You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Ashish Kumar Agrawal.
Triple-negative breast cancer (TNBC), the most aggressive and heterogenous type of cancer, lacks the expression of hormones like estrogen, progesterone, and human epidermal growth receptor-2, making chemotherapy the only treatment regimen against TNBC. To overcome the problem faced during chemotherapy, and to address the heterogeneity of TNBC, targeted therapy emerged based on the molecular profiling of TNBC. Several biomarkers are used as targets for the precision therapy in TNBC, including EGFR, VGFR, TP53, interleukins, insulin-like growth factor binding proteins, c-MET, androgen receptor, BRCA1, glucocorticoid, PTEN, ALDH1, etc.
- triple-negative breast cancer
- biomarkers
1. Introduction
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer, entailing no expression of estrogen receptor, progesterone receptor, and human epidermal receptor-2. It accounts for approximately 10–20% of total breast cancer cases and is found to be most prevalent in young African and Hispanic women [1]. According to the American Cancer Society and the National Cancer Institute, in 2020, approximately 276,480 new cases of TNBC occurred, wherein almost 42,170 women died [2]. TNBC is considered aggressive due to its heterogeneity, rapid metastasizing ability to the brain, lungs, and bones, and rapid onset of recurrence [3], which makes the treatment regimen difficult for TNBC. Moreover, as TNBC lacks the expression of hormones, endocrine therapy is out of the option, making chemotherapy the only treatment against TNBC [4]. From the molecular profiling, it came into focus that there are six molecular subtypes of TNBC, which include basal-like subtypes (BL1 and BL2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR) [5]. Further, on performing the genetic profiling of the molecular subtypes, it was found that these subtypes show either aberrant genetic expression or highly activated signaling pathways or receptors. For example, BL1 and BL2 subtypes show aberrant expressions of DNA-repair and cell-cycle regulating genes like MYC, PIK3CA, AKT2, CDK6, and BRCA2, and PTEN, RB1, and TP53, respectively. Similarly, MSL subtypes also show an aberrant expression of genes related to cell proliferation and stemness (ALDHA1, BCL2, BMP2, HOX, etc.) On the other hand, M and IM subtypes exhibit highly activated signaling pathways like Wnt, TGF-β, NK cell, IL-12, IL-7, etc. Moreover, LAR subtypes show highly activated androgen hormone-related signaling pathways [6]. It was thus inferred that the heterogeneous nature of TNBC might compromise the therapeutic efficacy of the chemotherapy.
Moreover, the conventional neoadjuvant chemotherapy exhibited pCR in 35–45% of TNBC patients only, and a majority of the TNBC patients showing responsiveness to conventional chemotherapy were limited to the non-metastatic stage [7]. Thus, to overcome such problems and make the treatment more precise, biomarkers have emerged as targeted therapeutic and diagnostic tools. Scientists are using cancer biomarkers to acquire knowledge regarding patients’ tumors to predict the personalized treatment regimens specific to particular TNBC subtypes. These predictive biomarkers include various germline and somatic mutations, genetic rearrangements, proteins, and metabolomics [8]. However, it was observed that none of the biomarkers achieve 100% in both sensitivity as well as specificity [9], and also that as the cancer treatment implements more combination therapy as compared to monotherapy, it becomes difficult to attach an identified biomarker with a single drug or target [10]. Hence, to increase specificity and to efficiently deliver multiple diagnostic and therapeutic molecules to a target site, nanoparticles (NPs) were developed based on their exclusive physiochemical characteristics. It was further observed that for improved sensitivity, and targetability, NPs are modulated to incorporate cancer-specific ligands having increased binding affinities towards TNBC biomarkers [9].
2. Biomarkers Derived from the Molecular Profiling of TNBC
Biomarkers are classified as reproducibly quantifiable biological variables. As defined by the National Institute of Health, clinically, they are considered measurable parameters used to evaluate the responses offered by therapeutic interventions. Additionally, they can be regarded as factors employed for early diagnosis, monitoring, and personalized treatment [11].
As discussed above, although in some TNBC patients targeted therapy does receive certain clinical benefits, the overall responses in TNBC patients remain limited. Such a scenario urges the need to develop more robust targeted approaches for improving the therapeutic outcomes in TNBC patients. The various biomarkers investigated as potential targets for TNBC include intracellular signalings like kinases, cell cycle, cell death regulation, and DNA damage (Figure 1) [12].
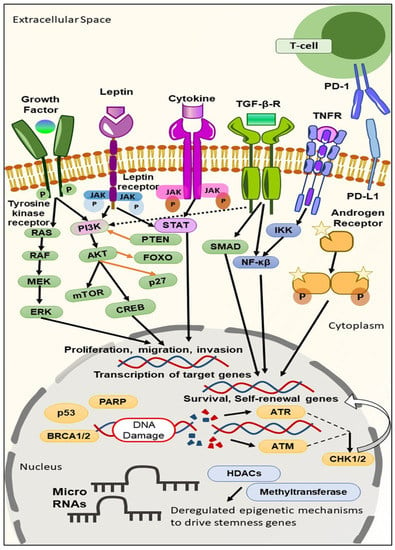
Figure 1. Different signaling pathways and epigenetic mechanisms that have been deregulated during TNBC progression and growth, contributing to stemness. Tyrosine kinase receptors promote tumorigenesis through Ras and the PI3K/AKT/mTOR signaling pathway. ERK phosphorylation, the activation of STAT, and the PI3K/AKT/mTOR pathway promote EMT, and regulate the proliferation, migration, and invasion of cancer cells. The activation of NF-κβ and the SMAD pathway promote the survival and self-renewal of genes. In the cytoplasm, the androgen receptor (AR) binds with the chaperone proteins and undergoes phosphorylation, which promotes the transcription of target genes in the nucleus. Within the nucleus, at the genetic level, the BRCA1/2, and p53 mutation, promote TNBC progression. PD-1/PD-L1 signaling suppresses CD8+ T activation, which in turn results in a tumor microenvironment and decreases tumor-infiltrating lymphocytes. The inactivation of PTEN, and FOXO, promote the PI3K/AKT/mTOR signaling pathway.
In recent times, a series of TNBC biomarkers have been evaluated. These TNBC biomarkers can be classified based on their usages as prognostic (biomarkers giving information regarding the overall outcome, regardless of the therapy), predictive (biomarkers providing information on the effect of a therapeutic intervention), and diagnostic (biomarkers confirming the presence of the disease) [13], based on the site where the biomarkers are found to be available like in the blood, cytoplasm, and nucleus, and on the surface of cells [10], or based on target expression in DNA, RNA, and proteins [14]. One biomarker can simultaneously be prognostic, predictive, and diagnostic [15]. It becomes reasonable to classify biomarkers based on the site where they are found, as this will aid in developing a strategy suitable for delivering the appropriate diagnostic and therapeutic moiety to the target site more efficiently. Moreover, if their area of availability is known, we can modulate or personalize the delivery or targeting system by changing their nature and characteristics.
2.1. TNBC Biomarkers on the Cell Surface
2.1.1. Folate Receptor
Cancer exhibits the overexpression of specific receptors, sometimes recognized as the biomarkers used to diagnose cancer. Folate receptor alpha (FRα) is one of the well-recognized prognostic biomarkers employed for the diagnosis of TNBC [16]. At the molecular level, folate plays an essential role in cell metabolism. FRα is a glycosylphosphatidylinositol (GPI) membrane-bound protein that exhibits increased binding affinity with folate, facilitating the increased transportation of folate into the cells. Hence, it was observed that the overexpression of FRα confers tumor growth via increased folate uptake, which may alter specific cellular signaling pathways and cause enhanced cell proliferation [17]. It was also found that folate sustains metabolic reactions, creating a suitable tumor microenvironment (TME) essential for the growth of cancer cells. Various genomic studies have revealed that 30% of the early-staged TNBC cases show the overexpression of FRα, whereas FRα is found to be overexpressed in 70–80% of stage IV metastatic TNBC cases [16].
2.1.2. Epidermal Growth Factor Receptor (EGFR)
The epidermal growth factor receptor (EGFR) belongs to the ErbB family of receptor tyrosine kinases (RTKs), delivering pivotal functions in cell physiology. EGFR was found to be overexpressed or mutated in 13–76% of TNBC cases. EGFR is an example of a prognostic biomarker [14][18][19]. In unstimulated conditions, EGFR remains dimerization-incompetent and auto-inhibited at the plasma membrane. On binding with the ligand, the receptor gets activated allosterically, undergoes dimerization, and facilitates autophosphorylation of the tyrosine residue, which finally triggers signaling cascades like cell growth, proliferation, metastasis, and angiogenesis [19].
2.1.3. Interleukin-3—Receptor α (IL-3Rα)
Interleukin-3 (IL-3) is a cytokine that is comprised of a heterodimeric receptor, consisting of an α-chain, which is the specific binding subunit, and a common β-chain, which is shared with GM-CSF (granulocyte-macrophage colony-stimulating factor) and IL-5 (interleukin-5) receptors. IL-3 is generated via activated T cells and mast cells and is associated with regulating hemopoietic pluripotent and expanding progenitor cells. It is a predictive biomarker. It was observed that on binding with its receptor, the IL-3 proceeds various biological processes such as the expression of adhesion molecules, proteins, and inflammatory and transcriptional factors. In addition, IL-3 plays a role in regulating cell survival and the proliferation of tumor-derived endothelial cells (TEC) and increasing the expression of the AKT signaling pathway and pro-tumorigenic and angiogenic receptors, thereby controlling the tumor microenvironment. From various clinical trials, it was observed that 55% of TNBC patients showed overexpression of IL-3R α. It was further observed that in TNBC, TECs release extracellular vesicles responsible for cell invasion, epithelial-to-mesenchymal transition (EMT), vascular mimicry (VM), and metastasis to secondary sites like the brain, bone, and lungs [20][21].
