing of enzymatic conversions favors 3-series thromboxanes (TXA3 vs. TXA2) and prostacyclines (PGI3 vs. PGI2), the effects of PGI prevail because of the relative power of these molecules. In vitro and in vivo experiments show that AA metabolizing P450-enzymes can use EPA and DHA as alternative substrates. Therefore, EPA/DHA supplementation shifts the P450-eicosanoid profile to EPA- and DHA-derived epoxy- and hydroxy-metabolites (17,18-epoxy-EPA and 19,20-epoxy-DHA) that are commonly identified as CYP eicosanoids [79][78]. These eicosanoids show protective vasoactive actions and antiarrhythmic properties in cardiomyocytes and are linked to the development of hypertension, myocardial infarction, pathological cardiac hypertrophy, stroke, kidney injury, and other inflammatory disorders [79,80][78][79]. Besides “classic oxylipins”, ω-3 PUFAs can generate some other oxylipins, designated “specialized pro-resolving mediators” (SPM), that participate in the resolution of inflammation and exert protective and beneficial effects on a variety of inflammatory diseases [81,82][80][81]. These compounds include resolvins, protectins, and maresins. EPA generates E-series resolvins (RvE1, RvE2, and RvE3) through the action of COX, while D-series resolvins (RvD1-D6), protectins, and maresins (including MaR1 and MaR2) are derived from DHA, through the actions of LOX [81][80]. Moreover, some ῳ-3 oxylipins, including protectin DX, maresin 1, and resolvin D1, display antioxidant capacity by regulating the expression of antioxidant