Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Peter Akah.
Globally, cancer is one of the deadliest diseases, needing a meticulous diagnosis and targeted treatment plan to achieve an initial prognosis, followed by precision and optimization in treatment. Nonselective targeting, difficulty in accurately monitoring treatment end-results, serious drug side-effects, and severity of disease resulting in metastasis are the key flaws of traditional techniques. Nanotechnology and nanoparticles possess special features to completely transform the field of diagnosis and treatment of cancer.
- cancer
- nanotheranostics
- nanocarrier
1. Introduction
According to the World Health Organization (WHO), cancer is one of the most important health challenges in both industrialized and developing countries. The rates of cancer morbidity and mortality are growing at an alarming level as a result of heightened pollution, aging population, and other factors. By 2030, the annual cancer incidence is predicted to reach 23.6 million [1]. The American Cancer Society rated the number of new cancer cases and deaths in the United States to be approximately 1.9 million and 608,000 in 2021 [2]. There are over 100 different forms of cancer, and they can affect any body part, including tissues and organs, due to rampant aberrant cell proliferation. The prostate and breast cancers are the most frequently diagnosed in men and women, respectively [3].
Breast cancer is the most often diagnosed cancer in women globally, accounting for one out of every four cancer cases. It is the prominent cause of cancer-related death in women. Breast cancer accounted for one out of every eight malignancies diagnosed in 2020, based on the expected 2.3 million new cases. Furthermore, statistics showed that breast cancer killed approximately 684,996 people in 2020, with a significant percentage of these fatalities happening in rural areas [4]. In 2022, 287,850 women and 2710 men were estimated to be diagnosed with breast cancer, with 43,250 women and 530 men estimated to die from the disease [5]. Today, the histopathologic type is frequently used as the first classification for breast cancer. The most common kind of breast cancer, invasive ductal carcinoma, accounts for most instances, but other, less common subtypes are nonetheless of interest due to their aggressiveness and prevalence in certain patient subpopulations (e.g., inflammatory breast cancer often occurs in younger patients) [6]. The tumor’s stage is typically the next significant worry. The original tumor in the breast (stage 1) often spreads to neighboring tissues and lymph nodes (stage 2–3) or distant organs as the disease advances (distant metastasis, i.e., stage 4) [7]. The most typical locations for breast cancer metastasis are the liver, lung, bone, and brain [8]. Due to the dramatic rise in mortality once the tumor has spread, staging is essential. Additionally, breast cancer is categorized according to its molecular subtypes, such as triple-negative, human epidermal growth factor receptor-2 (HER2), luminal A, and luminal B [9]. Hormone receptors (progesterone and estrogen) are expressed in luminal A and B. The most typical treatments for these two categories of breast cancer include estrogen antagonists such as tamoxifen and aromatase inhibitors. Estrogen and progesterone receptors are negative in breast cancers that are HER2-positive, whereas HER2 is overexpressed. The conventional treatment for this kind of breast cancer involves targeting HER2 with certain antibodies such as Trastuzumab. The most dangerous type of breast cancer is basal-like breast cancer, which is HER2- and hormone-negative (sometimes called triple-negative breast cancer, TNBC). TNBC currently has few treatment options available. The 5-year survival rate for metastatic breast cancer is below 30% [10]; despite significant breakthroughs in therapeutic approaches, 30% of patients with initial breast cancer may eventually proceed to the metastatic stage of this illness [11]. Exploring new therapies is, therefore, urgently needed to improve outcomes in various breast cancer subtypes.
Prostate cancer (PC or PCa), on the other hand, is the second most common cancer in men, with survival varying depending on the age and ethnicity of the victims, their daily routines, and the time since of detection. In the United States, prostate cancer is the most common malignancy and the second leading cause of cancer death in men [2]. It is estimated that 248,530 men were diagnosed with prostate cancer and 34,130 men died from the disease in 2021 [2]. The American cancer society speculated that in 2022 around 268,490 new cases and around 34,500 new deaths occurred because of PC in the USA [12]. PC is brought on by a mutation in the cells of the prostate gland, which causes them to multiply uncontrollably and eventually metastasize [13]. The etiology of prostate cancer is thought to be influenced by a number of factors, including radiation, toxins in the environment, age, genetics, and environmental pollutants. However, the precise mechanism is yet unknown [14]. A dismal prognosis of cancer is traced to the fact that PC is typically found when the cancer has spread to the bone or lymph, even though it is treatable if discovered early [15]. Prostate-specific antigen (PSA), which is frequently increased in prostate malignancies, is the most prevalent biomarker associated with prostate cancer [16]. Early detection of PC is essential for their prognosis because PC therapy largely relies on the stage of the cancer, with later stages being practically hard to cure [17]. It is exceedingly difficult to detect PC in patients at its early stage since it does not exhibit any symptoms, which is why it has a record of high mortality rate. If PC is to be cured, it has to be diagnosed at its budding stage. Traditional methods for diagnosing PC included rectal examination, tissue biopsy, and detection of prostate-specific antigen (PSA) from common biochemical assays [18,19,20][18][19][20].
Despite the fact that all three methods are frequently employed for PC detection, they all have some drawbacks. The sensitivity and specificity of the PSA are extremely low [21,22][21][22]. In a similar vein, DRE cannot offer early identification of PC regardless of the direct vision of the tumor gland status [23]. Additionally, the entire detection process is extremely uncomfortable and unpleasant due to direct touch with the patient’s prostate gland. The main downside of biopsies is the risk of infection from the bacterium inflaming the sick gland.
Generally, the cancer onus is increasing, putting forth enormous physical, psychological, and economic tension on persons, households, societies, and healthcare organizations. Several healthcare organizations in developing nations are ill-equipped to handle this onus, and many cancer sufferers around the world lack prompt access to high-quality diagnosis and therapy. However, with early diagnosis, superior therapies, and aftercare, cancer recovery rates have improved in nations with robust healthcare organizations.
Late detection and ineffective treatment account for the underlying determinants of greater death among cancer sufferers. Chemotherapy, surgery, radiation therapy, immunotherapy, and hormone therapy remain the conventional strategies in the treatment of cancer [1]. This strategy first requires diagnosing the patient, followed by treating the ailment or disorder using a well-known remedy. As a result, pharmaceutical and medical investigations have mostly relied on defining a disease type and then finding a drug or treatment modalities for that ailment [24]. However, due to their low specificity, these strategies are often constrained, as they might also harm normal tissue and/or the immune system, resulting in adverse effects. Furthermore, cancer therapies other than surgery can prompt resistance in tumor cells, reduced drug distribution, difficulty in permeating biological barriers, and inefficacy in tackling malignant ailment. Hence, the absence of standard modalities for therapeutic evaluation at the early stages is a significant drawback in outright cancer eradication [25]. As a result, anticancer investigations are always focused on finding more efficacious therapeutic techniques [26].