2.1.4. c-Kit
c-Kit, also known as CD117, is the receptor tyrosine kinase (RTK) for the stem cell factor (SCF), which is encoded by the proto-oncogene c-Kit situated on the 4q12 chromosome [22][23]. It is a prognostic biomarker. It was observed that c-Kit signaling plays a pivotal role in cellular differentiation [24], and that its gain-of-function mutation leads to the activation of various downstream pathways like the PI3K/AKT/mTOR, MAPK, and JAK/STAT transduction pathways [25]. It was observed that 25–45% of cases TNBC exhibits overexpression of c-Kit [22][26][27]. Earlier, sunitinib was employed against c-Kit-induced TNBC. Still, no improved results were observed, leading to the development of a new tyrosine kinase inhibitor that can block the functioning of c-Kit. Currently, dasatinib, sorafenib, and Nilotinib are used commercially to treat TNBC that exhibits overexpression of c-Kit [22][26].
2.1.5. c-Met
Similar to c-Kit, c-Met is also a prognostic biomarker. c-Met, also known as hepatocyte growth factor receptor (HGFR), is also a receptor tyrosine kinase that is encoded by c-Met proto-oncogene. It was observed that the activation of c-Met proto-oncogene via mutation, amplification, enhanced transcription, and increased ligand activation leads to increased cell growth, proliferation, invasion, and migration [28]. In ligand-activation mode, c-Met gets activated by binding with HGF in a paracrine method [29]. Such binding results in the autophosphorylation of tyrosine residues present within the kinase domain, further facilitating protein kinase cascade (PI3K/AKT/mTOR, ERK/mitogen-activated pathway, etc.) and resulting in cellular proliferation, invasion, migration, and angiogenesis [30]. The enhanced HGF levels were observed to be associated with reduced recurrence-free intervals and less survival outcomes [29]. Various studies revealed that overexpression of c-Met in TNBC is due to the increased copies of a c-Met proto-oncogene [31][32]. In both pre-clinical and clinical trials, it was observed that the combination of overexpression of c-Met proto-oncogene, and loss of p53, led to the development of the claudin-low subtype of TNBC [28].
2.1.6. Programmed Cell Death 1 Ligand (PD-L1)
Programmed cell death 1 ligand 1 (PD-L1) is a transmembrane protein in NK cells, B-cells, activated cytotoxic T cells, and vascular endothelial cells. CD274 encodes this protein and is a checkpoint regulator during the immune response. PD-L1 is a predictive biomarker. The binding of PD-1 with the PD-L1 ligand was observed to inhibit the IL-2 release, T-cell activation, and cell proliferation, thereby inhibiting the functioning of adaptive immune responses [33][34]. It was observed that the PD-1 binding regulates its tolerance to antigens and the expiration of immune responses, thereby restricting autoimmunity in a normal physiological situation. In contrast, in the presence of a tumor microenvironment, the binding serves as a pro-tumorigenic pathway that deactivates T-cells, further facilitating the escape of tumor cells from the immune surveillance. Various studies revealed that the activation of PD-L1 was controlled by multiple signaling pathways like the PI3K/AKT pathway, MAPK signaling pathway, JAK-STAT signaling pathway, aberrant WNT/β—catenin signaling pathway, NF-κβ signaling pathway, and hedgehog signaling pathway. It was observed that 20% of TNBC patients showed expression of PD-L1 [35].
2.1.7. Adenosine 2B Receptor (A2BR)
Adenosine receptors are G-protein coupled receptors that exist as four subtypes, namely Adora1 (A1R), Adora 2a (A2AR), Adora2b (A2BR), and Adora3 (A3R), which are characterized as either pertussis toxin-sensitive (A1R, and A3R), or pertussis toxin-insensitive (A2AR, and A2BR). A2BR is a type of prognostic biomarker. It was observed that cancer growth and progression depend on certain chemical messengers like cytokines, growth factors, and molecules like ATP and adenosine (Ado). Such findings also revealed that in the case of tumor hypoxia, the cell’s metabolic rate gets increased, demanding a high amount of ATP, which then gets metabolized to adenosine (Ado), further facilitating angiogenesis and inflammation, which are considered the two hallmark characteristics of cancer growth. It was observed that A2BR was found to be highly expressed in TNBC. It was further observed that A2BR mediates cAMP signaling, which inhibits the activation of T-cell receptors. This results in cell proliferation, invasion, and the secretion of anti-tumor cytokines like TNF-α and IFN-Υ. Interestingly, it was also revealed that Ado stimulated the expression of VEGF, which leads to enhanced intratumoral blood flow and angiogenesis by mediating purinergic P1 receptors, i.e., A2BR. Likewise, apart from VEGF, the activation of A2BR within the microvasculature also regulates the expression of other angiogenic factors like IL-8 and bFGF as well as results in the proliferation of cells which have an impact on cancer growth, invasion, and migration by inducing neo-vascularization within surrounding areas of cancer [36][37].
2.1.8. CD73
CD73 is a cell-surface glycosylphosphatidylinositol (GPI) that transforms extracellular adenosine monophosphate (AMP) into adenosine and inorganic phosphate [38][39]. It was observed that CD73 plays a role in regulating cancer growth and progression. CD73 is also a prognostic biomarker. On the genetic level, CD73 is considered an ectonucleotide, playing an important role in the purinergic CD39/CD73/adenosine signaling pathway. Further, it was observed that CD73 is responsible for the proliferation, migration, and angiogenesis of TNBC by mediating various signaling pathways, including the EGFR/Akt and VEGF/Akt signaling pathways. In addition to these, CD73 is also responsible for offering resistance to chemotherapy [38]. It was also observed that in TNBC, hypoxia induces the expression of CD73 by activating hypoxia-inducible factor-1α (HIF-1α), promoting epithelial-mesenchymal transition (EMT), cell invasion, and migration. Additionally, it was revealed that CD73-overexpressed TNBC is associated with poor outcomes due to immune evasion, as the adenosine safeguards the cancer cells from adaptive antitumor immune responses [39].
2.1.9. GABA Receptor π Subunit (GABRP)
GABRP is a prognostic biomarker. The GABRP gene encodes the π subunit of the GABA (gamma-aminobutyric acid) A receptor. Various studies found a correlation between the π subunit of the GABA A receptor and basal-like breast cancer subtypes including TNBC. It was revealed that the GABA-π subunit promotes cancer growth in cancer through ERK1/2 signaling. In addition, it was also revealed that breast cancer cell metastases to the brain show a GABAergic phenotype comprising the activation of the GABA A receptor, GABA transporters, and expression of GAD [40]. It was observed that 46–50% of TNBC patients exhibit brain metastasis, which correlates with poor survival. Brain metastasis involves cancer cell invasion, intravasation, and migration to brain cells by bypassing the blood-brain barrier (BBB) [41]. The RT–PCR study further revealed that the metastatic TNBC patients exhibited eight times higher GABRP expression than non–metastatic stage II–IV TNBC patients [42].
2.1.10. G–Protein-Coupled Receptor 161 (GPR161)
G–protein-coupled receptors (GPCRs) are found to be mutated or overexpressed in approximately 20% of all types of cancer, including TNBC, and are considered prognostic biomarkers. GPCRs are heptahelical membrane proteins essential in transducing signals from various ligands. From the genomic profiling, G–protein-coupled receptor 161 (GPR161) was found to be overexpressed in TNBC and is correlated with poor prognosis. GPR161 overexpression was associated with cell growth, proliferation, intracellular accumulation of E-cadherin, cell invasion, migration, and the development of multiacinar structures [43]. From various studies, it was observed that GPR161 knockdown diminishes cellular proliferation. Further, it was revealed that GPR161 forms a complex with multiple scaffold proteins, namely β-arrestin 2 in an ‘agonist-dependent’ manner [44] and Ile Gln motif-containing GTPase Activating Protein 1, and also binds with serine unit of IQGAP1, which overall leads to the activation of mTORC1, which is a sub-unit of the PI3K/AKT/mTOR signaling pathway, promoting cell proliferation and metastasis [45].
2.1.11. G–Protein-Coupled Kisspeptin Receptor (KISS1R)
G–protein-coupled kisspeptin receptor (KISS1R) is another type of GPCR associated with the progression of TNBC and is a prognostic biomarker. KISS1R, also known as GPR54, is a Gα–q/11–coupled GPCR, was found to be overexpressed in TNBC, and is associated with tumor invasion and migration [46]. In TNBC, the overexpression of KISS1R promotes EMT and results in tumor invasion by mediating MAPK and MT1-MMP signaling pathways and activating MMP-9 [47]. Further, KISS1R results in drug resistance by increasing the expression of ERK, AKT, and survivin [48]. It was also observed that the KISS1R pathway includes AXL as its signaling partner, and this was overexpressed in TNBC, depicting poor prognosis in TNBC patients [46].
2.1.12. Intercellular Adhesion Molecule-1 (ICAM-1)
Intercellular adhesion molecule-1 (ICAM-1) is a glycoprotein belonging to the immunoglobulin superfamily. It serves as an adhesion molecule. In addition to this, it elicits metastatic signaling [49]. ICAM-1 is a prognostic-type biomarker of TNBC. It was observed that ICAM-1 was upregulated in various cancers including TNBC. It was revealed that ICAM-1 forms a cross-linking that causes protein phosphorylation, modifications of the cytoskeleton, and the regulation of genes responsible for cell shape and migration [50]. It was observed from various studies that TNBC was associated with metastasis to the lungs, bone, and brain, and it was revealed that ICAM-1 overexpression resulted in lung metastasis of the TNBC cells. It was further observed that endothelial ICAM-1 facilitates the adhesion of leukocyte to endothelium via ICAM-1-LFA1 (lymphocyte function-associated antigen 1) and ICAM-1-Mac1 (macrophage-1 antigen) intercellular interactions, further mediating leukocyte transendothelial migration (TEM). ICAM-1 signaling also sustains the expression of CDK6 and other related pathways related to cell cycle and cell survival [51].
