Breast cancer is a common cancer in women and a leading cause of mortality. With the early diagnosis and development of therapeutic drugs, the prognosis of breast cancer has markedly improved. Chemotherapy is one of the predominant strategies for the treatment of breast cancer. Taxanes, including paclitaxel and docetaxel, are widely used in the treatment of breast cancer and remarkably decrease the risk of death and recurrence. Taxane resistance caused by multiple factors significantly impacts the effect of the drug and leads to poor prognosis. Long noncoding RNAs (lncRNAs) have been shown to play a significant role in critical cellular processes, and a number of studies have illustrated that lncRNAs play vital roles in taxane resistance.
- breast cancer
- long noncoding RNAs
- taxane resistance
1. Introduction
2. Mechanisms of Taxane Resistance in Breast Cancer
The taxane class is a series of derivatives synthesized by isolating the active antitumor component from plants and structurally modifying the active component obtained. Taxanes mainly include paclitaxel, docetaxel, and derivatives with a paclitaxel backbone structure. Paclitaxel is a taxane diterpene isolated from the bark of Taxus brevifolia [10,11,12][10][11][12]. Docetaxel is a semisynthetic product of precursors extracted from Taxus baccata L. that is structurally similar to paclitaxel [13]. Taxanes have been used to treat certain types of cancers. In particular, the use of taxanes in breast cancer is an important breakthrough that has greatly improved the prognosis of patients with breast cancer [14,15][14][15]. By binding to a hydrophobic cleft in β-tubulin [16], paclitaxel and docetaxel affect the dynamic balance between α- and β-tubulin dimers and microtubules, promoting the assembly of tubulin into microtubules and facilitating microtubule polymerization, blocking their depolymerization into subunits, causing cells to arrest in the G2 and M phases, and leading to abnormal mitosis or cessation of cell division, ultimately causing cell death [17,18,19][17][18][19]. Taxane-induced microtubule stabilization causes Bcl-2 phosphorylation, triggering a cascade of events leading to apoptosis [20]. Chemotherapy resistance is a major cause of cancer treatment failure, resulting in death in over 90% of patients with metastatic cancer [21]. As one of the standard strategies for breast cancer treatment, taxane resistance remains a major obstacle affecting prognosis. Therefore, understanding the mechanism of taxane resistance will help to identify biomarkers and develop new therapeutic approaches to overcome taxane resistance in breast cancer. The major mechanisms that mediate taxane resistance include (1) alteration of tubulin isotypes and mutations; (2) changes in microtubule-associated proteins (MAPs); (3) drug transport and efflux; (4) deregulation of cell death; (5) alterations in proliferation signaling pathways and the epithelial-to-mesenchymal transition (EMT) (Figure 1).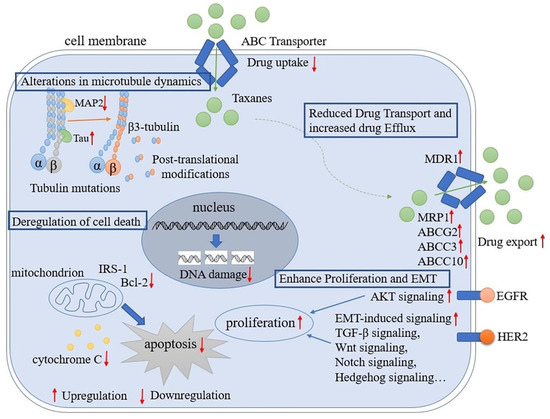
3. Long Noncoding RNAs
The transcription process involves production of both protein-coding messenger RNAs (mRNAs) and noncoding RNAs (ncRNAs) [107][22]. As a subclass of noncoding RNAs, lncRNAs consist of more than 200 nucleotides. Most lncRNAs have the same characteristics as mRNAs but are usually transcribed from fewer exons than coding RNAs and lack an open reading frame [108,109][23][24]. In general, lncRNAs are localized to the nucleus but can also be detected in the cytoplasm [110][25]. They are transcribed by RNA polymerase II, undergo 5′ capping, 3′ cleavage, and polyadenylation and produce mature lncRNAs through splicing, although the process is less efficient than that of mRNA splicing [108,111][23][26]. LncRNAs are typically classified into five categories based on their location relative to adjacent protein-coding genes, including intergenic lncRNAs (lincRNAs), antisense lncRNAs, sense lncRNAs, intronic lncRNAs, and