Nanomedicine is an interdisciplinary area that brings together nanotechnology, pharmaceutical science, and biomedical research to create new delivery frameworks for diagnostics and therapy [27,28][27][28]. The term “nano” refers to one-billionth of a meter. Any material employed in the formulation of medicine that results in a finished product from 1 to 100 nm in size is referred to as a nanoparticle [29]. The word “theranostics” refers to the concurrent convergence of diagnosis and therapy [30]. Therefore, nanotheranostics is the practice of combining the diagnostic and therapeutic activities of nanoscale materials or nanoparticles into a single entity (Figure 1). In cancer research, this technology has the possibility to transform the way wresearchers address the disease, not only in terms of treatment, but also in terms of diagnosis and prognosis.
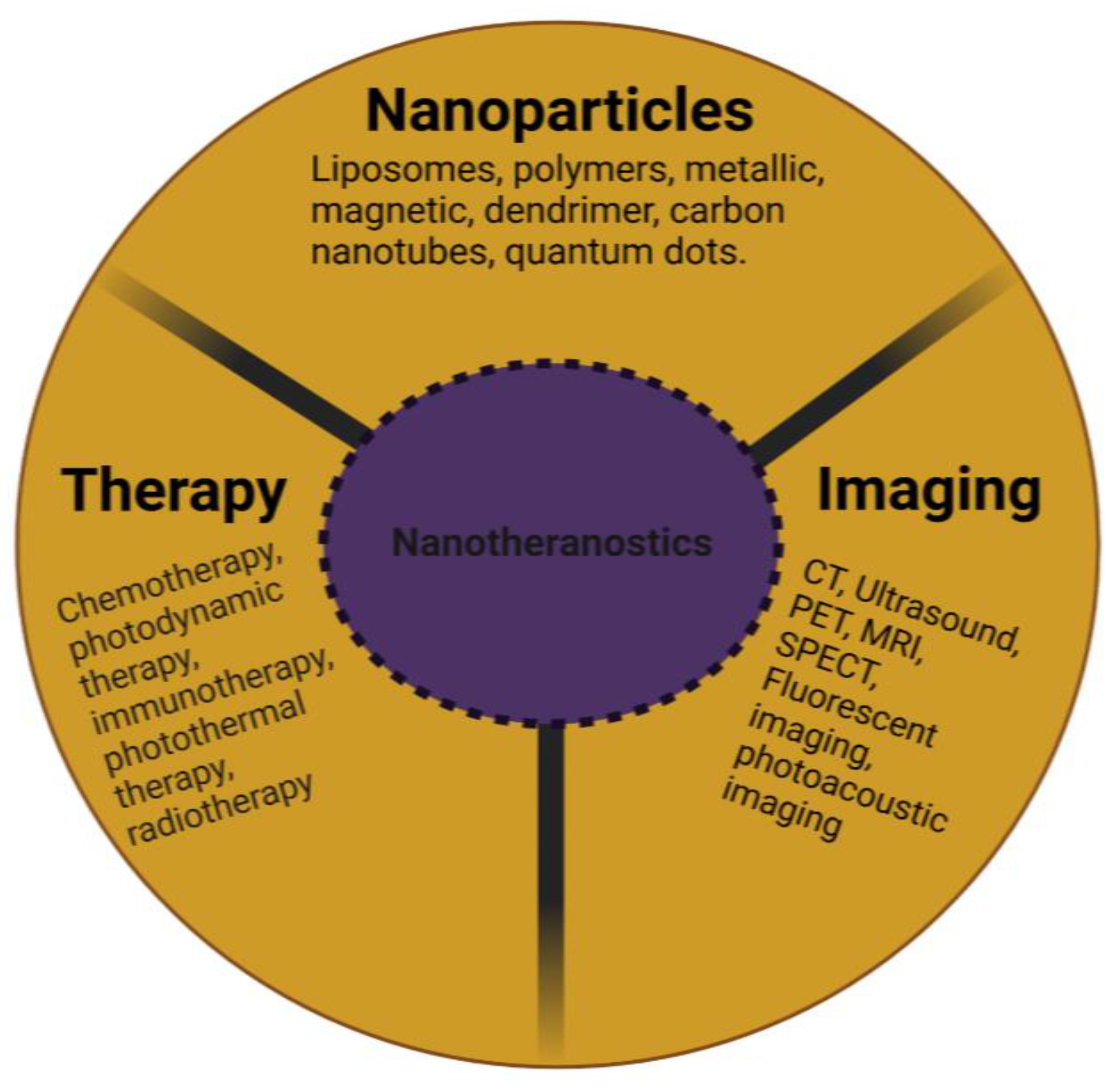
Figure 1. Schematic representation of the composition of a nanotheranostic system.
2. Nanotheranostic Platforms
The ideal nanoparticle should have biocompatible, biodegradable, nontoxic and non-immunogenic biological features, as well as the capacity to encapsulate both lipophobic and lipophilic drugs, diagnostic agents, and targeting moieties. These protect them against systemic inactivation and renal clearance in addition to enhancing their tumor targeting and real-time monitoring [31,32][31][32].
2.1. Liposomes
Liposomes and lipid nanocapsules (LNCs) are two lipid-based nanodelivery platforms. Liposomes, which are made up of a hydrophilic “core” surrounded by lipid bilayers, can contain hydrophobic or hydrophilic medicines in the lipid bilayer or the aqueous core, respectively [33] (Figure 2). Liposomes are frequently utilized as nanocarriers in drug delivery systems, while their size, biocompatibility, biodegradability, low toxicity, non-immunogenicity, and ability to encapsulate hydrophilic, lipophilic, and amphiphilic medicines give considerable benefits [34]. Liposomes possess the capability to incorporate both therapeutic and diagnostic agents which allow for a revolutionary use of liposomal delivery systems as theranostic platforms [35,36,37][35][36][37]. For instance, a hydrophilic gene probe for imaging hypoxia was loaded inside a PEGylated liposome that also contained a hydrophobic photosensitizer and the basic phospholipids lecithin and cholesterol. The liposomal transport of the probe was identified utilizing fluorescence imaging prior to undergoing therapeutic photodynamic therapy [38,39][38][39]. Liposomes and PEGylated liposomes were functionalized with gadolinium(III) diethylenetriamine pentaacetic acid salt, which served as a magnetic resonance imaging (MRI) contrast, and zinc phthalocyanine, which functioned as a model photosensitizer. The results showed how effective liposomal formulations could be as imaging agents [40]. A novel system containing near-infrared (NIR) carbon dots and cinobufagin, an anticancer drug, was developed and investigated as a potential anticancer nanotheranostic. Although cells could take up liposomes and deliver them to the tumor site, bioimaging of the created system was notably high. Furthermore, a significant anticancer activity and a protracted release pattern were observed [41]. As a preliminary measure of therapeutic response, multifunctional RNA-loaded magnetic liposomes were produced. The iron oxide-laden RNA liposomes activate dendritic cells after transferring RNA to them, thus enabling the prediction of tumor regression using MRI, according to Grippin et al. [42].