2.1.13. Leptin Receptor
Some studies revealed that the TNBC is also associated with weight gain (obesity), which in turn is associated with the excess secretion of adipokine protein, named Leptin (16kDa), by the adipocytes in response to obesity-related stimuli [52][53]. It was observed that 70–80% of TNBC cases show overexpression of leptin receptors. The leptin receptor is a prognostic-type biomarker of TNBC. It was found that leptin induces the growth and proliferation of cancer and mediates drug resistance [52]. It was observed that the binding of leptin to the leptin receptor facilitates the recruitment of JAK2 kinase, which later leads to the phosphorylation of STAT3 (pSTAT3), activating various downstream signaling pathways (Notch, JAK2, PI3K/AKT/mTOR, and MAPK), genes (Wnt4, ADHFE1, RDH5, etc.), and RBP-JK transcription factor, which is responsible for cell growth, proliferation, migration, and angiogenesis. Leptin binding to the leptin receptor also increases the progression of the cell cycle’s S-phase, apoptosis evasion, and chemoresistance [53].
2.1.14. Monocyte Chemoattractant Protein-1 (MCP-1)
In recent research, it came to light that obesity-related inflammations are also involved in cancer metastasis by producing specific chemokines. The Monocyte chemoattractant protein 1 (MCP-1) is one tumor-promoting chemokine associated with cancer progression. MCP-1 is also a prognostic biomarker. MCP-1 (12kD protein) belongs to the family of the C–C motif chemokine, which binds with the CCR2 receptor, which is a GPCR [54], where it recruits monocytes that later secrete CCL2 chemokine, resulting in tumor proliferation and invasion [55]. Additionally, it was demonstrated that overexpression of MCP-1 is associated with increased accumulation of M2 macrophages and their infiltration into the tumor microenvironment, mediating macrophage-driven angiogenesis [56]. It was observed that MCP-1 overexpression mediates cell invasiveness by activating the p44/42 MAP kinase (MAPK) signaling pathway [54].
2.1.15. Metabotropic Glutamate Receptor-1 (mGluR1)
Metabotropic glutamate receptors (mGluR1–mGluR2) are seven transmembrane domain receptors belonging to GPCRs that facilitate various responses of signaling molecules like chemokines, hormones, neurotransmitters, autocrine, and paracrine factors [57]. It was observed that out of eight mGluRs, mGluR1 and mGluR5 are associated with inducing strong pre-synaptic stimulation [58]. mGluR1 is a prognostic-type biomarker. It was further found that approximately 56% of TNBC patients showed overexpression of mGluR1 [59]. mGluR1, when coupled with Gαq-like protein, activates certain pro-proliferative signalings such as in phospholipase C (PLC), which facilitates the conversion of phosphatidylinositol into IP3 and DAG, activating MAPK and PI3K signaling cascade, which leads to various cellular functions like the regulation of the cell cycle and the activation of pro-survival and antiapoptotic proteins. It was also observed that, in TNBC, mGluR1 triggers the release of pro-inflammatory factors associated with metastasis of TNBC, namely TNF-β, IFN-α, and endothelial cells [60].
2.1.16. MDM2-Binding Protein (MTBP)
MDM2-binding protein (MTBP) is the transcriptional target of MYC oncogene, found to be overexpressed in various cancers including TNBC [61]. It is well known that MYC is a highly preserved oncogenic transcriptional factor that is overexpressed in various cancers and controls oncogenic behavior like increased cell differentiation, proliferation, metastasis, and apoptosis evasion. In addition to the stated activity, MTBP is associated with increased DNA replication [62]. MTBP is also a prognostic biomarker. Moreover, it was found that overexpression of MTBP prevents the self-ubiquitination of Mdm2, which causes Mdm2 stabilization and the enhanced degradation of the tumor suppressor gene, named p53, causing the growth and proliferation of TNBC. Additionally, it was noticed that on metastasis, the expression of MTBP gets downregulated temporarily; however, on getting localized into the metastasized site, the expression of MTBP gets upregulation, resulting in the proliferation of the TNBC cells [61].
2.1.17. Claudin Proteins
Claudins are tight junctional proteins existing between the epithelial cells, creating a barrier for the transport of macromolecules. However, in neoplastic cells, these tight junctions experience structural and functional defects which destroy them [63]. Various studies showed that 66.1% of TNBC cases show increased expression of claudin-4, along with an evident positive correlation with tumor size, nodal status, metastasis, and an expression of Ki-67 [63]. Claudin proteins are also considered prognostic-type biomarkers. Recently, it was found that in addition to claudin-4, claudin-3 and claudin-7 are also regarded as good prognostic factors in TNBC, and this was found relevant through their aberrant immunohistochemical expressions. It was further documented that increased expression of claudin-3 was correlated with the mutation of BRCA1 genes, and this further aids in testing BRCA mutation for TNBC patients [64]. There is another claudin protein named claudin-1 which, unlike the claudins mentioned above, serves as a tumor suppressor in TNBC. It was documented that the resurfacing of claudin-1 on TNBC cells induces apoptosis. From various clinical studies, it was observed that loss of expression of claudin-1 is associated with malignancy, invasiveness, and recurrence of TNBC [65].
2.1.18. Caveolin Proteins
Caveolins (Caveolin-1, 2, and 3) are scaffold proteins composed of cholesterol-enriched microdomains and play an essential role in tumor progression. It was further found that among various caveolins, caveolin-1 (Cav 1) plays a potential role in membrane trafficking, cell invasion and proliferation, cell migration, cell metastasis, and apoptosis, and belongs to the prognostic category of biomarkers. However, it was found that caveolin-1 can function as either a tumor suppressor or promoter, depending on the subtype of cancer in question. Cav-1 acts as an anti-proliferative factor in TNBC by arresting the cell cycle at the G2/M phase, which can be promoted by upregulating specific tumor suppressor genes, namely p21 and p27, and downregulating cyclin D2 [66]. In recent data, it was observed that the loss of normal Cav-1 is linked with the phosphorylation of AKT, TGF-β1, and acceleration of the aggressiveness of TNBC [67].
2.1.19. CCR5
CCR5 (C–C chemokine receptor type 5) is a seven-transmembrane GPCR highly expressed in TNBC patients. CCR5 is also a prognostic biomarker. One cohort study found that approximately 95% of TNBC patients were CCR5+, compared to the percentage of patients positive for CCR5 with other breast cancer subtypes [68]. It was observed that when the promoter region of CCR5 gets methylated, CCR5 protein results in overexpression [69]. It was further observed that overexpression of CCR5 results in increased Ca2+ signaling, which facilitates cellular migration in cancer cells. CCR5 also plays an important role in cell growth, proliferation, and the differentiation of immune cells by activating the PI3K signaling pathway, thereby inducing the activation of PDK1 and AKT [68]. Various studies showed that CCR5 overexpression is also positively associated with tumor immune cell infiltration via the activation of effector T-cells and tumor suppressor genes, and repression of YAP1 oncogenic pathways [69].
Recently, it was observed that blocking CCR5 results in anticancer activity. Such a phenomenon was showcased by the emergence of a humanized monoclonal antibody, Leronlimab (PRO 140), and CCR5 antagonist, maraviroc or vicriviroc. It was observed from the preclinical trial that the binding of Leronlimab to human CCR5 leads to the blockage of the CCR5-mediating signaling pathway, thereby preventing TNBC cell invasion [70][71].
Additionally, various in vitro and in vivo studies demonstrated that blocking or knocking down CCL5/CCR5 is harmful to metastatic tumors like TNBC, and thus limits their metastases. In May 2019, Leronlimab (PRO 140) was granted Fast Track Designation by the FDA for its application as a combination therapy with HAART for HIV-infected patients. Recently, Leronlimab has been filed as a drug of choice with the FDA for the treatment of CCR5+ mTNBC patients [72]. The filing was supported by the data from the second patient dosed with Leronlimab (Pro 140) under an emergency investigational new drug (IND) application granted by the FDA in September 2019. It was revealed that the TNBC patients receiving Leronlimab (PRO 140) exhibited no indication of metastases in the lungs and brain during the treatment [73]. In a similar context, phase Ib/II clinical study is ongoing for combining leronlimab with carboplatin (chemotherapy) for the treatment of CCR5+ mTNBC (NCT03838367). The preliminary studies showed an acceptable tolerability and efficacy with an increase in overall survival (OS) and progression-free survival (PFS) [7]. It was observed from the study that the patients who received leronlimab showed a significant 400–660% increase in 12-month PFS, as well as a 570–980% increase in 12-month OS, with a 72% decrease in circulating tumor cells [74].
Moreover, compassionate Use (NCT04313075) and the Basket Study (NCT04504942) were performed to evaluate the safety and efficacy profile of leronlimab at 12 months [75]. In compassionate study (NCT04313075) 2020, leronlimab (PRO 140) was combined with the treatment of physician’s choice (TPC) which included eribulin, gemcitabine, capecitabine, paclitaxel, nab-paclitaxel, vinorelbine, ixabepilone, or carboplatin for the treatment of CCR5+ mTNBC [76]. In the Basket Study (NCT04504942) of 2020, leronlimab (PRO 140) was administered to CCR5+ locally advanced or mTNBC patients. In this study leronlimab (PRO 140) was administered in continuation to the standard-of-care chemotherapy or radiotherapy [77].
In addition, the antibodies ipilimumab (NCT03546686) and tremelimumab (NCT02527434), which target CTLA4, Lacnotuzumab (NCT02435680, targeting CSF1/MCSF), tigatuzumab (NCT01307891, targeting human death receptor 5), utomilumab (NCT02554812, targeting CD137), and LAG525 (NCT03499899, targeting lymphocyte activation gene-3), are being actively analyzed for targeting TMNC (phase II clinical trial) [7].
In addition, the CCR5 antagonist blocks the CCR5 HIV co-receptor, which further leads to decreased in vitro invasion without affecting cell proliferation, and specifically, maraviroc decreases pulmonary metastasis [70][71].