bidirectional lncRNAs [112,113][27][28]. LncRNA expression is mainly tissue specific, suggesting that lncRNAs may play a functional role in physiological and biological processes [114][29]. Although the roles of most lncRNAs have not been verified, it has been shown that lncRNAs are involved in many areas of genome function, including epigenetics, gene transcription, splicing, and translation, as well as fundamental biological processes, such as cell cycle progression and differentiation [115][30]. In addition, lncRNAs perform cytoplasmic functions, mainly acting as miRNA sponges, regulating translation of specific mRNAs, and interacting with various signaling proteins [5]. Overall, lncRNAs are a group of diverse regulatory ncRNAs with different properties, locations, and mechanisms of action. The function of lncRNAs depends on their subcellular localization [113,116][28][31]. Several mechanisms of action have been proposed for lncRNAs (Figure 2).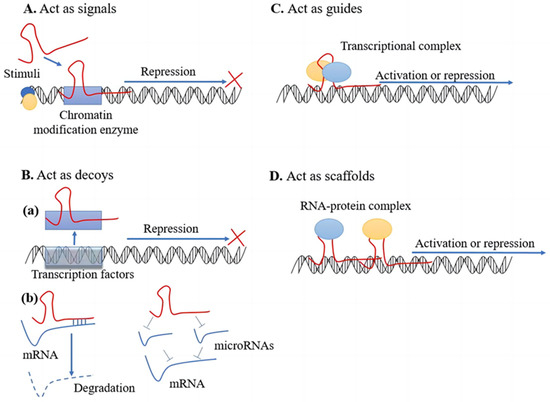
4. LncRNAs and Taxane Resistance
LncRNAs are recognized as important regulators of gene expression in cancer. Many lncRNAs have been implicated in cancer initiation and progression [108,128][23][43]. Abnormal expression of lncRNAs is closely related to tumor occurrence, metastasis, and tumor stage [129,130][44][45]. Furthermore, lncRNAs can directly or indirectly regulate a variety of pathways related to chemotherapy resistance, such as changes in drug efflux, inhibition of apoptosis pathways, and promotion of EMT [131,132,133][46][47][48]. As mentioned above, taxane resistance is a growing challenge in modern breast cancer chemotherapy, but the role of lncRNAs in mediating taxane resistance and susceptibility is not yet fully understood. However, several mechanisms are believed to be associated with taxane resistance induced by lncRNAs. First, ABC overexpression leads to enhanced drug efflux. ABC proteins, such as P-gp, ABCG2, and MRP, are frequently overexpressed in many types of cancers [134][49]. Multidrug resistance can be induced by overexpression of ABC efflux transporters due to specific lncRNAs [135][50]. A number of studies have shown that lncRNAs play a key role in increasing the outflow of a wide range of chemotherapeutic agents from a variety of cancer cells [136,137,138][51][52][53]. For example, UCA1 confers paclitaxel resistance through miR-129/ABCB1 axis in ovarian cancer [139][54]. In colorectal cancer, LINC00473 promotes paclitaxel resistance by activating the Bcl-2-related pathway and increases the LRP and MDR1 expression of MDR genes [140][55]. Second, many chemotherapeutic agents inhibit the proliferation of cancer cells by promoting apoptosis: lncRNAs associated to apoptotic pathways have been linked to multidrug resistance [132][47]. LncRNAs can protect cancer cells by inhibiting apoptosis. A previous study reported that after silencing LINC00511 expression, Bax and cleaved-caspase-3 increased with more cervical cancer cells arrested at the G1 phase [141][56]. In docetaxel-resistant lung adenocarcinoma cells, lncRNA CCAT1 was upregulated, and further study revealed that CCAT1 promotes chemoresistance by targeting the let-7c/Bcl-xl pathway [142][57]. Third, lncRNAs play a role in the EMT through signaling pathways, such as Wnt/β-Catenin and AKT/mTOR. Resistance/sensitivity to many chemotherapeutic agents has been associated with EMT-related lncRNAs [132][47]. In gastric cancer, lncRNA ZFAS1 can induce EMT via the Wnt/β-catenin pathway, thereby promoting chemotherapeutic tolerance [143][58]. Similarly, lncRNAs may mediate taxane resistance through these mechanisms in breast cancer (Figure 3).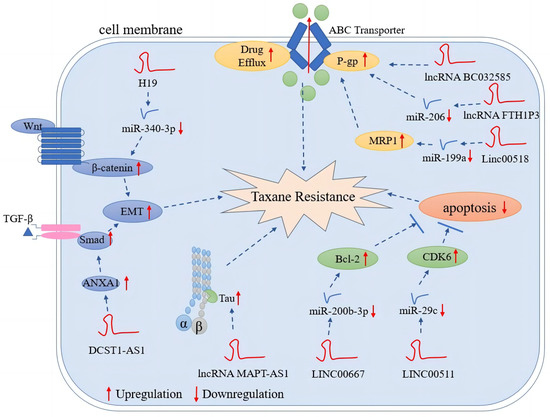
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249.
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055.
- Fabbri, M.; Girnita, L.; Varani, G.; Calin, G.A. Decrypting noncoding RNA interactions, structures, and functional networks. Genome Res. 2019, 29, 1377–1388.