proteins including catalase, superoxide dismutase, and glutathione peroxidase activity and attenuating lipid peroxidation and O2-generation [83,84][82][83]. In summary, the incorporation of ω-3 PUFAs in membrane phospholipids improves membrane fluidity and biophysical properties by changing lipid rafts and caveolae characteristics, thereby leading to modulation of protein–protein interaction and ion channel kinetics. These membrane changes involve also intracellular organelles and trigger a multiplicity of intracellular signaling mechanisms that can contribute to the antiatherosclerotic properties of ω-3 PUFAs and to the regulation of peripheral vascular tone (Figure 2).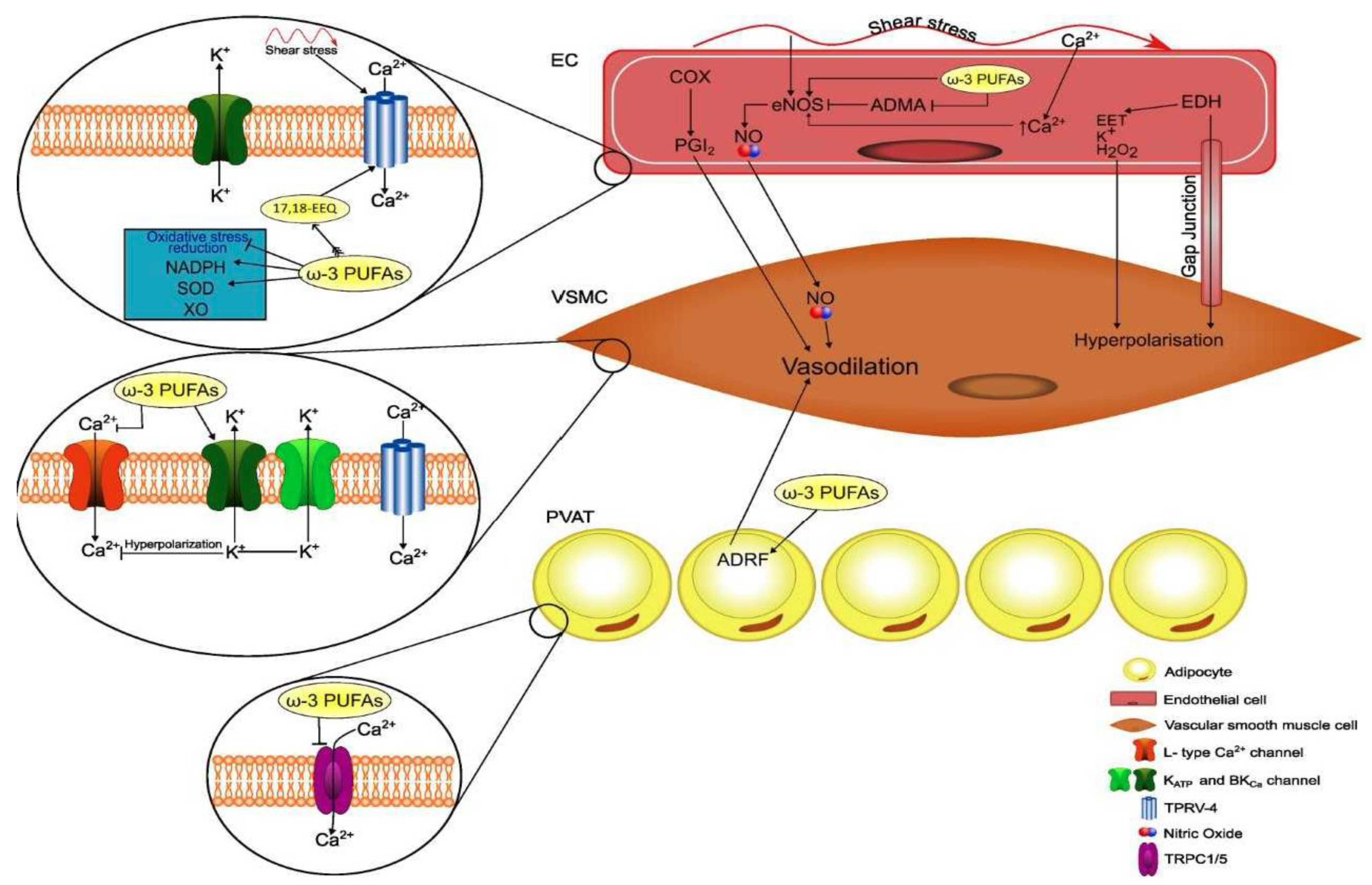
Figure 2. Schematic representation of actions of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) on cell membranes and intracellular signaling and their relevance for perivascular adipocytes and vascular cell functions. EEQ, epoxyeicosatetraenoic acid; NADPH, nicotinamide adenine dinucleotide; SOD, superoxide dismutase; XO, xanthine oxidase; PVAT, perivascular adipose tissue; ADRF, adventitium-derived relaxing factor; COX, cyclooxygenase; PGI2, prostacyclin; NO, nitric oxide; eNOS, endothelial nitric oxide synthase; ADMA, asymmetric dimethylarginine; EET, epoxyeicosatrienoic acid; endothelium-derived hyperpolarizing factor; BKCa, large-conductance voltage- and calcium-activated potassium channel; TRPV-4, transient receptor potential vanilloid-4; TRPC1/5, transient receptor potential cation.