2.1.20. Trop 2
Trophoblast cell surface antigen 2 (Trop-2) is an epithelial membrane surface glycoprotein that plays a pivotal role in cell growth, proliferation, and differentiation, and is found to be overexpressed in TNBC. It is both a prognostic as well as predictive type of biomarker. The overexpression of TNBC is associated with the transcription of various pro-oncogenes like NF-κβ, HOX, etc. The upregulation of Trop-2 was also initiated with the inactivation of TP63/TP53L, ERG, FOXP3, and other transcriptional factors. It was observed that overexpression of Trop-2 knocks out the TACSTD2 gene, further aiding in increased cell growth and proliferation [78]. Apart from regulating transcriptional factors, Trop-2 takes part in Ca2+ signaling where it mobilizes calcium into the cells, activating the MAPK, NF-κB, and RAF pathways, and increasing the expression levels of phosphorylated ERK1, ERK2, and FOXM1, thus resulting in enhanced cell proliferation, cell invasion, and metastasis. It was further noticed that the direct interaction of Trop-2 with β-catenin stimulated stem-cell-like properties. Clinically, it was found that antibody-drug conjugates (ADCs) serve as a therapeutic target for Trop-2 [79].
2.2. TNBC Biomarkers in the Cytoplasm
2.2.1. PI3K/AKT/mTOR Pathway
The PI3K/AKT/mTOR pathway is the pathway responsible for establishing a balance between two signaling molecules, namely phosphatidylinositol (4,5)—bisphosphate (PIP2) and phosphatidylinositol (3,4,5)—trisphosphate (PIP3), and acting antagonistically with PTEN (phosphatase and tensin homolog) [80]. It was observed that when the growth factors stimulate the signaling pathway, the phosphatidylinositol 4,5-bisphosphate 3- kinase catalytic subunit alpha isoform (PI3KCA) gets activated. The levels of PIP3 get increased, eventually driving the phosphorylation of protein kinase B (AKT) and other downstream functionalities like cell division, differentiation, and survival [81][82]. Further, it was observed that on hyperactivation of the PI3K signaling pathway, various oncogenes (PIK3CA, AKT, and mTOR) get activated, and tumor suppressor genes (PIK3R1, INPP4B, PTEN, TSC1, TSC2, and LKB) get inactivated [83]. The PI3K/AKT/mTOR signaling pathway is a predictive type of biomarker. It was observed that 10% of TNBC patients exhibit PI3KCA mutation while 30–50% of TNBC patients exhibit loss of PTEN expression [82]. The loss-of-function mutation of PTEN includes frameshift mutation and truncated mutation or homozygous deletion, which causes the loss of its functions, i.e., tumor suppression and the hyperactivation of AKT, which furthers lead to cell proliferation, resistance to apoptosis, and the switching of p27 from tumor suppressor to an oncogene [84][85].
2.2.2. Androgen Receptor (AR)
The androgen receptor (AR) belongs to the steroid receptor family and is a nuclear transcription factor. AR is usually found in the cytoplasm and translocates to the nucleus when bound with a ligand. It further gets attached to the androgen-related elements and facilitates cell proliferation [86]. It was observed that AR is overexpressed in 30–35% of TNBC cases [14]. AR is a prognostic biomarker of TNBC. It was further revealed that AR plays an essential role in the progression of TNBC. However, the impact of AR signaling on the prognosis of the TNBC patient remained controversial. It was indicated that being a transcriptional factor, AR controls specific genes associated with particular cell processes, such as stimulating or suppressing cell growth and cell death, etc. [87]. It was further observed that AR overexpression is associated with LAR-subtype TNBC. It was further observed that AR-positive TNBC exhibits decreased Ki-67 index and poor sensitivity to chemotherapy [14].
2.2.3. Aldehyde Dehydrogenase 1 (ALDH1)
Aldehyde dehydrogenase 1 (ALDH1) is a stem-cell-related marker in the cytoplasm of tumor-initiating cells [88]. ALDH1 was found to be overexpressed in TNBC patients and is associated with metastasis (tumor grade) and resistance to chemotherapy (taxane- and epirubicin-based) [89]. ALDH1 is a predictive-type biomarker. It was observed that ALDH1 is associated with cancer stem cells and results in early differentiation [90]. Further, on genomic profiling, it was demonstrated that SMAD4 was the transcription factor of ALDH1. SMAD4 facilitates the TGF-β signaling pathway and regulates genes associated with stemness like Twist1, Snail, and Slug. Thus, it could be inferred that ALDH1 plays a role in cellular differentiation, invasion, tumor development, apoptosis, and immune response [91].
2.2.4. HOX Genes
Abnormal expression of the HOX genes was found to be associated with the growth and proliferation of breast cancer. The HOX genes are considered prognostic biomarkers. The HOX genes are classified into four groups: HOXA, HOXB, HOXC, and HOXD. It was observed that the HOX genes were overexpressed in the primary cancer site with a prominent chance of metastasis. It was revealed that HOXB7 facilitates TNBC progression by activating the TGF-β/SMAD3 signaling pathway through SMAD3 phosphorylation [92]. Moreover, it was observed that HOX genes were regulated by the hypermethylation of the CpGs and epigenetic methylation, which further led to breast cancer tumorigenesis. It was further observed that primary TNBC cells showed three-fold overexpression of HOXB7 compared to normal breast cells [93]. It was also revealed that, contrary to the tumorigenic property of HOXB7, HOXD8 was considered a tumor suppressor gene. It was observed that HOXD8 overexpression diminishes the phosphorylation of AKT and mTOR, which further inactivates the AKT/mTOR signaling pathway and decreases tumor growth and proliferation [94].
2.2.5. Protein Kinase D1 (PKD1)
Protein Kinase D1 (PKD1), belonging to the Ca2+/calmodulin-dependent protein kinase (CAMPK) superfamily, is a serine/threonine kinase found to be expressed in almost all tissues. It is found to be overexpressed in cancers, including TNBC. It is a prognostic type of biomarker. It was observed that the activation of PKD1 was mediated in two ways: first was by the phosphorylation of two serine residues (S738/742) located at the activation loop of the catalytic core of protein kinase C (PKC), and the second was through the autophosphorylation of carboxy-terminal of the serine residue (S910). The activation of PKD1 further facilitates various oncogenic activities like cell proliferation, cell survival, migration, and membrane trafficking [95][96].
2.2.6. 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphate-4 (PFKFB4)
The most well-known characteristic of cancer cells is the huge production of lactate and pyruvate due to increased glycolysis despite oxygen availability. In this context, PFKFB4 plays an important role in glucose catabolism by regulating glycolytic flux. Moreover, it was observed that PFKFB4 facilitates hostile TME, which promotes the development of tumors in distant sites [97]. PFKFB4 is a prognostic biomarker of TNBC. One of the two primary isoenzymes of the family, namely PFKFB4, was found to be overexpressed in TNBC, and is associated with the regulation of the cell cycle, apoptosis, and autophagy. It was further found that the regulation of PFKFB4 expression was activated by HIF-1, which facilitates the cell to adapt to hypoxia and upregulates the expression of genes responsible for conducting glycolysis. Additionally, PFKFB4 was found to regulate the G1/S phase transition by enhancing the level of CDK6 and phosphorylating Rb. There is some evidence linked with the oncogenic activity of PFKFB4 which states that in TNBC, PFKFB4 regulates cell survival by regulating AKT signaling, the activity of caspase 3/7, and levels of ROS [98].
2.3. TNBC Biomarkers in the Nucleus
2.3.1. BRCA Genes
BRCA genes (BRCA1 and BRCA2) are tumor suppressor genes. They are predictive biomarkers of TNBC. They also repair DNA damage, recombine DNA strands, control cell-cycle checkpoints, and regulate apoptotic and transcriptional factors. It was observed that the mutation of BRCA genes leads to the impairment of their functioning as in the impairment of the DNA-repairing and recombination mechanism, disruption in controlling cell-cycle checkpoints, and dysregulation of apoptotic and transcriptional factors, and that such mutations are associated with the progression of breast cancer and ovarian cancer. According to some studies, it was revealed that TNBC shows the mutation of BRCA1 genes (BRCA1Mut), while BRCA2 mutation tends to exhibit similar pathological characteristics as those of normal BRCA genes. It was observed from the epidemiological survey that 5–10% of newly diagnosed TNBC cases in Western countries are associated with BRCA1 (8.5%) and BRCA2 (2.7%) genes (BRCA1/2), and from the current meta-analysis, it was further revealed that such mutation is affiliated with a 40–57% lifetime risk of female TNBC. It was further shown that TNBC patients with BRCA1/2 carriers exhibited a high risk of contralateral breast cancer (≈50%) [99][100].
2.3.2. TP53
One of the important reasons for the failure of TNBC therapy is the lack of identifiable targeted molecular alterations responsible for TNBC progression, therapy resistance, and relapse. The current studies found that ≈80% of TNBC cases showed mutations of the TP53 gene that further led to the production of a mutant p53 protein. Like BRCA genes, TP53 genes are also considered tumor suppressor genes that modulate cell cycle functioning, repair DNA strands and apoptosis, and are predictive biomarkers (69). It was further observed that most TP53 mutation occurs at the DNA-binding domain, resulting in a dysfunctional cell cycle [101]. It was also found that TP53 mutation is associated with the overexpression of CDK7, an essential component of CDK-activating kinase, and plays a pivotal role in cell division and transcription [102].