- Luo, Y.; Tang, W.; Xiang, S.; Feng, J.; Zu, X. Non-coding RNAs in breast cancer: Implications for programmed cell death. Cancer Lett. 2022, 550, 215929.
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36.
- Pandya, G.; Kirtonia, A.; Sethi, G.; Pandey, A.K.; Garg, M. The implication of long non-coding RNAs in the diagnosis, pathogenesis and drug resistance of pancreatic ductal adenocarcinoma and their possible therapeutic potential. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188423.
- Singh, D.; Assaraf, Y.G.; Gacche, R.N. Long non-coding RNA mediated drug resistance in breast cancer. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 2022, 63, 100851.
- Liu, Y.; Ao, X.; Wang, Y.; Li, X.; Wang, J. Long Non-Coding RNA in Gastric Cancer: Mechanisms and Clinical Implications for Drug Resistance. Front. Oncol. 2022, 12, 841411.
- Deng, H.; Zhang, J.; Shi, J.; Guo, Z.; He, C.; Ding, L.; Tang, J.H.; Hou, Y. Role of long non-coding RNA in tumor drug resistance. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 11623–11631.
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495.
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martinez, R.A.; Canovas-Diaz, M.; de Diego Puente, T. A Compressive Review about Taxol((R)): History and Future Challenges. Molecules 2020, 25, 5986.
- Arnst, J. When Taxol met tubulin. J. Biol. Chem. 2020, 295, 13994–13995.
- Bissery, M.C.; Nohynek, G.; Sanderink, G.J.; Lavelle, F. Docetaxel (Taxotere): A review of preclinical and clinical experience. Part I: Preclinical experience. Anti-Cancer Drugs 1995, 6, 338–363.
- Alken, S.; Kelly, C.M. Benefit risk assessment and update on the use of docetaxel in the management of breast cancer. Cancer Manag. Res. 2013, 5, 357–365.
- Qi, W.X.; Shen, Z.; Lin, F.; Sun, Y.J.; Min, D.L.; Tang, L.N.; He, A.N.; Yao, Y. Paclitaxel-based versus docetaxel-based regimens in metastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. Curr. Med. Res. Opin. 2013, 29, 117–125.
- Snyder, J.P.; Nettles, J.H.; Cornett, B.; Downing, K.H.; Nogales, E. The binding conformation of Taxol in beta-tubulin: A model based on electron crystallographic density. Proc. Natl. Acad. Sci. USA 2001, 98, 5312–5316.
- Yvon, A.M.; Wadsworth, P.; Jordan, M.A. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 1999, 10, 947–959.
- Amin, A.; Gali-Muhtasib, H.; Ocker, M.; Schneider-Stock, R. Overview of major classes of plant-derived anticancer drugs. Int. J. Biomed. Sci. 2009, 5, 1.
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta 2008, 1785, 96–132.
- Pienta, K.J. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin. Oncol. 2001, 28 (Suppl. S15), 3–7.
- Rueff, J.; Rodrigues, A.S. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint. Methods Mol. Biol. 2016, 1395, 1–18.
- Orom, U.A.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010, 143, 46–58.
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118.
- Guo, C.J.; Ma, X.K.; Xing, Y.H.; Zheng, C.C.; Xu, Y.F.; Shan, L.; Zhang, J.; Wang, S.; Wang, Y.; Carmichael, G.G.; et al. Distinct Processing of lncRNAs Contributes to Non-conserved Functions in Stem Cells. Cell 2020, 181, 621–636.e22.
- Mukherjee, N.; Calviello, L.; Hirsekorn, A.; de Pretis, S.; Pelizzola, M.; Ohler, U. Integrative classification of human coding and noncoding genes through RNA metabolism profiles. Nat. Struct. Mol. Biol. 2017, 24, 86–96.
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789.
- Lin, W.; Zhou, Q.; Wang, C.Q.; Zhu, L.; Bi, C.; Zhang, S.; Wang, X.; Jin, H. LncRNAs regulate metabolism in cancer. Int. J. Biol. Sci. 2020, 16, 1194–1206.
- Tsagakis, I.; Douka, K.; Birds, I.; Aspden, J.L. Long non-coding RNAs in development and disease: Conservation to mechanisms. J. Pathol. 2020, 250, 480–495.
- Liu, Y.; Sharma, S.; Watabe, K. Roles of lncRNA in breast cancer. Front. Biosci. 2015, 7, 94–108.
- Yousefi, H.; Maheronnaghsh, M.; Molaei, F.; Mashouri, L.; Reza Aref, A.; Momeny, M.; Alahari, S.K. Long noncoding RNAs and exosomal lncRNAs: Classification, and mechanisms in breast cancer metastasis and drug resistance. Oncogene 2020, 39, 953–974.
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551.
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914.
- Tian, D.; Sun, S.; Lee, J.T. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell 2010, 143, 390–403.