3. ω-3 PUFAs and Blood Pressure
The effect of ω-3 PUFAs on BP has been well characterized in the past three decades across multiple trials, systematic reviews, and meta-analyses, most of which included both hypertensive and normotensive individuals. Overall, meta-analyses have shown that relatively high doses of ω-3 PUFAs, usually more than 3 g/day, lead to small albeit meaningful BP reductions, particularly in subjects with untreated hypertension. The first meta-analysis appeared in 1993 and included 17 controlled clinical trials (6 in untreated hypertensive subjects without any other comorbidity and 11 in normotensives) reporting a reduction in systolic BP (SBP) and diastolic BP (DBP) that was significant only in hypertensives (−5.5 and −3.5 mm Hg, respectively) [85][84], with a median ω-3 PUFA dose of 5 g/day that was administered for a median of 8 weeks. Another meta-analysis that included 31 controlled clinical trials with 1356 healthy or hypertensive participants confirmed a significant reduction in SBP and DBP only in hypertensive patients (−3.4 and −2 mm Hg, respectively) who took an average ω-3 PUFA dose of 4.8 g/day in the form of fish or fish oil for 3 to 24 weeks [86][85]. Another meta-analysis included a total of 36 studies, 22 of which had a double-blind design [87][86]. Fish oil reduced SBP by 2.1 mm Hg and DBP by 1.6 mm Hg with effects that tended to be greater in hypertensive subjects older than 45 years. Effects of ω-3 PUFAs on BP were the object of further subsequent meta-analyses [88[87][88][89][90][91],89,90,91,92], all showing a small but significant reduction in BP levels. These results have been recently corroborated by an umbrella meta-analysis [93][92] that included 10 meta-analyses of 131 studies carried out between 1989 and 2021 with ω-3 PUFA supplements across studies of 2.2 to 6 g/day and duration of exposure from 4 to 29 weeks. This meta-analysis has confirmed that ω-3 PUFA decreases SBP (−1.19 mm Hg; 95% CI: −1.76, −0.62, p < 0.001) and DBP levels (−0.91 mm Hg, 95% CI: −1.35, −0.47; p < 0.001) with effects that were more prominent in older hypertensive subjects who were supplemented with doses greater than 2 g/day for more than 10 weeks. Although results of meta-analyses indicate an effect of ω-3 PUFA supplementation in reducing BP, important variability of findings in individual studies reflecting heterogeneity in the source (fish, fish oil, capsules enriched with ethyl-ester forms of DHA and EPA, powders), the relative content of EPA and DHA, duration of exposure, and differences in terms of comorbidities and additional treatments should not be overlooked [94][93]. In addition, as for any other dietary intervention study, assessment of compliance to