2.3.3. Activating Transcription Factor 4 (ATF4)
Activating transcription factor 4 (ATF4) belongs to the ATF/CREB family and functions as a transcription factor. It is found to be overexpressed in various tumors, including in TNBC tumors. ATF4 is a prognostic biomarker. It was observed that when a cell experiences stress, like hypoxia, endoplasmic reticulum stress (ERS), or nutrient deprivation, the integrated stress response (ISR) gets activated, and this helps in preserving homeostasis. Further, the ISR activation results in the reduction of global protein synthesis through the phosphorylation of eIF2α (eukaryotic translation initiation factor 2 alpha), which ultimately leads to the activation of ATF4, which controls the fate of the cancer cells by regulating cell growth, proliferation, invasion, migration, autophagy, and resistance to chemotherapy. It was further confirmed that the phosphorylation of eIF2α was initiated via double-stranded RNA-dependent protein kinase (PKR, EIF2AK2), endoplasmic reticulum kinase (PERK, EIF2AK3), general control nonderepressible 2 kinases (GCN2, EIF2AK4), and heme-regulated inhibitor (HRI, EIF2AK1) [103]. It was observed that in TNBC, overexpression of ATF4 was correlated with poor OS after diagnosis (approximately 37 months) [104]. Genetic profiling further revealed that the overexpression of ATF4 was associated with the canonical SMAD-dependent TGF-β pathway. The study depicted that the depletion of ATF4 further reduced the activity of TGF-β and diminished the expression of the SMAD2/3/4 pathway, indicating the existence of a feedback loop between ATF4 and the TGF-β pathway. Such findings further demonstrated that TGF-β and SMAD2/3/4 constitute the upstream signaling pathway of ATF4, regulating the positive feedback loop of the TGF-β pathway associated with TNBC aggressiveness [103].
2.3.4. ETS Translocation Variant4 (ETV4)
ETS translocation variant (ETV4) is a transcription factor belonging to the PEA3 subfamily of ETS (E–26). It was observed that in cancer cells, ETV4 is expressed at a higher level compared to normal cells. ETV4 also acts as an oncogenic protein that can enhance cancer growth, progression, and metastasis. Further analysis showed that EGFR induces nuclear translocation of ETV4 by activating matrix metalloproteinase (MMP)–9 and 14. It was observed that 57% of TNBC cases exhibit overexpression of ETV4 proteins. ETV4 is a prognostic biomarker. ETV4 protein overexpression was also associated with lymph nodes and lymphovascular invasion [105]. Additionally, ETV4 mediates the expression of MMP13, which plays an important role in proliferation, invasion, and migration [106].
2.3.5. Forkhead Box M1 (FOXM1)
Forkhead Box M1 (FOXM1) belongs to the family of fork-head/winged-helix proteins associated with various biological processes like cell proliferation, differentiation, DNA damage repair, and angiogenesis [107]. Various studies revealed that FOXM1 was found to be overexpressed in cancers including TNBC. Notably, 85% of TNBC patients show overexpression of FOXM1, which serves as a prognostic biomarker. In TNBC, it was observed that FOXM1 promotes cell progression and invasion by direct binding with eEF2K [108]. Wei et al., 2015 further revealed that FOXM1 regulates EMT by activating the SNAIL gene. Studies also demonstrated that FOXM1 activation or overexpression also excises other biological processes in TNBC via reprogramming energy metabolism, inflammation, apoptosis evasion, promoting genomic instability, and enabling replicative mortality [109].
2.3.6. Glucocorticoids
In recent times, steroid receptors have emerged as potential prognostic and predictive-type biomarkers in TNBC. Among various steroid receptors, glucocorticoid receptors (GRs) are overexpressed in TNBC. It was observed that the binding of GC to GRs facilitates the translocation of GC to the nucleus, where it undergoes dimerization and enhances the transcription of GC-inducible genes, which results in anti-apoptotic activity and multi-drug resistance [110]. Preclinical studies revealed that GRs’ antiproliferative effect was mediated via BRCA1, where their activity resulted in the phosphorylation of downstream signaling pathways like MAPK. However, some pieces of evidence indicate that the long-term activity of GRs diminishes the expression of BRCA1, whereas the accumulation of free GRs enhances the expression of BRCA1. Such statements require evidence circumventing the specific mechanisms of GRs so that they can be employed as applicable proteomic biomarkers in the therapy of TNBC [111]. Recent studies have revealed that an increased mortality rate was observed in TNBC patients that exhibited overexpression of GRs because GR overexpression can activate the oncogenes, leading to the suppression of the tumor suppressor gene and preventing apoptosis and resulting in unfavorable clinical outcomes. Further, it was observed that in addition to MDR, GR overexpression is also associated with increased recurrence [112].
2.4. TNBC Biomarkers in the Blood
2.4.1. Vascular Endothelial Growth Factor (VEGF)
Cancer cells require oxygen and nutrients for their growth and angiogenesis, i.e., the formation of new blood vessels aids in providing these substrates. The critical facilitator of angiogenesis is vascular endothelial growth factor (VEGF), mostly induced by hypoxia. Hence, VEGF can be considered an appealing target for developing anticancer therapeutics and is a prognostic biomarker of TNBC [18]. VEGFs were found to be overexpressed in 30–60% of TNBC patients [14]. It was also observed that VEGFR gets activated in the presence of a mutated p53 gene. The activated VEGFR further stimulates the JAK2/STAT3 signaling pathway and increases proliferation, migration, angiogenesis, and chemoresistance [113]. It was depicted that in cancer, VEGF gets glycosylated and further binds with VEGFR, stimulates the process of angiogenesis, and increases the permeability of neighboring blood vessels and lymphatics, ultimately resulting in increased cancer metastasis [14].
2.4.2. Interleukin-8 (IL-8)
The progression of TNBC needs the simultaneous expression of interleukin 8 (IL-8). In TNBC, IL-8 acts as a predictive biomarker. From the xenograft animal model, it was observed that inhibiting the expression of IL-8 facilitated the suppression of cell progression, colony formation, migration, etc. [114]. IL-8 is a pro-inflammatory multifunctional chemokine that binds with the chemokine receptors, namely CXCR1 and CXCR2, and as a result the various signaling pathways like mitogen-activated protein kinase (MAPK) and Akt get activated. Additionally, it was observed that the production of IL-8 can be regulated via various factors like lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), and IL-1β. In cancer, IL-8 overexpression is associated with the induction of cyclin D1 and B1, resulting in increased tumor progression, angiogenesis, and cell invasion and migration [114][115]. In TNBC, IL-8 production is associated with hypoxic conditions and aids in recruiting mesenchymal stem cells (MSCs) to the primary site of TNBC, creating a microenvironment around the tumor. Such aberrant physiological conditions increase multi-drug resistance (MDR) and metastatic risk [10].
Although researchers have described the role of each biomarker in the progression of TNBC, it was recently observed that scientists are endorsing a combination of biomarkers for better and more efficient results. In such a scenario, the specificity and the sensitivity of individual biomarkers are kept optimum so that they can result in a better prognosis or an effective diagnosis. Hence, before selecting a biomarker or combination of biomarkers, the family history of the concerned patient, and their lifestyle, should be considered. Additionally, specific ideal characteristics of biomarkers recorded include the expression of biomarkers in the early stage of the disease and the ability to discriminate the diseased population from the healthy population. All these factors and criteria result in effective therapy and diagnosis [116].
In Table 1, researchers have listed various clinical studies that have been completed or are ongoing involving the participation of biomarkers in the treatment of TNBC.
Table 1.
Clinical trials of biomarkers in TNBC.
| Agents | Biomarkers (Targeting Moiety) | Clinical Phase | Identifier |
|---|---|---|---|
| APR-246 + Pembrolizumab | TP53 + PD-1 | I/II | NCT04383938 |
| Ribociclib + Bicalutamide | CDK4/6 | I/II | NCT03090165 |
| Taselisib + Enzalutamide | PI3K/AKT/mTOR | I/II | NCT02457910 |
| Alpelisib + Enzalutamide | PI3K/AKT/mTOR | I | NCT03207529 |
| Olaparib + Carboplatin/Paclitaxel | PARP | I | NCT00516724 |
| MEDI4736 + Olaparib and/or Cediranib | PD-L1 + PARP + VEGFR | I/II | NCT02484404 |
| Olaparib + Durvalumab | PARP + PD-L1 | II | NCT03801369 |
| Talazoparib | PARP | II | NCT03901469 |
| Olaparib + Onalespib | PARP + HSP90 | I | NCT02898207 |
| HX008 + Niraparib | PD-1 + PARP | II | NCT04508803 |
| Prexasertib | CHK1 | II | NCT02873975 |
| IDX-1197 | PARP | I/II | NCT04174716 |
| Avelumab | PD-L1 | II | NCT02554812 |
| Nivolumab + Bicalutamide + Ipilimumab | PD-1 + AR + CTLA4 | II | NCT03650894 |
| Avelumab + Binimetinib, Utomilumab, or anti-OX40 antibody | PD-L1 + MEK ½, CD 137 or OX40 | II | NCT03971409 |
| Atezolizumab in different combinations | PD-L1 in different combinations, including chemotherapy, ADC, CD40, IL6R, VEGFA, and AKT | I/II | NCT03424005 |
| Spartalizumab + LAG525 in combination with NIR178, Capmatinib, MCS110, or Canakinumab | PD-1 + LAG-3 in combination with anti- adenosine A2A receptor, Met receptor, CSF-1 or IL1β | I | NCT03742349 |
| Sacituzumab govitecan + Talazoparib | ADC + PARP | I/II | NCT04039230 |
| AMXI-5001 | PARP and a microtubule polymerization inhibitor | I/II | NCT04503265 |
| BKM120/BYL719 + Olaparib | PI3K + PARP | I | NCT01623349 |
References
- Shohdy, K.S.; Almeldin, D.S.; Fekry, M.A.; Ismail, M.A.; AboElmaaref, N.A.; ElSadany, E.G.; Hamza, B.M.; El-Shorbagy, F.H.; Ali, A.S.; Attia, H.; et al. Pathological responses and survival outcomes in patients with locally advanced breast cancer after neoadjuvant chemotherapy: A single-institute experience. J. Egypt. Natl. Cancer Inst. 2021, 33, 39.
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507.
- Wahba, H.A.; El-Hadaad, H.A. Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med. 2015, 12, 106–116.
- Won, K.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261.
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61.
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121.
- Mo, H.; Renna, C. Biomarker-Driven Targeted Therapies in Solid Tumor Malignancies. J. Hematol. Oncol. Pharm. 2021, 11, 84–91.
