Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Steven V. Pacia.
Sub-scalp Implantable Telemetric EEG (SITE) devices are under development for the treatment of epilepsy. However, beyond epilepsy, continuous EEG analysis could revolutionize the management of patients suffering from all types of brain disorders.
- SITE
- sub-scalp
- implantable EEG
- telemetric-EEG
1. SITE Device and Electrode Implantation
Minimally invasive SITE electrodes are implanted into the subdermal or subgaleal spaces over the cranium [65][1]. Electrodes may be inserted in a lateral to medial direction toward the vertex or from posterolateral to anterolateral to cover the temporal region [2,3][2][3]. In either case, electrode number and spatial resolution are limited to ensure minimally invasive procedures and long-term patient comfort [66,67][4][5]. Subgaleal device and electrode placement between the temporalis muscle insertions may result in less muscle artifact than laterally placed electrodes under the temporalis muscles [2,65][1][2]. Figure 1 shows a coronal MRI of a six-contact, subgaleal EEG electrode inserted above the left temporalis muscle and directed toward the cranial vertex. For patients where bi-hemispheric EEG sampling is necessary, the distal electrode may be inserted over the vertex contralaterally, leaving the proximal electrode ipsilateral, near the incision site. Alternatively, bi-hemispheric EEG sampling may be accomplished through bilateral device implantation [68][6].
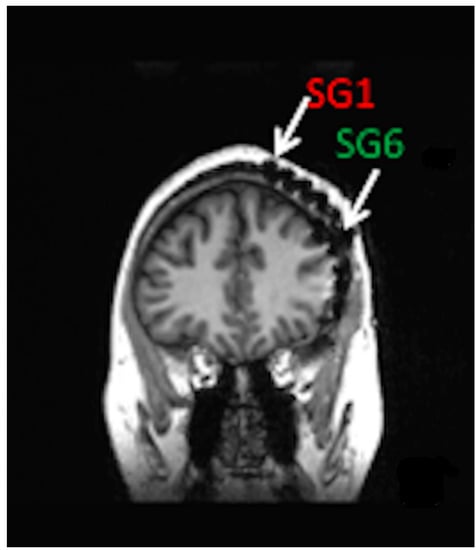
Figure 1. Coronal MRI of patient with left hemisphere intracranial EEG electrode placement with simultaneously placed 6-contact, subgaleal EEG electrode with the most distal contact, SG1 near the vertex and most proximal contact, and SG6 laterally at the site of electrode placement. For bi-hemispheric sampling, SG1 would be directed contralaterally past the vertex, with SG6 remaining over the left hemisphere.
Fully implantable SITE systems may be preferable to those with external batteries, eliminating the need for patient compliance [65][1]. Depending on the desired amount of EEG sampling and wireless signal transmission, implanted batteries are expected to last between two and three years before requiring replacement. SITE devices that use an external battery, similar to cochlear implants, have rechargeable batteries but require ongoing patient compliance to ensure continuous EEG sampling.
The operative procedure for fully implantable SITE devices may be performed in the outpatient setting under local anesthesia. Following a single scalp incision, a ribbon retractor shaped to approximate the curvature of the skull may be used to bluntly dissect a subgaleal pocket between the galea and the pericranium. Because the subgaleal space is a potential space, the body of the device and electrode array may be inserted into the pocket without concern for migration [2]. For partially implantable devices, only the electrode and transmitter need to be implanted, resulting in a smaller subgaleal dissection track [3]. However, maintaining proximity to the post-auricular external power source requires electrode insertion to pass under the temporalis muscle. Fully implantable SITE systems, as shown in Figure 2, may be deployed between the insertions of the temporalis muscles, over the cranium, essentially eliminating temporalis and mandibular muscle artifact.
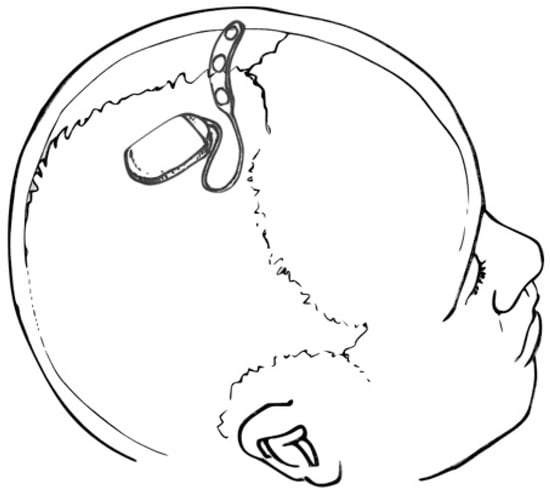
Figure 2. Drawing depicting a SITE device and multi-contact strip electrode placed over the cranial vertex (within the subgaleal space—not shown). Courtesy of artist/surgeon Werner K. Doyle, MD.