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; Horlings, H.M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621–629.
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283.
- Wang, J.; Gong, C.; Maquat, L.E. Control of myogenesis by rodent SINE-containing lncRNAs. Genes Dev. 2013, 27, 793–804.
- Wang, K.; Sun, T.; Li, N.; Wang, Y.; Wang, J.X.; Zhou, L.Y.; Long, B.; Liu, C.Y.; Liu, F.; Li, P.F. MDRL lncRNA regulates the processing of miR-484 primary transcript by targeting miR-361. PLoS Genet. 2014, 10, e1004467.
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157.
- Hanly, D.J.; Esteller, M.; Berdasco, M. Interplay between long non-coding RNAs and epigenetic machinery: Emerging targets in cancer? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2018, 373, 20170074.
- Ahmad, P.; Bensaoud, C.; Mekki, I.; Rehman, M.U.; Kotsyfakis, M. Long Non-Coding RNAs and Their Potential Roles in the Vector-Host-Pathogen Triad. Life 2021, 11, 56.
- Janakiraman, H.; House, R.P.; Gangaraju, V.K.; Diehl, J.A.; Howe, P.H.; Palanisamy, V. The Long (lncRNA) and Short (miRNA) of It: TGFbeta-Mediated Control of RNA-Binding Proteins and Noncoding RNAs. Mol. Cancer Res. 2018, 16, 567–579.
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186.
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981.
- Vitiello, M.; Tuccoli, A.; Poliseno, L. Long non-coding RNAs in cancer: Implications for personalized therapy. Cell. Oncol. 2015, 38, 17–28.
- Bartonicek, N.; Maag, J.L.; Dinger, M.E. Long noncoding RNAs in cancer: Mechanisms of action and technological advancements. Mol. Cancer 2016, 15, 43.
- Jiang, W.; Xia, J.; Xie, S.; Zou, R.; Pan, S.; Wang, Z.W.; Assaraf, Y.G.; Zhu, X. Long non-coding RNAs as a determinant of cancer drug resistance: Towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 2020, 50, 100683.
- Qu, Y.; Tan, H.Y.; Chan, Y.T.; Jiang, H.; Wang, N.; Wang, D. The functional role of long noncoding RNA in resistance to anticancer treatment. Ther. Adv. Med. Oncol. 2020, 12, 1758835920927850.
- Bin, X.; Hongjian, Y.; Xiping, Z.; Bo, C.; Shifeng, Y.; Binbin, T. Research progresses in roles of LncRNA and its relationships with breast cancer. Cancer Cell Int. 2018, 18, 179.
- Cui, H.; Zhang, A.J.; Chen, M.; Liu, J.J. ABC Transporter Inhibitors in Reversing Multidrug Resistance to Chemotherapy. Curr. Drug Targets 2015, 16, 1356–1371.
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164.
- Mou, S.J.; Yang, P.F.; Liu, Y.P.; Xu, N.; Jiang, W.W.; Yue, W.J. BCLAF1 promotes cell proliferation, invasion and drug-resistance though targeting lncRNA NEAT1 in hepatocellular carcinoma. Life Sci. 2020, 242, 117177.
- Song, L.; Zhou, Z.; Gan, Y.; Li, P.; Xu, Y.; Zhang, Z.; Luo, F.; Xu, J.; Zhou, Q.; Dai, F. Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATbeta/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. J. Cell. Biochem. 2019, 120, 9656–9666.
- Zhu, Q.N.; Wang, G.; Guo, Y.; Peng, Y.; Zhang, R.; Deng, J.L.; Li, Z.X.; Zhu, Y.S. LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. Oncotarget 2017, 8, 91990–92003.
- Wang, J.; Ye, C.; Liu, J.; Hu, Y. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem. Biophys. Res. Commun. 2018, 501, 1034–1040.
- Wang, L.; Zhang, X.; Sheng, L.; Qiu, C.; Luo, R. LINC00473 promotes the Taxol resistance via miR-15a in colorectal cancer. Biosci. Rep. 2018, 38, BSR20180790.
- Mao, B.D.; Xu, P.; Xu, P.; Zhong, Y.; Ding, W.W.; Meng, Q.Z. LINC00511 knockdown prevents cervical cancer cell proliferation and reduces resistance to paclitaxel. J. Biosci. 2019, 44, 44.
- Chen, J.; Zhang, K.; Song, H.; Wang, R.; Chu, X.; Chen, L. Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells. Oncotarget 2016, 7, 62474–62489.
- Xu, W.; He, L.; Li, Y.; Tan, Y.; Zhang, F.; Xu, H. Silencing of lncRNA ZFAS1 inhibits malignancies by blocking Wnt/beta-catenin signaling in gastric cancer cells. Biosci. Biotechnol. Biochem. 2018, 82, 456–465.