- Ye, F.; Zhao, Y.; El-Sayed, R.; Muhammed, M.; Hassan, M. Advances in nanotechnology for cancer biomarkers. Nano Today 2018, 18, 103–123.
- Fleisher, B.; Clarke, C.; Ait-Oudhia, S. Current advances in biomarkers for targeted therapy in triple-negative breast cancer. Breast Cancer Targets Ther. 2016, 8, 183–197.
- da Silva, J.L.; Cardoso Nunes, N.C.; Izetti, P.; de Mesquita, G.G.; de Melo, A.C. Triple negative breast cancer: A thorough review of biomarkers. Crit. Rev. Oncol. Hematol. 2020, 145, 102855.
- Deng, X.; Zheng, C.; Tang, F.; Rosol, T.J.; Shao, Z.-M. Editorial: Triple-negative breast cancer: Heterogeneity, tumor microenvironment and targeted therapy. Front. Oncol. 2022, 12, 1026566.
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015, 4, 256–269.
- Sukumar, J.; Gast, K.; Quiroga, D.; Lustberg, M.; Williams, N. Triple-negative breast cancer: Promising prognostic biomarkers currently in development. Expert Rev. Anticancer. Ther. 2021, 21, 135–148.
- Chang, J.Y.H.; Ladame, S. Diagnostic, prognostic, and predictive biomarkers for cancer. In Bioengineering Innovative Solutions for Cancer; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–21.
- DeCarlo, A.; Malardier-Jugroot, C.; Szewczuk, M.R. Next-Generation Multimodality of Nanomedicine Therapy: Folic Acid Conjugated Copolymer and Folate Receptor Interactions Disrupt Receptor Functionality Resulting in Dual Therapeutic AntiCancer Potential in Triple-Negative Breast Cancer. Preprints 2020, 2020080316.
- Norton, N.; Youssef, B.; Hillman, D.W.; Nassar, A.; Geiger, X.J.; Necela, B.M.; Liu, H.; Ruddy, K.J.; Polley, M.-Y.C.; Ingle, J.N.; et al. Folate receptor alpha expression associates with improved disease-free survival in triple negative breast cancer patients. NPJ Breast Cancer 2020, 6, 4.
- Ferrara, N. VEGF as a Therapeutic Target in Cancer. Oncology 2005, 69, 11–16.
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of theEGFRin cancer. Mol. Oncol. 2017, 12, 3–20.
- Koni, M.; Castellano, I.; Venturelli, E.; Sarcinella, A.; Lopatina, T.; Grange, C.; Cedrino, M.; Femminò, S.; Cossu-Rocca, P.; Orrù, S.; et al. Interleukin-3-Receptor-α in Triple-Negative Breast Cancer (TNBC): An Additional Novel Biomarker of TNBC Aggressiveness and a Therapeutic Target. Cancers 2022, 14, 3918.
- Lopatina, T.; Grange, C.; Cavallari, C.; Navarro-Tableros, V.; Lombardo, G.; Rosso, A.; Cedrino, M.; Pomatto, M.A.C.; Koni, M.; Veneziano, F.; et al. Targeting IL-3Rα on tumor-derived endothelial cells blunts metastatic spread of triple-negative breast cancer via extracellular vesicle reprogramming. Oncogenesis 2020, 9, 90.
- López-Mejía, J.A.; Tallabs-Utrilla, L.F.; Salazar-Sojo, P.; Mantilla-Ollarves, J.C.; Sánchez-Carballido, M.A.; Rocha-Zavaleta, L. c-Kit Induces Migration of Triple-Negative Breast Cancer Cells and Is a Promising Target for Tyrosine Kinase Inhibitor Treatment. Int. J. Mol. Sci. 2022, 23, 8702.
- Nogi; Toriumi, Y.; Fukushima, H.; Uchida, K.; Nogi, H.; Kobayashi, T.; Suzuki, M.; Tabei, I.; Kawase, K. EGFR as paradoxical predictor of chemosensitivity and outcome among triple-negative breast cancer. Oncol. Rep. 1994, 21, 413–417.
- Shams, T.M.; Shams, M.E. Overexpression of c-KIT (CD117) in triple-negative breast cancer. Egypt. J. Pathol. 2011, 31, 113–117.
- Jansson, S.; Bendahl, P.-O.; Grabau, D.A.; Falck, A.-K.; Fernö, M.; Aaltonen, K.; Rydén, L. The Three Receptor Tyrosine Kinases c-KIT, VEGFR2 and PDGFRα, Closely Spaced at 4q12, Show Increased Protein Expression in Triple-Negative Breast Cancer. PLoS ONE 2014, 9, e102176.
- Zhu, Y.; Wang, Y.; Guan, B.; Rao, Q.; Wang, J.; Ma, H.; Zhang, Z.; Zhou, X. C-kit and PDGFRA gene mutations in triple negative breast cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 4280–4285.
- Johansson, I.; Aaltonen, K.E.; Ebbesson, A.; Grabau, D.; Wigerup, C.; Hedenfalk, I.; Rydén, L. Increased gene copy number of KIT and VEGFR2 at 4q12 in primary breast cancer is related to an aggressive phenotype and impaired prognosis. Genes Chromosom. Cancer 2011, 51, 375–383.
- Gaule, P.B.; Crown, J.; O’Donovan, N.; Duffy, M.J. cMET in triple-negative breast cancer: Is it a therapeutic target for this subset of breast cancer patients? Expert. Opin. Ther. Targets 2014, 18, 999–1009.
- Breen, L.; Gaule, P.B.; Canonici, A.; Walsh, N.; Collins, D.M.; Cremona, M.; Hennessy, B.T.; Duffy, M.J.; Crown, J.; Donovan, N.O.; et al. Targeting c-Met in triple negative breast cancer: Preclinical studies using the c-Met inhibitor, Cpd A. Investig. New Drugs 2020, 38, 1365–1372.
- Ho-Yen, C.M.; Jones, J.L.; Kermorgant, S. The clinical and functional significance of c-Met in breast cancer: A review. Breast Cancer Res. 2015, 17, 52.
- Gonzalez-Angulo, A.M.; Chen, H.; Karuturi, M.S.; Chavez-MacGregor, M.; Tsavachidis, S.; Meric-Bernstam, F.; Do, K.-A.; Hortobagyi, G.N.; Thompson, P.A.; Mills, G.B.; et al. Frequency of mesenchymal-epithelial transition factor gene (MET) and the catalytic subunit of phosphoinositide-3-kinase (PIK3CA) copy number elevation and correlation with outcome in patients with early stage breast cancer. Cancer 2012, 119, 7–15.
- Yi, Y.W.; You, K.; Bae, E.J.; Kwak, S.-J.; Seong, Y.-S.; Bae, I. Dual inhibition of EGFR and MET induces synthetic lethality in triple-negative breast cancer cells through downregulation of ribosomal protein S6. Int. J. Oncol. 2015, 47, 122–132.
- Soliman, H.; Khalil, F.; Antonia, S. PD-L1 Expression Is Increased in a Subset of Basal Type Breast Cancer Cells. PLoS ONE 2014, 9, e88557.
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 15–24.
- Cocco, S.; Piezzo, M.; Calabrese, A.; Cianniello, D.; Caputo, R.; Di Lauro, V.; Fusco, G.; Di Gioia, G.; Licenziato, M.; De Laurentiis, M. Biomarkers in Triple-Negative Breast Cancer: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 4579.
- Neumann, C.A.; Levine, K.; Oesterreich, S. Targeting adenosine receptor 2B in triple negative breast cancer. J. Cancer Metastasis Treat. 2018, 4, 13.
- Fernandez-Gallardo, M.; González-Ramírez, R.; Sandoval, A.; Felix, R.; Monjaraz, E. Adenosine Stimulate Proliferation and Migration in Triple Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0167445.
- Lafont, V.; Michaud, H.-A.; Bonnefoy, N. CD73: A new biomarker in triple-negative breast cancer. Transl. Cancer Res. 2018, 7, S594–S596.
- Petruk, N.; Tuominen, S.; Åkerfelt, M.; Mattsson, J.; Sandholm, J.; Nees, M.; Yegutkin, G.G.; Jukkola, A.; Tuomela, J.; Selander, K.S. CD73 facilitates EMT progression and promotes lung metastases in triple-negative breast cancer. Sci. Rep. 2021, 11, 6035.
- Sizemore, G.M.; Sizemore, S.T.; Seachrist, D.D.; Keri, R.A. GABA(A) Receptor Pi (GABRP) Stimulates Basal-like Breast Cancer Cell Migration through Activation of Extracellular-regulated Kinase 1/2 (ERK1/2). J. Biol. Chem. 2014, 289, 24102–24113.
- Azizi-Tabesh, G.; Kamaliyan, Z.; Darbeheshti, F.; Omranipour, R.; Soleimani, V.; Alipour, N.; Mirfakhraie, R.; Yassaee, V.R. Overexpression of GABRP Gene in Triple Negative Breast Cancer: Molecular Mechanisms and Interpretation. Int. J. Cancer Manag. 2021, 14, e119130.
- Juvale, I.I.A.; Hassan, Z.; Has, A.T.C. The Emerging Roles of π Subunit-Containing GABAA Receptors in Different Cancers. Int. J. Med Sci. 2021, 18, 3851–3860.
- Feigin, M.E.; Xue, B.; Hammell, M.C.; Muthuswamy, S.K. G-protein–coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc. Natl. Acad. Sci. USA 2014, 111, 4191–4196.
- Feigin, M.E.; Xue, B.; Hammell, M.; Muthuswamy, S.K. Abstract A236: The orphan G-protein coupled receptor GPR161 is an oncogene in triple negative breast cancer. Mol. Cancer Ther. 2013, 12, A236.
- Gutierrez, A.N.; McDonald, P.H. GPCRs as Emerging Cancer Drug Targets: Target Validation of oGPCR, GPR161 and its Role in Triple Negative Breast Cancer. FASEB J. 2019, 33, 674.4.