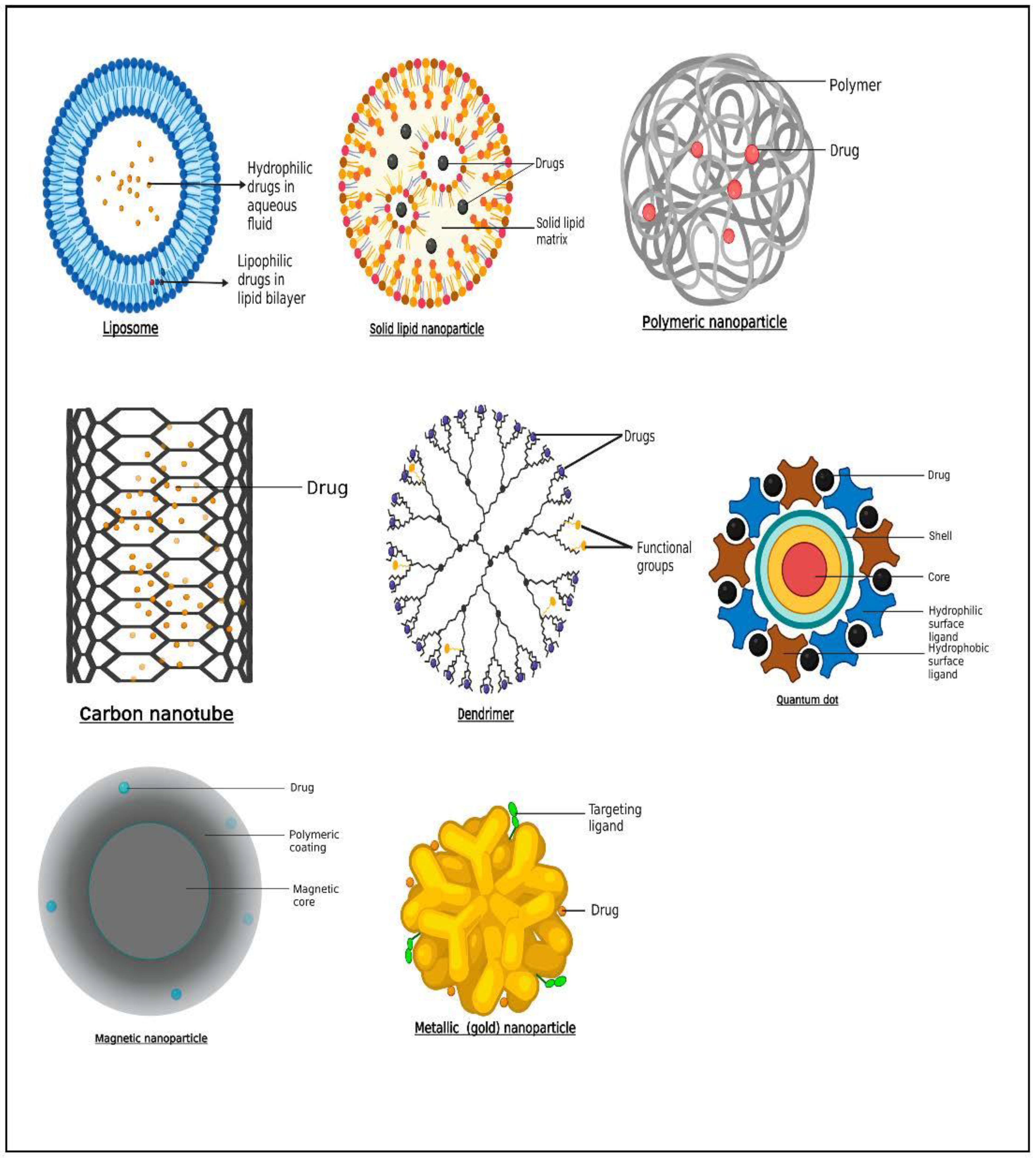
Figure 2. Schematic representation of some nanocarriers employed for theranostic purposes in breast and prostate cancer.
Currently, several kinds of cancer drugs have been applied to this lipid-based system using a variety of preparation methods. Among them, liposomal formulations of the anthracyclines doxorubicin (Doxil, Myocet) and daunorubicin (DaunoXome) are approved for the treatment of metastatic breast cancer and AIDS-related Kaposi’s sarcoma [44,45][43][44]. The research group of Rizzitelli [46,47][45][46] developed long-circulating liposomes loading both gadoteridol and the chemotherapeutic drug doxorubicin. After systemic injection, they successfully achieved a local tumor drug release from liposomes by ultrasound. This strategy significantly improved the therapeutic efficacy in a breast tumor model, demonstrated by almost complete tumor regression.
Yari et al. [48][47] also reported the development of a prostate-specific membrane antigen (PSMA)-tagged liposome for specific targeting of advanced prostate cancer tumoral cells. Their results showed the efficiency of active targeting of prostate cancer cells with PSMA ligand. Studies have demonstrated improved efficacy of liposomal drugs in prostate cancer models. For example, the combined effect of liposomal forms of curcumin and resveratrol more significantly inhibited tumor growth and induced apoptosis in PTEN-CaP8 cell lines, with a concomitant reduction in prostatic adenocarcinoma in vivo in PTEN mice [49][48]. Additionally, curcumin-loaded liposomes decorated with PSMA antibodies tested in LNCaP and C4-2B efficiently inhibited cellular proliferation at a very low dose (5–10 µM) compared to free curcumin [50][49]. Recently, epirubicin encapsulated by propylene glycol liposomes (EPI-PG-liposomes) was established as being effective in overcoming MDR in BC [51][50]. Another recent study also demonstrated that engineered liposomes using arginine8–glycine–aspartic acid (R8GD) encapsulated with daunorubicin and emodin selectively deposit at the tumor site, thereby demonstrating a distinct anti-BC effect [52][51].