prescription is particularly problematic and only a minority of studies were controlled with measurement of ω-3 PUFA content in cellular membranes. WResearchers prospectively followed a group of uncomplicated hypertensive patients who were advised to eat a meal of farmed fish fed with PUFA-enriched chow three times a week for 6 months. Ambulatory BP monitoring and ω-3 PUFA content in red blood cell plasma membranes were evaluated at baseline and at the end of the study. Twenty-four-hour and nighttime BP were significantly reduced only in those patients who had increased membrane ω-3 PUFA content with an effect that was more pronounced in those with lower baseline ω-3 PUFA content [95][94] (Figure 3).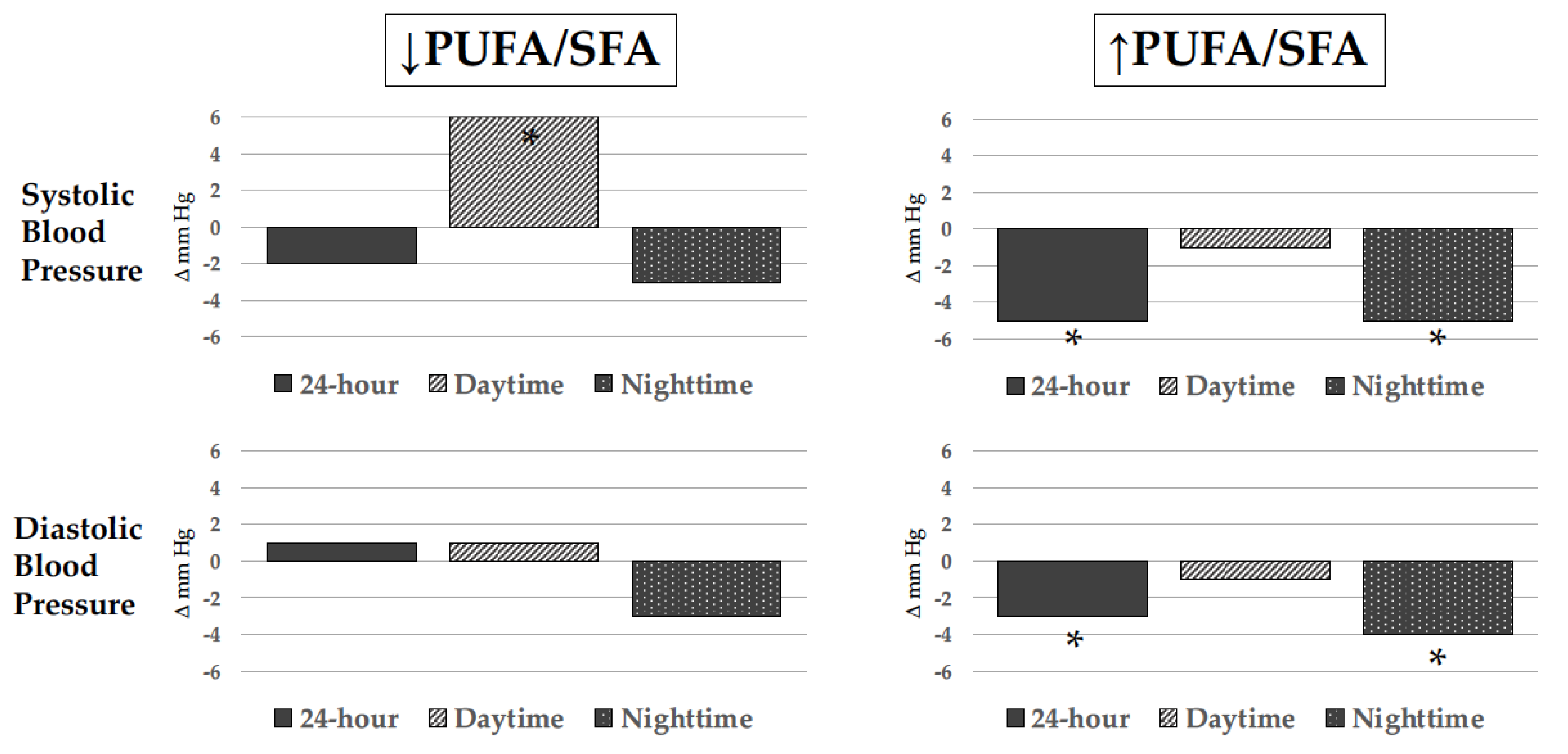
Figure 3. Changes in blood pressure levels as measured by noninvasive ambulatory blood pressure monitoring (ABPM) in hypertensive patients who ate 3 weekly meals of trout rich in polyunsaturated fatty acids for 6 months. Changes are represented according to change (↑ increase; ↓ decrease) in erythrocyte cell membrane polyunsaturated to saturated fatty acid ratio (PUFA/SFA). Only hypertensive patients with an increased PUFA/SFA had significant 24-hour and nighttime blood pressure reduction. * p < 0.05.
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Erratum in: Eur. Heart J. 2019, 40, 475.
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.J.; Collins, K.J.; Dennison Himmelfarb, C.; De Palma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115, Erratum in Hypertension 2018, 71, e140–e144.
- World Health Organization (WHO). World Health Organization Obesity and Overweight Fact Sheet; World Health Organization: Geneva, Switzerland, 2016.
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260.
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014.
- Ward, R. Familial aggregation and genetic epidemiology of blood pressure. In Hypertension: Pathophysiology, Diagnosis and Management; New York Raven Press: New York, NY, USA, 1990; Volume 1, pp. 81–100.
- Bang, H.O.; Dyerberg, J.; Hjoorne, T. The composition of food consumed by Greenland Eskimos. Acta Med. Scand. 1976, 200, 69–73.
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in northwestern Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661.
- Middaugh, J.P. Cardiovascular deaths among Alaskan Natives, 1980–1986. Am. J. Public Health 1990, 80, 282–285.
- Newman, W.P.; Middaugh, J.P.; Propst, M.T.; Roger, D.R. Atherosclerosis in Alaska Natives and Non-Natives. Lancet 1993, 341, 1056–1057.
- Weinberg, R.L.; Brook, R.D.; Rubenfire, M.; Eagle, K.A. Cardiovascular Impact of Nutritional Supplementation with Omega-3 Fatty Acids: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 593–608.