- Blake, A.; Dragan, M.; Tirona, R.G.; Hardy, D.B.; Brackstone, M.; Tuck, A.B.; Babwah, A.V.; Bhattacharya, M. G protein-coupled KISS1 receptor is overexpressed in triple negative breast cancer and promotes drug resistance. Sci. Rep. 2017, 7, srep46525.
- Dragan, M.; Nguyen, M.-U.; Guzman, S.; Goertzen, C.; Brackstone, M.; Dhillo, W.S.; Bech, P.R.; Clarke, S.; Abbara, A.; Tuck, A.B.; et al. G protein-coupled kisspeptin receptor induces metabolic reprograming and tumorigenesis in estrogen receptor-negative breast cancer. Cell Death Dis. 2020, 11, 106.
- Guzman, S.; Brackstone, M.; Radovick, S.; Babwah, A.V.; Bhattacharya, M.M. KISS1/KISS1R in Cancer: Friend or Foe? Front. Endocrinol. 2018, 9, 437.
- Rosette, C.; Roth, R.B.; Oeth, P.; Braun, A.; Kammerer, S.; Ekblom, J.; Denissenko, M.F. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis 2005, 26, 943–950.
- Guo, P.; Huang, J.; Wang, L.; Jia, D.; Yang, J.; Dillon, D.A.; Zurakowski, D.; Mao, H.; Moses, M.A.; Auguste, D.T. ICAM-1 as a molecular target for triple negative breast cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14710–14715.
- Taftaf, R.; Liu, X.; Singh, S.; Jia, Y.; Dashzeveg, N.K.; Hoffmann, A.D.; El-Shennawy, L.; Ramos, E.K.; Adorno-Cruz, V.; Schuster, E.J.; et al. ICAM1 initiates CTC cluster formation and trans-endothelial migration in lung metastasis of breast cancer. Nat. Commun. 2021, 12, 4867.
- Otvos, L.; Kovalszky, I.; Riolfi, M.; Ferla, R.; Olah, J.; Sztodola, A.; Nama, K.; Molino, A.; Piubello, Q.; Wade, J.D.; et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur. J. Cancer 2011, 47, 1578–1584.
- Lipsey, C.C.; Harbuzariu, A.; Robey, R.W.; Huff, L.M.; Gottesman, M.M.; Gonzalez-Perez, R.R. Leptin Signaling Affects Survival and Chemoresistance of Estrogen Receptor Negative Breast Cancer. Int. J. Mol. Sci. 2020, 21, 3794.
- Dutta, P.; Sarkissyan, M.; Paico, K.; Wu, Y.; Vadgama, J.V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 2018, 170, 477–486.
- Kanga, K.J.; Mendonca, P.; Soliman, K.F.; Ferguson, D.T.; Darling-Reed, S.F. Effect of Diallyl Trisulfide on TNF-α-induced CCL2/MCP-1 Release in Genetically Different Triple-negative Breast Cancer Cells. Anticancer. Res. 2021, 41, 5919–5933.
- Cranford, T.L.; Velázquez, K.T.; Enos, R.T.; Bader, J.E.; Carson, M.S.; Chatzistamou, I.; Nagarkatti, M.; Murphy, E.A. Loss of monocyte chemoattractant protein-1 expression delays mammary tumorigenesis and reduces localized inflammation in the C3(1)/SV40Tag triple negative breast cancer model. Cancer Biol. Ther. 2017, 18, 85–93.
- Banda, M.; Speyer, C.L.; Semma, S.N.; Osuala, K.O.; Kounalakis, N.; Torres, K.E.T.; Barnard, N.J.; Kim, H.J.; Sloane, B.F.; Miller, F.R.; et al. Metabotropic Glutamate Receptor-1 Contributes to Progression in Triple Negative Breast Cancer. PLoS ONE 2014, 9, e81126.
- Speyer, C.L.; Smith, J.S.; Banda, M.; Devries, J.A.; Mekani, T.; Gorski, D.H. Metabotropic glutamate receptor-1: A potential therapeutic target for the treatment of breast cancer. Breast Cancer Res. Treat. 2011, 132, 565–573.
- Bastiaansen, A.E.M.; Timmermans, A.M.; Smid, M.; van Deurzen, C.H.M.; Hulsenboom, E.S.P.; der Smissen, W.J.C.P.-V.; Foekens, R.; Trapman-Jansen, A.M.A.C.; Smitt, P.A.E.S.; Luider, T.M.; et al. Metabotropic glutamate receptor 1 is associated with unfavorable prognosis in ER-negative and triple-negative breast cancer. Sci. Rep. 2020, 10, 22292.
- Sexton, R.E.; Hachem, A.H.; Assi, A.A.; Bukhsh, M.A.; Gorski, D.H.; Speyer, C.L. Metabotropic glutamate receptor-1 regulates inflammation in triple negative breast cancer. Sci. Rep. 2018, 8, 16008.
- Iwakuma, T.; Tochigi, Y.; Van Pelt, C.S.; Caldwell, L.C.; Terzian, T.; Parant, J.M.; Chau, G.P.; Koch, J.G.; Eischen, C.M.; Lozano, G. Mtbp haploinsufficiency in mice increases tumor metastasis. Oncogene 2007, 27, 1813–1820.
- Grieb, B.C.; Eischen, C.M. MTBP and MYC: A Dynamic Duo in Proliferation, Cancer, and Aging. Biology 2022, 11, 881.
- Naimi, A.; Zare, N.; Amjadi, E.; Soltan, M. High claudin-4 antigen expression in triple-negative breast cancer by the immunohistochemistry method. J. Res. Med Sci. 2022, 27, 20.
- Gowrikumar, S.; Singh, A.B.; Dhawan, P. Role of Claudin Proteins in Regulating Cancer Stem Cells and Chemoresistance-Potential Implication in Disease Prognosis and Therapy. Int. J. Mol. Sci. 2019, 21, 53.
- Geoffroy, M.; Kleinclauss, A.; Kuntz, S.; Grillier-Vuissoz, I. Claudin 1 inhibits cell migration and increases intercellular adhesion in triple-negative breast cancer cell line. Mol. Biol. Rep. 2020, 47, 7643–7653.
- Qian, X.-L.; Pan, Y.-H.; Huang, Q.-Y.; Shi, Y.-B.; Huang, Q.-Y.; Hu, Z.-Z.; Xiong, L.-X. Caveolin-1: A multifaceted driver of breast cancer progression and its application in clinical treatment. OncoTargets Ther. 2019, 12, 1539–1552.
- Yeong, J.; Thike, A.A.; Ikeda, M.; Lim, J.C.T.; Lee, B.; Nakamura, S.; Iqbal, J.; Tan, P.H. Caveolin-1 expression as a prognostic marker in triple negative breast cancers of Asian women. J. Clin. Pathol. 2017, 71, 161–167.
- Jiao, X.; Nawab, O.; Patel, T.; Kossenkov, A.V.; Halama, N.; Jaeger, D.; Pestell, R.G. Recent Advances Targeting CCR5 for Cancer and Its Role in Immuno-Oncology. Cancer Res. 2019, 79, 4801–4807.
- Wang, X.; Han, Y.; Peng, J.; He, J. CCR5 is a prognostic biomarker and an immune regulator for triple negative breast cancer. Aging 2021, 13, 23810–23830.
- Velasco-Velázquez, M.; Jiao, X.; De La Fuente, M.; Pestell, T.G.; Ertel, A.; Lisanti, M.P.; Pestell, R.G. CCR5 Antagonist Blocks Metastasis of Basal Breast Cancer Cells. Cancer Res. 2012, 72, 3839–3850.
- Cristofanilli, M.; Chittoria, N.; Ehsani, S.; Rui, H.; Dolezal, M.; Stork-Sloots, L.; de Snoo, F.; Recknor, C.; Abramson, V. Abstract P5-17-08: A phase Ib/II study of leronlimab combined with carboplatin in patients with CCR5+ metastatic triple-negative breast cancer (mTNBC). Cancer Res. 2022, 82, P5–P17.
- Stewart, J. Leronlimab FDA Approval Status. 2021. Available online: https://www.drugs.com/history/leronlimab.html (accessed on 4 May 2023).
- Mumal, I. CytoDyn Files for FDA Breakthrough Therapy Status for Leronlimab for Metastatic TNBC. Available online: https://breastcancer-news.com/2020/01/14/cytodyn-files-for-breakthrough-therapy-designation-for-leronlimab-for-mtnbc/ (accessed on 4 May 2023).
- Pelosci, A. Leronlimab Provides a Significant Survival Benefit in Metastatic Triple-Negative Breast Cancer. Available online: https://www.cancernetwork.com/view/leronlimab-provides-a-significant-survival-benefit-in-metastatic-triple-negative-breast-cancer (accessed on 27 April 2023).
- Brett, E.; Duscher, D.; Pagani, A.; Daigeler, A.; Kolbenschlag, J.; Hahn, M. Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment. Int. J. Mol. Sci. 2022, 23, 14159.
- CytoDyn, I. A Compassionate Use Study of Leronlimab in Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04313075 (accessed on 1 May 2023).
- CytoDyn, I. Basket Study of Leronlimab (PRO 140) in Patients With CCR5+ Locally Advanced or Metastatic Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04504942 (accessed on 1 May 2023).
- Jeon, Y.; Jo, U.; Hong, J.; Gong, G.; Lee, H.J. Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer 2022, 22, 1014.
- Izci, H.; Punie, K.; Waumans, L.; Laenen, A.; Wildiers, H.; Verdoodt, F.; Desmedt, C.; Ardui, J.; Smeets, A.; Han, S.N.; et al. TROP-2 expression in triple-negative breast cancer: Correlations with tumor-infiltrating lymphocytes, histological subtypes and survival. Res. Sq. 2022, 1–22.