2.2. Solid Lipid Nanoparticles
Particles having a solid lipid matrix and a mean size of 50–500 nm are known as solid lipid nanoparticles (SLNs) (Figure 2). The liquid lipid (oil) in the structure of an oil-in-water emulsion is substituted for a solid lipid, or perhaps a combination of solid lipids, to produce SLNs. According to Lima et al. [54][52], one crucial characteristic of SLNs is that they remain solid at both room temperature and body temperature. Surface active agents as stabilizers in combination with the lipid and drug are present in the formulation. SLNs have the benefits of preserving labile medications from breakdown, improved physicochemical stability, ease of formulation, increased targeted delivery at tumor site, improved therapeutic outcome, and reduced systemic adverse effects [55][53]. Furthermore, because of the solid matrix, there is more flexibility in adjusting the release rate, a reduced breakdown rate, and no residual organic solvents during formulation [56][54]. Due to their composition of physiological lipids, solid lipid nanoparticles provide excellent opportunities to insert pharmaceuticals into nanosized targeted vehicles with high biotolerability and minimal biotoxicity [57][55]. According to Wissing et al. [58][56], SLNs are often made up of solid form lipids such as wax, triglycerides with a greater degree of purity, free fatty acids, free fatty alcohols, complex glyceride blends, and other well-known physiologic lipids. SLNs have many advantages, including the ability to include both hydrophilic and lipophilic medications, a nontoxic profile, and high drug stability [57][55]. Garg et al. [59][57] demonstrated in an in vivo study that there was accumulation of drug within breast cancer tissue after intravenous administration of methotrexate-loaded SLNs relative to methotrexate alone. Furthermore, significant lifespan prolongation was observed in mice treated with methotrexate-loaded SLNs. In another study by Guney et al. [60][58], tamoxifen, a hormonal compound, was loaded into SLNs for drug-resistant breast cancer cells. The findings revealed the cytotoxic and aggressive activity of tamoxifen-SLN was higher in the drug-resistant breast cancer cells than the free tamoxifen.
The biodistribution of resveratrol solid lipid nanoparticle (RSV-SLN) was found to be extremely high in prostate cells and accumulate 7.56 times greater than that of RSV solution. The developed RSV-SLN can be applied as potential carrier for delivery of drug of chemotherapeutics with an extended systemic circulation and targeting efficiency at tumor site [61][59]. Akanda et al. [62][60] showed that adjusting the process parameters (e.g., pressure/temperature) and using different lipids increased the anticancer activity of SLNs loaded with retinoic acid (RTA). The result further revealed that RTA-SLNs incubated in LNCap cell lines exhibited decreased cell viability and higher drug concentrations (e.g., 9.53% at 200 g/mL), but blank SLNs exhibited no cytotoxicity.
2.3. Polymers
Polymers of natural origin can be remodeled by chemical reactions to produce semisynthetic polymers, e.g., methylcellulose. Several polymers of natural sources that are employed in the synthesis of nanocarriers include chitosan, dextran, albumin, heparin, gelatin, and collagen [63,64][61][62]. These polymers possess biodegradable, biocompatible, non-immunogenic, and nontoxic features. Polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), and polystyrene are some examples of synthetic polymers. In terms of drug distribution and therapy, these are comparable to natural polymers [65][63]. The ability to surface-modify polymer-based drug delivery systems allows for precise localization of therapeutic and/or diagnostic agents, enhancing the sensitivity, specificity, and efficacy of the therapeutic and diagnostic approach. They can also be easily modified to improve the theranostic conjugates’ biocompatibility and solubility [66][64]. Natural and synthetic macromolecules have both been employed as nanotheranostics. In a documented study for diagnosis and distribution of doxorubicin (DOX) in tumor cells, Zhao et al. [67][65] employed peptide aptamer-targeted polymers. As potential nanocarriers, these nanopolymers may penetrate biological membranes, preserve treatment payload, and distribute it to target tissue.
Glycol chitosan was coupled with the photosensitizer protoporphyrin IX and PEG to produce a polymeric core–shell nanosystem that can be activated by the plasma membrane. Jia et al. [68][66] reported that the generated nanoagents boosted tumor-related in vivo fluorescence and accumulated in tumor cells. Zhu et al. [69][67] developed bioresponsive and bright HA–iodixanol nanogels for application as targeted X-ray computed tomography imaging and chemotherapeutic drugs. To specifically target MCF-7 human breast tumors, the chemotherapeutic drug paclitaxel was added to the nanogels. It was demonstrated that the nanogel had a high rate of cell absorption and suppressed tumor growth. Moreover, MCF-7 breast tumors in nude mice were imaged using enhanced computed tomography (CT), and fluorescence showed that nanogels were disseminated throughout the whole tumor, suggesting deep tumor penetration [69][67]. In a study by Yu et al. [70][68], deoxycholic acid–HA–methotrexate was used to transport ICG and doxorubicin. The produced NPs demonstrated intracellular doxorubicin uptake by the CD44/folate receptor. The use of nanotheranostic technology for imaging-guided chemo-photothermal combination therapy, per the authors, is superior to more traditional approaches [70][68]. In a separate study, ZnS fluorescent quantum dots were altered using carboxymethylcellulose (CMC) to produce a nanocolloidal system, which was subsequently linked with doxorubicin. Nanocolloids with an average size of 3.6 nm were discovered to have both a photoluminescence emission property and to be biocompatible. The resulting system can, therefore, potentially serve as a fluorescent nanoprobe and drug nanocarrier with inhibitory ability [71][69]. The issues with using polymeric nanoparticles are related to the organic solvents that were used to formulate them, which might be present in the final product as residues and cause hazardous consequences (Figure 2).
The chemotherapy medicines docetaxel (DTX) and quercetin (QU) were combined to create chemically modified polymeric nanocapsules (NCs) that were specifically designed to target prostate cancer (PCa). Luteinizing hormone-releasing hormone (LHRH) ligands were attached to poly(propylene glycol-co-glycolide) (PLGA) utilizing polyethylene glycol (PEG) as a carrier to accomplish active targeting. An increase in cellular inhibitory activity and a considerable rise in cellular absorption of LHRH-targeted NCs were demonstrated by in vitro tests. The outcomes of tumor location and anticancer activity in in vivo tests complemented the in vitro findings, revealing the advantageous effects of NCs containing the combination of DTX and QU in the fight against PCa [72][70].
2.4. Metallic and Magnetic Nanoparticles
Nanoparticles of inorganic sources such as superparamagnetic iron oxides, gold nanoparticles (Figure 2), and other metallic and non-metallic nanoparticles or nanoclusters, increase radiation efficiency and tumor detection. Inorganic silver oxide (AgO) nanoparticles offer a great deal of promise for application as anticancer agents. Furthermore, they have antibacterial, antioxidant, anti-inflammatory, and anti-angiogenic properties [73][71]. Organic molecules such as proteins, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) can be delivered using gold nanoparticles [74][72]. Ionic or covalent interactions, as well as adhesion, are all easy ways to affix drugs. In order to improve the stability and half-life of metallic nanoparticles, polyethylene glycol (PEG) can be bonded to their surfaces. The physical characteristics of the gold nanoparticles and their potential for theranostic applications are influenced by their varied morphology, which can take the form of spheres, cubes, rods, clusters, or threads, necessitating strict form control [75][73]. Utilizing radioactive gold nanoparticles is another theranostic strategy (198AuNP and 199AuNP). Beta particles are employed to destroy tumor tissue, whereas gamma rays are used to acquire pictures for scintigraphy or single-photon emission computed tomography/computed tomography (SPECT/CT) [76][74]. Both radionuclides are beta (β) and gamma (γ) emitters. Target-specific drug delivery was shown by Kim et al. [77][75] to kill prostate cancer cells more efficiently than untargeted cells when doxorubicin was loaded onto aptamer-conjugated gold nanoparticles. By attaching gadolinium (Gd)(III) complexes and ligands that target PSMA to the surface of gold nanoparticles, Luo et al. [78][76] created gold nanoparticles for MR-guided prostate cancer targeted therapy. Higher binding affinity was produced as a result of the surface alteration, which quadrupled the rate of r1 relaxation. The outcomes demonstrated improved uptake of gold nanoparticles by prostate cancer cells expressing PSMA, good MRI contrast in vitro and in vivo, and improved suppression of prostate cancer following radiotherapy by binding of gold and Gd(III). The good selectivity of Au/Gd(III)–PSMA NPs for PSMA-expressing prostate cancer cells with improved cellular MR contrast and in vitro radiosensitization was validated [79,80][77][78]. PSMA-targeted gold nanoparticles’ ability to selectively target tumors enabled precise radiation therapy with a low irradiation dosage and little harm to healthy tissues [78][76].