- Colussi, G.L.; Baroselli, S.; Sechi, L. Omega-3 polyunsaturated fatty acids decrease plasma lipoprotein(a) levels in hypertensive subjects. Clin. Nutr. 2004, 23, 1246–1247.
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’Donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: A systematic review and meta-regression analysis of randomized controlled trials. Circulation 2019, 140, 1308–1317.
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118.
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: Findings from the China national stroke screening and prevention project (CNSSPP). BMC Public Health 2019, 19, 1500.
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563.
- Budoff, M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am. J. Cardiol. 2016, 118, 138–145.
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1352.
- Thomsen, M.; Varbo, A.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Low nonfasting triglycerides and reduced all-cause mortality: A mendelian randomization study. Clin. Chem. 2014, 60, 737–746.
- Sacks, F.M.; Carey, V.J.; Fruchart, J.C. Combination lipid therapy in type 2 diabetes. N. Engl. J. Med. 2010, 363, 692–695.
- The ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574.
- Guyton, J.R.; Slee, A.E.; Anderson, T.; Fleg, J.L.; Goldberg, R.B.; Kashyap, M.L.; Marcovina, S.M.; Nash, S.D.; O’Brien, K.D.; Weintraub, W.S.; et al. Relationship of lipoproteins to cardiovascular events: The AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 2013, 62, 1580–1584.
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22.
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461.
- Dietary Guidelines for Americans. US Department of Health and Human Services and US Department of Agriculture. 2015; Volume 7. Available online: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed on 5 April 2023).
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757.
- Minihane, A.M. Fish oil omega-3 fatty acids and cardio-metabolic health, alone or with statins. Eur. J. Clin. Nutr. 2013, 67, 536–540.
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972.
- Colussi, G.; Catena, C.; Baroselli, S.; Nadalini, E.; Lapenna, R.; Chiuch, A.; Sechi, L.A. Omega-3 fatty acids: From biochemistry to their clinical use in the prevention of cardiovascular disease. Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 13–21.
- Burdge, G.C. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168.
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56.
- Nettleton, J.A. Omega-3 fatty acids: Comparison of plant and seafood sources in human nutrition. J. Am. Diet. Assoc. 1991, 91, 331–337.
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S.
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420.
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic and metabolism in young men. Br. J. Nutr. 2002, 88, 355–364.
- Lin, Y.H.; Salem, N., Jr. Whole body distribution of deuterated linoleic and alpha-linolenic acids and their metabolites in the rat. J. Lipid Res. 2007, 48, 2709–2724.
- Cook, H.; McMaster, C. Fatty acid desaturation and chain elongation in eukaryotes. New Compr. Biochem. 2002, 36, 181–204.
- Wada, M.; De Long, C.J.; Hong, Y.H.; Rieke, C.J.; Song, I.; Sidhu, R.S.; Yuan, C.; Warnock, M.; Schmaier, A.H.; Yokoyama, C.; et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J. Biol. Chem. 2007, 282, 22254–22266.
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53.
- Liou, Y.A.; King, D.J.; Zibrik, D.; Innis, S.M. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J. Nutr. 2007, 137, 945–952.
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688.
- Harris, W.S.; Von Schacky, C. The omega-3 index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220.
- McDonnell, S.L.; French, C.B.; Baggerly, C.A.; Harris, W.S. Cross-sectional study of the combined associations of dietary and supplemental eicosapentaenoic acid + docosahexaenoic acid on omega-3 index. Nutr. Res. 2019, 71, 43–55.
- Dempsey, M.; Rockwell, M.S.; Wentz, L.M. The influence of dietary and supplemental omega-3 fatty acids on the omega-3 index: A scoping review. Front. Nutr. 2023, 10, 1072653.
- Sherratt, S.C.R.; Mason, R.P. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by X-ray diffraction. Chem. Phys. Lipids 2018, 212, 73–79.
- Williams, J.A.; Batten, S.E.; Harris, M.; Rockett Drew, B.; Shaikh Raza, S.; Stillwell, W.; Wassall, S.R. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J. 2012, 103, 228–237.