2. Therapeutic Monitoring
EEG monitoring with SITE devices presents an unprecedented opportunity for therapeutic monitoring. Continuous EEG screening and automated cloud-based analysis provide real-time access to therapeutic responses, not possible with routine laboratory-based EEG. For example, after establishing baseline TBR or alpha peak frequency for an ADHD patient, the effects of medication or cognitive behavioral therapy on the EEG may be assessed immediately, allowing for timely provider intervention. Similarly, instead of repeated polysomnograms or following oxygen saturation to assess the efficacy of OSA treatments, SITE EEG may provide both nightly sleep staging as well as verification of improved waking cerebral function and alertness, which is the ultimate goal of OSA treatment.
3. Overcoming Spatial Sampling Limitations
Due to the lower spatial resolution of SITE systems, the complex modeling of cortical sources and cerebral networks that require high-density electrode arrays are less appropriate for SITE devices [9][7]. Similarly, EEG microstate analysis which attempts to define very brief 80 to 100 msec stable global patterns of scalp potential topographies used to characterize large-scale neural networks would also be challenging for limited channel electrode arrays [72][8]. However, resting-state studies often lead to the discovery of limited cortical locations or regions of interest that characterize disease progression or therapeutic response. For example, short-term EEG studies using standard 10–20 electrode placements, for hemispheric asymmetry analysis, frequently identify asymmetries in single electrode pairs, like P3–P4 [73][9]. Targeted SITE electrode implantation, based on well-conceived based-line, high-density EEG studies, obviates the need for whole-brain EEG coverage, allowing for efficient data acquisition and focused analysis.
4. Sleep EEG Analysis
Sleep offers a structured and consistent opportunity to follow brain function over time. Discrete sleep oscillations, like sleep spindles that characterize stage 2, NREM sleep, are associated with memory consolidation [74,75][10][11]. Moreover, alterations in sleep spindle frequency and architecture occur in AD [76][12], schizophrenia, Autism [77][13], and PD [78][14]. For example, a study of sixty-eight PD patients without dementia were compared with forty- seven healthy controls to determine if sleep spindle analysis predicts dementia [78][14]. Sleep spindle density and amplitude were reduced in the eighteen patients who later developed dementia compared with the controls and PD patients who did not develop memory disorders. In addition to sleep spindle analysis, spectral density and topography features of synchronous low-frequency NREM sleep oscillations correlate with prognosis for memory and cognitive disorders, including AD [79][15].
More recently, closer analysis of sleep spindles shows significant intra-subject heterogeneity, leading to new analysis strategies to integrate continuous variables like frequency, amplitude, duration, and topography to identify patient-unique sleep biomarkers [80][16]. For example, instead of averaging spindle activity across sleep stages, Stokes and colleagues considered the complex temporal evolution of transient events, like spindles, as a dynamic process and developed a continuous sleep metric that proved to have strong night-to-night stability for individuals [81][17]. By coupling slow oscillation power with phase to create a power-phase representation for a full-night sleep recording, it becomes possible to create an EEG phenotype for an individual. The technique was applied to seventeen patients with schizophrenia, confirming previously established sleep spindle deficits but also discovering unique transient NREM events in low alpha and theta ranges that characterized each patient’s sleep. By applying dynamic analysis techniques like these to SITE, sleep biomarkers may reflect therapeutic response and disease progression not only for schizophrenia but for all cognitive disorders. Additionally, because sleep complexes and spindles are seen at the mid-line, SITE electrodes placed at the cranial vertex are appropriate for sleep EEG.
References
- Haneef, Z.; Yang, K.; Sheth, S.A.; Aloor, F.Z.; Aazhang, B.; Krishnan, V.; Karakas, C. Sub-scalp electroencephalography: A next-generation technique to study human neurophysiology. Clin. Neurophysiol. 2022, 141, 77–87.