Magnetic nanoparticles are thought to be safe because, in the serum iron pool, they are swiftly degraded and transformed into hemoglobin or other metabolic products [81,82][79][80]. Inorganic nanoparticles (IONPs), which are paramagnetic, can be used as imaging agents to identify and track pathological conditions, as well as release drugs [83][81]. This is accomplished by applying an external magnetic field to the target tissue. In tissues containing nanoparticles, selective cell death occurs, which lessens the detrimental effects. Superparamagnetic iron oxide nanoparticles (SPIONs), perfluorohexane, and paclitaxel were added to a carboxyl-modified PEGylated poly skeleton, on which the herceptin antibody was changed to show an HER—specific surface [84][82]. The perfluorohexane was vaporized, and paclitaxel was subsequently released after these SPIONs were activated by an NIR laser, which enabled the translation of laser energy into thermal energy. Consequently, this nanotheranostic incorporates a combination of chemotherapy and photothermal therapy for the treatment of breast cancer [84][82].
2.5. Carbon Nanotubes
Carbon nanomaterials are mostly made of carbon and come in the shape of hollow spheres, ellipsoids, or tubes with nanometer-sized diameters. Carbon nanotubes (CNTs) are remarkable in that the atoms’ bonds are extremely tough, allowing the tubes to exhibit extraordinary aspect ratios (Figure 2). CNTs come in a variety of shapes and sizes, but they are usually classified as single-walled (SWNTs) or multi-walled nanotubes (MWNTs). SWNTs are similar to a conventional straw in appearance. There is one layer, or wall, to it. Carbon nanotubes with multiple walls are made up of layered tubes with progressively smaller diameters. They can consist of as little as one exterior and one inner tube (a double-walled nanotube) to as many as 100 tubes (walls). Interatomic forces keep each tube apart from its neighbors. CNTs are fascinating because of their mechanical durability and electrical characteristics [85][83]. CNTs were also shown to be appropriate for theranostic utilization due to their vast surface area, capacity to enclose therapeutic/imaging agents, and adaptability to surface changes [86,87,88][84][85][86]. Chemotherapeutic agents such as doxorubicin and paclitaxel, as well as nucleic acids such as antisense oligonucleotides and siRNAs, have recently been included in nanotubes by several experts [89][87]. Moreover, many studies are looking at the possibility of using nanotubes as diagnostic agents. In order to attach paclitaxel (PTX) to the water-soluble carbon nanotubes, a branching polyethylene glycol chain was created by chemically altering single-walled carbon nanotubes with an ester bond. This substance demonstrated little toxicity and 10 times more tumor absorption than standard Taxol® in the mouse 4T1 breast triple-negative breast cancer (TNBC) model. The PEGylation was likely responsible for the enhanced circulation since it improved penetration and retention [90][88] and resulted in greater tumor growth suppression. Another fascinating application of these carbon nanotubes is based on photothermal-induced ablation, in which the nanotubes promote cell membrane permeabilization and necrosis, removing both the tumor mass and the breast cancer (BC) stem cells. This raises the possibility of this being a successful remedial option for tumor resistance and preventing recurrence [91][89].
A theranostic MWCNT was created by Das et al. [92][90] by combining acid-oxidized MWCNTs with four different functional moieties: a fluorochrome (Alexa-fluor, AF488/647), a targeting agent (folic acid), a radionuclide (Technitium-99m), and methotrexate. According to the cellular uptake investigations, human cancer cells (A549) and MCF-7 cells that express the folate receptor endocytose selectively internalize theranostic MWCNTs. In order to load the anticancer agent PTX, human serum albumin (HSA) nanoparticles were conjugated to create a single-walled carbon nanotube (SWNT)-based drug delivery system [93][91]. According to Shao et al. [93][91], the PTX formulated with SWNT-HSA inhibited proliferation in MCF-7 breast cancer cells more effectively than PTX made with HSA nanoparticle alone (cell viability of 63% versus 70% in 48 h and 53% versus 62% in 72 h).
2.6. Dendrimers
Dendrimers are monodispersed organic compounds that can create various polydisperse molecules (Figure 2). Low cytotoxicity, high solubility, chemical stability, and selective accumulation in tumor cells characterize the drug dendrimer conjugate [94][92]. Dendrimers’ cytotoxicity is the main cause for worry, although it depends on the type of polymer they are made of. It is frequently necessary to make specific structural changes, or new biocompatible polymers must be used to provide more physiologically acceptable alternatives [95][93]. The external active functional groups of dendrimers allow for the conjugation of biomolecules or contrast compounds to the surface while simultaneously loading medicines within. Dendrimers have been used to transport a variety of cargo, but nucleic acids and small molecules are the two types of cargo that have received the most attention [96,97][94][95]. For these reasons, charged polymers such as poly(ethylenimine) (PEI) and poly(amidoamine) (PAMAM) are widely utilized. Clinical trials are currently being conducted for a variety of dendrimer-based products, including contrast agents, topical gels, transfection agents, and theranostic agents [97,98,99][95][96][97]. Sweet lemon peel-derived carbon quantum dots and PAMAM dendrimers were used to treat triple-negative breast cancer. The system also contained the arginine–glycine–aspartic acid (RGD) peptide, targeting integrin, which was overexpressed in the specific cancer. This technology can successfully bind to cancer cells, according to research by Gosh et al. [100][98], making it a distinct theranostic option.