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.R.; Chattopadhyay, A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim. Biophys. Acta 2016, 1858, 3131–3140.
- Faber, J.; Berkhout, M.; Vos, A.P.; Sijben, J.W.C.; Calder, P.C.; Garssen, J.; Van Helvoort, A. Supplementation with a fish oil-enriched, high-protein medicalfood leads to rapid incorporation of EPA into white blood cells and modulates immune responses within one week in healthy men and women. J. Nutr. 2011, 141, 964–970.
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342.
- Vidgren, H.M.; Agren, J.J.; Schwab, U.; Rissanen, T.; Hänninen, O.; Uusitupa, M.I. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 1997, 32, 697–705.
- Laude, A.J.; Prior, I.A. Plasma membrane microdomains: Organization, function and trafficking. Mol. Membr. Biol. 2004, 21, 193–205.
- Hancock, J.F. Lipid rafts: Contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 2006, 7, 456–462.
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572.
- Andersonl, R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998, 67, 199–225.
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 770–776.
- Ikonen, E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001, 13, 470–477.
- Layne, J.; Majkova, Z.; Smart, E.J.; Toborek, M.; Hennig, B. Caveolae: A regulatory platform for nutritional modulation of inflammatory diseases. J. Nutr. Biochem. 2011, 22, 807–811.
- Dart, C. Lipid microdomains and the regulation of ion channel function. J. Physiol. 2010, 588, 3169–3178.
- Grossfield, A.; Feller, S.E.; Pitman, M.C. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. USA 2006, 103, 4888–4893.
- Xiao, Y.F.; Ke, Q.; Wang, S.Y.; Auktor, K.; Yang, Y.; Wang, G.K.; Morgan, J.P.; Leaf, A. Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na. channels. Proc. Natl. Acad. Sci. USA 2001, 98, 3606–3611.
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698.
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94.
- Im, D.S. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur. J. Pharmacol. 2016, 785, 36–43.
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castilo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163.
- Haneklaus, M.; O’Neill, L.A.; Coll, R.C. Modulatory mechanisms controlling the NLRP3 inammasome in inammation: Recent developments. Curr. Opin. Immunol. 2013, 25, 40–45.
- Jump, D.B. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr. Opin. Lipidol. 2008, 19, 242–247.
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792.
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317.
- Hertz, R.; Magenheim, J.; Berman, I.; Bar-Tana, J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature 1998, 392, 512–516.
- Fan, Y.Y.; Spencer, T.E.; Wang, N.; Moyer, M.P.; Chapkin, R.S. Chemopreventive n-3 fatty acids activate RXRalpha in colonocytes. Carcinogenesis 2003, 24, 1541–1548.
- de Urquiza, A.M.; Liu, S.; Sjoberg, M.; Zetterström, R.H.; Griffiths, W.; Sjövall, J.; Perlmann, T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 2000, 290, 2140–2144.
- Schroeder, F.; Petrescu, A.D.; Huang, H.; Atshaves, B.P.; McIntosh, A.L.; Martin, G.G.; Hosteler, H.A.; Vespa, A.; Landrock, D.; Landrock, K.K.; et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 2008, 43, 1–17.
- Wolfrum, C.; Borrmann, C.M.; Borchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha- and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328.
- Needleman, P.; Truk, J.; Jakschik, B.A.; Morrison, A.R.; Lefkowith, J.B. Arachidonic acid metabolism. Annu. Rev. Biochem. 1986, 55, 69–102.
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82.
- Biscione, F.; Pignalberi, C.; Totteri, A.; Messina, F.; Altamura, G. Cardiovascular effects of omega-3 free fatty acids. Curr. Vasc. Pharmacol. 2007, 5, 163–172.
- Das, U.N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhytmic, anty-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008, 7, 37.
- Westphal, C.; Konkel, A.; Schunck, W.-H. Cytochrome P-450 enzymes in the bioactivcation of polyunsaturated fatty acids and their role in cardiovascular disease. Adv. Exp. Med. Biol. 2015, 851, 151–187.