- Pacia, S.V.; Doyle, W.K.; Friedman, D.; Bacher, D.H.; Kuzniecky, R.I. Intracranial EEG Validation of Single-Channel Subgaleal EEG for Seizure Identification. J. Clin. Neurophysiol. 2022, 39, 283–288.
- Duun-Henriksen, J.; Baud, M.; Richardson, M.P.; Cook, M.; Kouvas, G.; Heasman, J.M.; Friedman, D.; Peltola, J.; Zibrandtsen, I.C.; Kjaer, T.W. A new era in electroencephalographic monitoring? Subscalp devices for ultra–long-term recordings. Epilepsia 2020, 61, 1805–1817.
- Weisdorf, S.; Duun-Henriksen, J.; Kjeldsen, M.J.; Poulsen, F.R.; Gangstad, S.W.; Kjær, T.W. Ultra-long-term subcutaneous home monitoring of epilepsy-490 days of EEG from nine patients. Epilepsia. 2019, 60, 2204–2214.
- Castillo Rodriguez, M.L.A.; Brandt, A.; Schulze-Bonhage, A. Differentiation of subclinical and clinical electrographic events in long-term electroencephalographic recordings. Epilepsia 2022.
- Stirling, R.E.; Maturana, M.I.; Karoly, P.J.; Nurse, E.S.; McCutcheon, K.; Grayden, D.B.; Ringo, S.G.; Heasman, J.M.; Hoare, R.J.; Lai, A.; et al. Seizure Forecasting Using a Novel Sub-Scalp Ultra-Long Term EEG Monitoring System. Front. Neurol. 2021, 12, 713794.
- Babiloni, C.; Barry, R.J.; Başar, E.; Blinowska, K.J.; Cichocki, A.; Drinkenburg, W.H.I.M.; Klimesch, W.; Knight, R.T.; da Silva, F.L.; Nunez, P.; et al. International Federation of Clinical Neurophysiology (IFCN)—EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies. Clin. Neurophysiol. 2020, 131, 285–307.
- Zhang, K.; Shi, W.; Wang, C.; Li, Y.; Liu, Z.; Liu, T.; Li, J.; Yan, X.; Wang, Q.; Cao, Z.; et al. Reliability of EEG microstate analysis at different electrode densities during propofol-induced transitions of brain states. Neuroimage 2021, 231, 117861.
- Metzen, D.; Genç, E.; Getzmann, S.; Larra, M.F.; Wascher, E.; Ocklenburg, S. Frontal and parietal EEG alpha asymmetry: A large-scale investigation of short-term reliability on distinct EEG systems. Anat. Embryol. 2022, 227, 725–740.
- De Gennaro, L.; Ferrara, M. Sleep spindles: An overview. Sleep Med. Rev. 2003, 7, 423–440.
- Fogel, S.M.; Smith, C.T. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev. 2011, 35, 1154–1165.
- Katsuki, F.; Gerashchenko, D.; Brown, R.E. Alterations of sleep oscillations in Alzheimer’s disease: A potential role for GABAergic neurons in the cortex, hippocampus, and thalamus. Brain Res. Bull. 2022, 187, 181–198.
- Page, J.; Lustenberger, C.; Fröhlich, F. Nonrapid eye movement sleep and risk for autism spectrum disorder in early development: A topographical electroencephalogram pilot study. Brain Behav. 2020, 10, e01557.
- Latreille, V.; Carrier, J.; Lafortune, M.; Postuma, R.B.; Bertrand, J.-A.; Panisset, M.; Chouinard, S.; Gagnon, J.-F. Sleep spindles in Parkinson’s disease may predict the development of dementia. Neurobiol. Aging 2015, 36, 1083–1090.
- Grigg-Damberger, M.M.; Foldvary-Schaefer, N. Sleep Biomarkers Help Predict the Development of Alzheimer Disease. J. Clin. Neurophysiol. 2022, 39, 327–334.
- Gonzalez, C.; Jiang, X.; Gonzalez-Martinez, J.; Halgren, E. Human Spindle Variability. J. Neurosci. 2022, 42, 4517–4537.
- Stokes, P.A.; Rath, P.; Possidente, T.; He, M.; Purcell, S.; Manoach, D.S.; Stickgold, R.; Prerau, M.J. Transient oscillation dynamics during sleep provide a robust basis for electroencephalographic phenotyping and biomarker identification. Sleep 2022, 46, zsac223.
More