For the simultaneous delivery of cisplatin and doxorubicin, Guo et al. [101][99] produced a novel type of PAMAM dendrimer nanoparticle modified by HA (HA@PAMAM-Pt-Dox). HA@PAMAM-Pt-Dox demonstrated high potency in increasing the chemotherapeutic efficacy of cisplatin and DOX, according to the study’s findings. In both the androgen-sensitive prostate cancer cell line LNCaP and the androgen-independent prostate cancer cell line DU145, curcumin has been shown to have the ability to inhibit in vitro prostate cancer cell proliferation [102[100][101][102],103,104], as well as in vivo tumor growth in an LNCaP xenograft mouse model [105][103]. Using PAMAM dendrimers, Chittasupho et al. [106][104] created CXCR4-targeted dendrimers that were combined with the anticancer drug doxorubicin. The dendrimers were taken up by the T47D and BT-549-Luc breast carcinoma cells in a concentration- and time-dependent manner. In comparison to free doxorubicin and unloaded dendrimers, the polymeric dendrimer loaded with doxorubicin had a stronger cytotoxicity. Doxorubicin-loaded PAMAM dendrimers that conjugate with pluronic F68 incorporation were created by Wang et al. [107][105]. The in vivo and in vitro anticancer investigations showed that caveolae-mediated endocytosis boosted the antitumor activity of doxorubicin pluronic F68-PAMAM dendrimers against MCF-7/ADR cancer cells. By controlling gene expression and mitochondrial activity, they substantially enhanced apoptosis [107][105].
Dendrimers are used to deliver medications to patients with a variety of illnesses, including breast and prostate cancers, in a regulated and focused manner [94][92]. They are improvable, which means they can have their surfaces tweaked using targeted ligands including carrying both the therapeutic and the diagnostic agents [108,109][106][107].
2.7. Quantum Dots
Quantum dots (QDs) are light-emitting nanocrystals with a diameter of 2–10 nm manufactured from semiconductor substances such as selenides or cadmium or zinc sulfides. Depending on their size and makeup, they have distinct optical and electrical characteristics. QDs are safe, physiologically inert, and low-toxicity compounds employed as nanosensors and nanocarriers [110][108]. QDs are more durable than fluorescent proteins at varying temperatures and pH levels, making them ideal for long-term fluorescence tracking and image-guided treatment, and as potential substitutes for molecular fluorophores such as fluorescein and rhodamine [111][109]. QDs are attractive semiconductor nanoparticles for use in cancer nanotheranostics because of their unique capacity to see the tumor site (Figure 2). Functionalizing QD with targeting molecules, such as aptamers, may increase their affinity for certain body regions and enable targeted drug delivery [112][110]. Huang et al. [113][111] created a nanotheranostic platform by coating QDs with a polymer containing paclitaxel (a standard anticancer agent) and lipoic acid. Between the polymer and the drug, an ester bond was formed. This approach has been shown to be effective in the detection and treatment of cancer cell lines in vitro. AbdElhamid et al. [114][112] created highly fluorescent QDs that were layer by layer combined with gelatin/chondroitin and shown anti-breast cancer effectiveness, as well as non-immunogenicity. In this investigation, imaging and medication distribution were performed at the same time, typifying the nanotheranostic technique.
For intravenous castrate-resistant prostate cancer (CRPC) therapy, Jiang et al. [115][113] created a multifunctional enzalutamide-loaded graphene oxide nanosystem. Enzalutamide was then added to the 200 nm graphene quantum dot derivatives that were originally created via the disulfide cross-linking of graphene quantum dots. Tumor-targeting peptides and PEG were then used to further functionalize the created graphene quantum nano-drug system. This nano-drug carrier had a potent prostate cancer-targeting capacity which could be quickly internalized by CRPC cells through endocytosis, decrease the proliferation of prostate cancer cells, and lessen enzalutamine’s in vivo side-effects. The high photostability and brightness of QDs have led to their application in nanodevices for the detection of breast and prostate cancer. QD-conjugated protein microarrays for PSA detection with less nonspecific binding were created by Gokarna et al. [116][114]. Carbon quantum dots (CQDs) that were nitrogen-doped were created via a hydrothermal process by Samimi et al. [117][115]. In order to target breast cancer cells, the resultant nanoparticles were coupled with quinic acid. Electrostatic interactions allowed gemcitabine to be loaded onto the resultant nanoparticles. Through the use of the MCF-7 cell line, cell viability was assessed. Quinic acid-conjugated N-CQDs demonstrated intriguing properties such as outstanding luminous characteristics and high tumor accumulation when taken as a whole, highlighting them as great candidates for multifunctional theranostic agents [117][115].
References
- NCI. Available online: https://www.cancer.gov/about-cancer/understanding (accessed on 30 July 2020).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- WHO. Breast 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf (accessed on 2 June 2021).
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541.
- Altekruse, S.F.; Kosary, C.L.; Krapcho, M.; Neyman, N.; Aminou, R.; Waldron, W.; Ruhl, J.; Howlader, N.; Tatalovich, Z.; Cho, H.; et al. SEER Cancer Statistics Review, 1975–2007; National Cancer Institute: Bethesda, MD, USA, 2010. Available online: https://seer.cancer.gov/archive/csr/1975_2007/ (accessed on 17 May 2017).
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154.
- Weigelt, B.; Peterse, J.L.; van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602.
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120.
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of Outcome in Metastatic Breast Cancer: Insights from a Real-World Scenario. Oncology 2014, 19, 608–615.
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112.
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201.
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917.
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89.
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L.; Dvm, A.J. Cancer statistics for adolescents and young adults, 2020. CA A Cancer J. Clin. 2020, 70, 443–459.
- Perdonà, S.; Cavadas, V.; Di Lorenzo, G.; Damiano, R.; Chiappetta, G.; Del Prete, P.; Franco, R.; Azzarito, G.; Scala, S.; Arra, C.; et al. Prostate cancer detection in the ‘‘grey area” of prostate-specific antigen below 10 ng/ml: Head-to-head comparison of the updated PCPT calculator and Chun’s nomogram, two risk estimators incorporating prostate cancer antigen 3. Eur. Urol. 2011, 59, 81–87.
- Shinohara, K. Improving cancer detection by prostate biopsy: The role of core number and site. Nat. Rev. Endocrinol. 2006, 3, 526–527.
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154.
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of Prostate-Specific Antigen in Serum as a Screening Test for Prostate Cancer. N. Engl. J. Med. 1991, 324, 1156–1161.
- Schröder, F.H.; van der Cruijsen-Koeter, I.; de Koning, H.J.; Vis, A.N.; Hoedemaeker, R.F.; Kranse, R. Prostate cancer detection at low prostate specific antigen. J. Urol. 2000, 163, 806–812.
- Lee, K.H.; Kang, B.J.; Jeun, M.; Jang, G.H.; Song, S.H.; Jeong, I.G.; Kim, C.-S.; Searson, P.C. Diagnosis of prostate cancer via nanotechnological approach. Int. J. Nanomed. 2015, 10, 6555–6569.
- Nie, S.; Xing, Y.; Kim, G.J.; Simons, J.W. Nanotechnology Applications in Cancer. Annu. Rev. Biomed. Eng. 2007, 9, 257–288.
- Melancon, M.P.; Stafford, R.J.; Li, C. Challenges to effective cancer nanotheranostics. J. Control. Release 2012, 164, 177–182.
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300.
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574.
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37.