- Schunk, W.-H. EPA and/or DHA? A test question on the principles and opportunities in utilizing the therapeutic potential of omega-3 fatty acids. J. Lipid Res. 2016, 57, 1608–1611.
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101.
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inammation: Dual anti-inammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361.
- Wang, H.J.; Jung, T.W.; Kim, J.W.; Kim, J.A.; Lee, Y.B.; Hong, S.H.; Roh, E.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Protectin DX prevents H2O2-mediated oxidative stress in vascular endothelial cells via an AMPK-dependent mechanism. Cell Signal 2019, 53, 14–21.
- Sun, Q.; Wu, Y.; Zhao, F.; Wang, J. Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 9634803.
- Appel, L.J.; Miller, E.R., 3rd; Seidler, A.J.; Whelton, P.K. Does supplementation of diet with ‘fish oil’ reduce blood pressure? A meta-analysis of controlled clinical trials. Arch. Intern. Med. 1993, 153, 1429–1438.
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533.
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.; Kok, F.J. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499.
- Dickinson, H.O.; Mason, J.M.; Nicolson, D.J.; Campbell, F.; Beyer, F.C.; Beyer, F.R.; Cook, J.V.; Williams, B.; Ford, G.A. Lifestyle interventions to reduce raised blood pressure: A systematic review of randomized controlled trials. J. Hypertens. 2006, 24, 215–233.
- Campbell, F.; Dickinson, H.O.; Critchley, J.A.; Ford, G.A.; Bradburn, M. A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur. J. Prev. Cardiol. 2013, 20, 107–120.
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-chain omega-3 fatty acids eicosapen-taenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014, 27, 885–896.
- AbuMweis, S.; Jew, S.; Tayyem, R.; Agraib, L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: A meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet. 2018, 31, 67–84.
- Guo, X.F.; Li, K.L.; Li, J.M.; Li, D. Effects of EPA and DHA on blood pressure and inflammatory factors: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 3380–3393.
- Musazadeh, V.; Kavyani, Z.; Naghshbandi, B.; Dehghan, P.; Vajdi, M. The beneficial effects of omega-3 polyunsaturated fatty acids on controlling blood pressure: An umbrella meta-analysis. Front. Nutr. 2022, 18, 85451.
- Minihane, A.M.; Armah, C.K.; Miles, E.A.; Madden, J.M.; Clark, A.B.; Caslake, M.J.; Packard, C.J.; Kofler, B.M.; Lietz, G.; Curtis, P.J.; et al. Consumption of fish oil providing amounts of eicosapentaenoic acid and docosahexaenoic acid that can be obtained from the diet reduces blood pressure in adults with systolic hypertension: A retrospective analysis. J. Nutr. 2016, 146, 516–523.
- Colussi, G.; Catena, C.; Dialti, V.; Pezzutto, F.; Mos, L.; Sechi, L.A. Fish meal supplementation and ambulatory blood pressure in patients with hypertension: Relevance of baseline membrane fatty acid composition. Am. J. Hypertens. 2014, 27, 471–481.
- Yang, B.; Shi, M.Q.; Li, Z.H.; Yang, J.J.; Li, D. Fish, Long-chain n-3 PUFA and incidence of elevated blood pressure: A meta-analysis of prospective cohort studies. Nutrients 2016, 8, 58.
- Chen, J.; Sun, B.; Zhang, D. Association of dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007–2014. Nutrients 2019, 11, 1232.
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta- analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913.
- Hardy, S.T.; Loehr, L.R.; Butler, K.R.; Chakladar, S.; Chang, P.P.; Folsom, A.R.; Heiss, G.; MacLehose, R.F.; Matsushita, K.; Avery, C.L. Reducing the blood pressure-related burden of cardiovascular disease: Impact of achievable improvements in blood pressure prevention and control. J. Am. Heart Assoc. 2015, 4, e002276.
More