- Al-Saad, S.; Donnem, T.; Al-Shibli, K.; Persson, M.; Bremnes, R.M.; Busund, L.-T. Diverse prognostic roles of Akt isoforms, PTEN and PI3K in tumor epithelial cells and stromal compartment in non-small cell lung cancer. Anticancer. Res. 2009, 29, 4175–4183.
- West, L.; Vidwans, S.J.; Campbell, N.P.; Shrager, J.; Simon, G.R.; Bueno, R.; Dennis, P.A.; Otterson, G.A.; Salgia, R. A Novel Classification of Lung Cancer into Molecular Subtypes. PLoS ONE 2012, 7, e31906.
- Ellis, H.; Ma, C.X. PI3K Inhibitors in Breast Cancer Therapy. Curr. Oncol. Rep. 2019, 21, 110.
- Pascual, J.; Turner, N.C. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann. Oncol. 2019, 30, 1051–1060.
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296.
- Xie, Y.; Naizabekov, S.; Chen, Z.; Tokay, T. Power of PTEN/AKT: Molecular switch between tumor suppressors and oncogenes. Oncol. Lett. 2016, 12, 375–378.
- Rampurwala, M.; Wisinski, K.B.; O’Regan, R. Role of the androgen receptor in triple-negative breast cancer. Clin. Adv. Hematol. Oncol. H&O 2016, 14, 186–193.
- Gerratana, L.; Basile, D.; Buono, G.; De Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; De Santo, I.; Del Mastro, L.; et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat. Rev. 2018, 68, 102–110.
- Kim, S.J.; Kim, Y.S.; Jang, E.D.; Seo, K.J.; Kim, J.S. Prognostic Impact and Clinicopathological Correlation of CD133 and ALDH1 Expression in Invasive Breast Cancer. J. Breast Cancer 2015, 18, 347–355.
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032.
- Panigoro, S.S.; Kurnia, D.; Kurnia, A.; Haryono, S.J.; Albar, Z.A. ALDH1 Cancer Stem Cell Marker as a Prognostic Factor in Triple-Negative Breast Cancer. Int. J. Surg. Oncol. 2020, 2020, 7863243.
- Yamada, A.; Suzuki, C.; Shima, H.; Kida, K.; Adachi, S.; Yamamoto, S.; Narui, K.; Tanabe, M.; Shimizu, D.; Taniguchi, R.; et al. Aldehyde Dehydrogenase 1-related Genes in Triple-negative Breast Cancer Investigated Using Network Analysis. Anticancer. Res. 2020, 40, 6733–6742.
- de Bessa Garcia, S.; Araújo, M.; Pereira, T.; Freitas, R. HOXB7 Overexpression Leads Triple-Negative Breast Cancer Cells to a Less Aggressive Phenotype. Biomedicines 2021, 9, 515.
- Paço, A.; Leitão-Castro, J.; Freitas, R. Epigenetic Regulation of CDH1 Is Altered after HOXB7-Silencing in MDA-MB-468 Triple-Negative Breast Cancer Cells. Genes 2021, 12, 1575.
- Zhang, Y.; Yu, Y.; Su, X.; Lu, Y. HOXD8 inhibits the proliferation and migration of triple-negative breast cancer cells and induces apoptosis in them through regulation of AKT/mTOR pathway. Reprod. Biol. 2021, 21, 100544.
- Spasojevic, C.; Marangoni, E.; Vacher, S.; Assayag, F.; Meseure, D.; Château-Joubert, S.; Humbert, M.; Karam, M.; Ricort, J.M.; Auclair, C.; et al. PKD1 is a potential biomarker and therapeutic target in triple-negative breast cancer. Oncotarget 2018, 9, 23208–23219.
- Merzoug-Larabi, M.; Youssef, I.; Bui, A.T.; Legay, C.; Loiodice, S.; Lognon, S.; Babajko, S.; Ricort, J.-M. Protein Kinase D1 (PKD1) Is a New Functional Non-Genomic Target of Bisphenol A in Breast Cancer Cells. Front. Pharmacol. 2020, 10, 1683.
- Dai, T.; Rosario, S.R.; Katsuta, E.; Dessai, A.S.; Paterson, E.J.; Novickis, A.T.; Gomez, E.C.; Zhu, B.; Liu, S.; Wang, H.; et al. Hypoxic activation of PFKFB4 in breast tumor microenvironment shapes metabolic and cellular plasticity to accentuate metastatic competence. Cell Rep. 2022, 41, 111756.
- Cai, Y.-C.; Yang, H.; Shan, H.-B.; Su, H.-F.; Jiang, W.-Q.; Shi, Y.-X. PFKFB4 Overexpression Facilitates Proliferation by Promoting the G1/S Transition and Is Associated with a Poor Prognosis in Triple-Negative Breast Cancer. Dis. Markers 2021, 2021, 8824589S.
- Peshkin, B.N.; Alabek, M.L.; Isaacs, C. BRCA1/2 mutations and triple negative breast cancers. Breast Dis. 2011, 32, 25–33.
- Hahnen, E.; Hauke, J.; Engel, C.; Neidhardt, G.; Rhiem, K.; Schmutzler, R.K. Germline Mutations in Triple-Negative Breast Cancer. Breast Care 2017, 12, 15–19.
- Mitri, Z.I.; Abuhadra, N.; Goodyear, S.M.; Hobbs, E.A.; Kaempf, A.; Thompson, A.M.; Moulder, S.L. Impact of TP53 mutations in Triple Negative Breast Cancer. NPJ Precis. Oncol. 2022, 6, 64.
- Peng, J.; Yang, M.; Bi, R.; Wang, Y.; Wang, C.; Wei, X.; Zhang, Z.; Xie, X.; Wei, W. Targeting Mutated p53 Dependency in Triple-Negative Breast Cancer Cells Through CDK7 Inhibition. Front. Oncol. 2021, 11, 664848.
- González-González, A.; Muñoz-Muela, E.; Marchal, J.A.; Cara, F.E.; Molina, M.P.; Cruz-Lozano, M.; Jiménez, G.; Verma, A.; Ramírez, A.; Qian, W.; et al. Activating Transcription Factor 4 Modulates TGFβ-Induced Aggressiveness in Triple-Negative Breast Cancer via SMAD2/3/4 and mTORC2 Signaling. Clin. Cancer Res. 2018, 24, 5697–5709.
- Wang, M.; Lu, Y.; Wang, H.; Wu, Y.; Xu, X.; Li, Y. High ATF4 Expression Is Associated With Poor Prognosis, Amino Acid Metabolism, and Autophagy in Gastric Cancer. Front. Oncol. 2021, 11, 740120.
- Yuan, Z.-Y.; Dai, T.; Wang, S.-S.; Peng, R.-J.; Li, X.-H.; Qin, T.; Song, L.-B.; Wang, X. Overexpression of ETV4 protein in triple-negative breast cancer is associated with a higher risk of distant metastasis. OncoTargets Ther. 2014, 7, 1733–1742.
- Dumortier, M.; Ladam, F.; Damour, I.; Vacher, S.; Bièche, I.; Marchand, N.; de Launoit, Y.; Tulasne, D.; Chotteau-Lelièvre, A. ETV4 transcription factor and MMP13 metalloprotease are interplaying actors of breast tumorigenesis. Breast Cancer Res. 2018, 20, 73.
- Saba, R.; Alsayed, A.; Zacny, J.P.; Dudek, A.Z. The Role of Forkhead Box Protein M1 in Breast Cancer Progression and Resistance to Therapy. Int. J. Breast Cancer 2016, 2016, 9768183.
- Huang, S.; Hu, P.; Lakowski, T.M. Bioinformatics driven discovery of small molecule compounds that modulate the FOXM1 and PPARA pathway activities in breast cancer. Pharm. J. 2022, 1–12.
- Tan, Y.; Wang, Q.; Xie, Y.; Qiao, X.; Zhang, S.; Wang, Y.; Yang, Y.; Zhang, B. Identification of FOXM1 as a specific marker for triple-negative breast cancer. Int. J. Oncol. 2018, 54, 87–97.
- Kanai, A.; McNamara, K.M.; Iwabuchi, E.; Miki, Y.; Onodera, Y.; Guestini, F.; Khalid, F.; Sagara, Y.; Ohi, Y.; Rai, Y.; et al. Significance of glucocorticoid signaling in triple-negative breast cancer patients: A newly revealed interaction with androgen signaling. Breast Cancer Res. Treat. 2020, 180, 97–110.
- Vilasco, M.; Bracaps; Communal, L.; Hugon-Rodin, J.; Penault-Llorca, F.; Mourra, N.; Wu, Z.; Forgez, P.; Gompel, A. Loss of glucocorticoid receptor activation is a hallmark of BRCA1-mutated breast tissue. Breast Cancer Res. Treat. 2013, 142, 283–296.
- Buschmann, D.; González, R.; Kirchner, B.; Mazzone, C.; Pfaffl, M.; Schelling, G.; Steinlein, O.; Reithmair, M. Glucocorticoid receptor overexpression slightly shifts microRNA expression patterns in triple-negative breast cancer. Int. J. Oncol. 2018, 52, 1765–1776.
- Zhu, X.; Zhou, W. The Emerging Regulation of VEGFR-2 in Triple-Negative Breast Cancer. Front. Endocrinol. 2015, 6, 159.
- Fu, S.; Lin, J. Blocking Interleukin-6 and Interleukin-8 Signaling Inhibits Cell Viability, Colony-forming Activity, and Cell Migration in Human Triple-negative Breast Cancer and Pancreatic Cancer Cells. Anticancer. Res. 2018, 38, 6271–6279.
- Kim, S.; Lee, J.; Jeon, M.; Lee, J.E.; Nam, S.J. MEK-dependent IL-8 induction regulates the invasiveness of triple-negative breast cancer cells. Tumor Biol. 2015, 37, 4991–4999.
- Verma, M.; Patel, P.; Verma, M. Biomarkers in Prostate Cancer Epidemiology. Cancers 2011, 3, 3773–3798.
More