- Banthia, P.; Gambhir, L.; Sharma, A.; Daga, D.; Kapoor, N.; Chaudhary, R.; Sharma, G. Nano to rescue: Repository of nanocarriers for targeted drug delivery to curb breast cancer. 3 Biotech 2022, 12, 1–23.
- Funkhouser, J. Reinventing Pharma: The theranostic revolution. Curr. Drug Discov. 2002, 2, 17–19.
- Asem, H.; Malmström, E. Polymeric Nanoparticles Explored for Drug-Delivery Applications. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; pp. 315–331.
- Kröger, A.P.P.; Hamelmann, N.M.; Juan, A.; Lindhoud, S.; Paulusse, J.M.J. Biocompatible Single-Chain Polymer Nanoparticles for Drug Delivery—A Dual Approach. ACS Appl. Mater. Interfaces 2018, 10, 30946–30951.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Rehman, A.U.; Akram, S.; Seralin, A.; Vandamme, T.; Anton, N. Lipid nanocarriers: Formulation, properties, and applications. In Smart Nanocontainers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–382.
- Bombelli, C.; Stringaro, A.; Borocci, S.; Bozzuto, G.; Colone, M.; Giansanti, L.; Sgambato, R.; Toccaceli, L.; Mancini, G.; Molinari, A. Efficiency of Liposomes in the Delivery of a Photosensitizer Controlled by the Stereochemistry of a Gemini Surfactant Component. Mol. Pharm. 2010, 7, 130–137.
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677.
- Xing, H.; Hwang, K.; Lu, Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352.
- Zhang, K.; Zhang, Y.; Meng, X.; Lu, H.; Chang, H.; Dong, H.; Zhang, X. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials 2018, 185, 301–309.
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 2020, 156, 105576.
- Skupin-Mrugalska, P.; Sobotta, L.; Warowicka, A.; Wereszczynska, B.; Zalewski, T.; Gierlich, P.; Jarek, M.; Nowaczyk, G.; Kempka, M.; Gapinski, J.; et al. Theranostic liposomes as a bimodal carrier for magnetic resonance imaging contrast agent and photosensitizer. J. Inorg. Biochem. 2018, 180, 1–14.
- Ren, W.; Chen, S.; Liao, Y.; Li, S.; Ge, J.; Tao, F.; Huo, Q.; Zhang, Y.; Zhao, Z. Near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the anticancer agent cinobufagin in vivo and in vitro. Colloids Surfaces B Biointerfaces 2019, 174, 384–392.
- Grippin, A.J.; Wummer, B.; Wildes, T.; Dyson, K.; Trivedi, V.; Yang, C.; Sebastian, M.; Mendez-Gomez, H.R.; Padala, S.; Grubb, M.; et al. Dendritic Cell-Activating Magnetic Nanoparticles Enable Early Prediction of Antitumor Response with Magnetic Resonance Imaging. ACS Nano 2019, 13, 13884–13898.
- Markman, M. Pegylated liposomal doxorubicin in the treatment of cancers of the breast and ovary. Expert Opin. Pharmacother. 2006, 7, 1469–1474.
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anti-Cancer Drugs 1999, 10, 767–776.
- Rizzitelli, S.; Giustetto, P.; Cutrin, J.; Castelli, D.D.; Boffa, C.; Ruzza, M.; Menchise, V.; Molinari, F.; Aime, S.; Terreno, E. Sonosensitive theranostic liposomes for preclinical in vivo MRI-guided visualization of doxorubicin release stimulated by pulsed low intensity non-focused ultrasound. J. Control. Release 2015, 202, 21–30.
- Rizzitelli, S.; Giustetto, P.; Faletto, D.; Castelli, D.D.; Aime, S.; Terreno, E. The release of Doxorubicin from liposomes monitored by MRI and triggered by a combination of US stimuli led to a complete tumor regression in a breast cancer mouse model. J. Control. Release 2016, 230, 57–63.
- Yari, H.; Nkepang, G.; Awasthi, V. Surface Modification of Liposomes by a Lipopolymer Targeting Prostate Specific Membrane Antigen for Theranostic Delivery in Prostate Cancer. Materials 2019, 12, 756.
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8.
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123.
- Zhao, Y.-Z.; Dai, D.-D.; Lu, C.-T.; Chen, L.-J.; Lin, M.; Shen, X.-T.; Li, X.-K.; Zhang, M.; Jiang, X.; Jin, R.-R.; et al. Epirubicin loaded with propylene glycol liposomes significantly overcomes multidrug resistance in breast cancer. Cancer Lett. 2013, 330, 74–83.
- Fu, M.; Tang, W.; Liu, J.-J.; Gong, X.-Q.; Kong, L.; Yao, X.-M.; Jing, M.; Cai, F.-Y.; Li, X.-T.; Ju, R.-J. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J. Drug Target. 2020, 28, 245–258.
- Lima, A.M.; Pizzol, C.D.; Monteiro, F.B.; Creczynski-Pasa, T.B.; Andrade, G.P.; Ribeiro, A.O.; Perussi, J.R. Hypericin encapsulated in solid lipid nanoparticles: Phototoxicity and photodynamic efficiency. J. Photochem. Photobiol. B Biol. 2013, 125, 146–154.
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504.
- Fathi, M.; Mozafari, M.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27.
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196.
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid Lipid Nanoparticles for Parenteral Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272.
- Garg, N.K.; Singh, B.; Jain, A.; Nirbhavane, P.; Sharma, R.; Tyagi, R.K.; Kushwah, V.; Jain, S.; Katare, O.P. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Colloids Surfaces B Biointerfaces 2016, 146, 114–126.
- Eskiler, G.G.; Cecener, G.; Dikmen, G.; Egeli, U.; Tunca, B. Solid lipid nanoparticles: Reversal of tamoxifen resistance in breast cancer. Eur. J. Pharm. Sci. 2018, 120, 73–88.
- Sharma, A.N.; Upadhyay, P.K.; Dewangan, H.K. Development, evaluation, pharmacokinetic and biodistribution estimation of resveratrol-loaded solid lipid nanoparticles for prostate cancer targeting. J. Microencapsul. 2022, 39, 563–574.
- Akanda, M.H.; Rai, R.; Slipper, I.J.; Chowdhry, B.Z.; Lamprou, D.; Getti, G.; Douroumis, D. Delivery of retinoic acid to LNCap human prostate cancer cells using solid lipid nanoparticles. Int. J. Pharm. 2015, 493, 161–171.
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063.
- Grossman, J.H.; McNeil, S.E. Nanotechnology in Cancer Medicine. Phys. Today 2012, 65, 38–42.
- Gardel, M.L. Synthetic polymers with biological rigidity. Nature 2013, 493, 619.
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881.
- Zhao, Y.; Houston, Z.H.; Simpson, J.D.; Chen, L.; Fletcher, N.L.; Fuchs, A.V.; Blakey, I.; Thurecht, K.J. Using Peptide Aptamer Targeted Polymers as a Model Nanomedicine for Investigating Drug Distribution in Cancer Nanotheranostics. Mol. Pharm. 2017, 14, 3539–3549.
- Jia, H.-R.; Jiang, Y.-W.; Zhu, Y.-X.; Li, Y.-H.; Wang, H.-Y.; Han, X.; Yu, Z.-W.; Gu, N.; Liu, P.; Chen, Z.; et al. Plasma membrane activatable polymeric nanotheranostics with self-enhanced light-triggered photosensitizer cellular influx for photodynamic cancer therapy. J. Control. Release 2017, 255, 231–241.
- Zhu, Y.; Wang, X.; Chen, J.; Zhang, J.; Meng, F.; Deng, C.; Cheng, R.; Feijen, J.; Zhong, Z. Bioresponsive and fluorescent hyaluronic acid-iodixanol nanogels for targeted X-ray computed tomography imaging and chemotherapy of breast tumors. J. Control. Release 2016, 244, 229–239.
- Yu, F.; Zhu, M.; Li, N.; Ao, M.; Li, Y.; Zhong, M.; Yuan, Q.; Chen, H.; Fan, Z.; Wang, Y.; et al. Imaging-guided synergistic targeting-promoted photo-chemotherapy against cancers by methotrexate-conjugated hyaluronic acid nanoparticles. Chem. Eng. J. 2020, 380, 122426.
- Mansur, A.A.; Caires, A.J.; Carvalho, S.M.; Capanema, N.S.; Carvalho, I.C.; Mansur, H.S. Dual-functional supramolecular nanohybrids of quantum dot/biopolymer/chemotherapeutic drug for bioimaging and killing brain cancer cells in vitro. Colloids Surfaces B Biointerfaces 2019, 184, 110507.
- Shitole, A.A.; Sharma, N.; Giram, P.; Khandwekar, A.; Baruah, M.; Garnaik, B.; Koratkar, S. LHRH-conjugated, PEGylated, poly-lactide-co-glycolide nanocapsules for targeted delivery of combinational chemotherapeutic drugs Docetaxel and Quercetin for prostate cancer. Mater. Sci. Eng. C 2020, 114, 111035.
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534.
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R., Jr.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149.
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233.
- Xu, H.; Jiang, S.; Wang, J.; Li, X.; Wu, T.; Xu, P.; Santos-Oliveira, R.; Zhang, A. Radioactive Gold Nanoparticle in Two Forms (19879Au GNPs and 99mTc-GNPs) for Lung Cancer Antiproliferative Induction and Intralesional Imaging: A Proof of Concept. Anti-Cancer Agents Med. Chem. 2020, 20, 1648–1653.
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer−Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696.
- Luo, D.; Johnson, A.; Wang, X.; Li, H.; Erokwu, B.O.; Springer, S.; Lou, J.; Ramamurthy, G.; Flask, C.A.; Burda, C.; et al. Targeted Radiosensitizers for MR-Guided Radiation Therapy of Prostate Cancer. Nano Lett. 2020, 20, 7159–7167.
- Cunningham, C.; de Kock, M.; Engelbrecht, M.; Miles, X.; Slabbert, J.; Vandevoorde, C. Radiosensitization effect of gold nanoparticles in proton therapy. Front. Public Health. 2021, 9, 699822.
- Bouché, M.; Hsu, J.C.; Dong, Y.C.; Kim, J.; Taing, K.; Cormode, D.P. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconj. Chem. 2020, 31, 303–314.
- Yang, S.-J.; Huang, C.-H.; Wang, C.-H.; Shieh, M.-J.; Chen, K.-C. The Synergistic Effect of Hyperthermia and Chemotherapy in Magnetite Nanomedicine-Based Lung Cancer Treatment. Int. J. Nanomed. 2020, 15, 10331–10347.
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Chen, S.; Tiwari, S.; Shi, K.; Zhang, S.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815.
- Ferreira, M.; Sousa, J.; Pais, A.; Vitorino, C. The Role of Magnetic Nanoparticles in Cancer Nanotheranostics. Materials 2020, 13, 266.
- Guo, Y.; Wang, X.-Y.; Chen, Y.-L.; Liu, F.-Q.; Tan, M.-X.; Ao, M.; Yu, J.-H.; Ran, H.-T.; Wang, Z.-X. A light-controllable specific drug delivery nanoplatform for targeted bimodal imaging-guided photothermal/chemo synergistic cancer therapy. Acta Biomater. 2018, 80, 308–326.
- Arndt, M.; Nairz, O.; Voss-Andreae, J.; Keller, C.; Van der Zouw, G.; Zeilinger, A. Wave-particle duality of C60. Nature 1999, 401, 680–682.
- McDevitt, M.R.; Chattopadhyay, D.; Kappel, B.J.; Jaggi, J.S.; Schiffman, S.R.; Antczak, C.; Njardarson, J.T.; Brentjens, R.; Scheinberg, D.A. Tumor Targeting with Antibody-Functionalized, Radiolabeled Carbon Nanotubes. J. Nucl. Med. 2007, 48, 1180–1189.
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717.
- Fubini, B.; Ghiazza, M.; Fenoglio, I. Physio-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology 2010, 4, 347–363.
- Fabbro, C.; Ali-Boucetta, H.; Da Ros, T.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. 2012, 48, 3911–3926.
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug Delivery with Carbon Nanotubes for In vivo Cancer Treatment. Cancer Res. 2008, 68, 6652–6660.
- Burke, A.R.; Singh, R.N.; Carroll, D.L.; Wood, J.C.S.; D’Agostino, R.B.; Ajayan, P.M.; Torti, F.M.; Torti, S.V. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012, 33, 2961–2970.
- Das, M.; Datir, S.R.; Singh, R.P.; Jain, S. Augmented anticancer activity of a targeted, intracellularly activatable, theranostic nanomedicine based a fluorescent and radiolabeled, methotrxate-folic acid-multiwalled carbon nanotube conjugate. Mol. Pharm. 2013, 10, 2543–2557.
- Shao, W.; Paul, A.; Rodes, L.; Prakash, S. A New Carbon Nanotube-Based Breast Cancer Drug Delivery System: Preparation and In Vitro Analysis Using Paclitaxel. Cell Biochem. Biophys. 2015, 71, 1405–1414.
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res Lett. 2014, 9, 1–10.
- Yan, X.; Yang, Y.; Sun, Y. Dendrimer Applications for Cancer Therapies. J. Phys. Conf. Ser. 2021, 1948, 012205.
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer Advances for the Central Nervous System Delivery of Therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13.
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401.
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185.
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617.
- Ghosh, S.; Ghosal, K.; Mohammad, S.A.; Sarkar, K. Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy. Chem. Eng. J. 2019, 373, 468–484.
- Guo, X.L.; Kang, X.X.; Wang, Y.Q.; Zhang, X.J.; Li, C.J.; Liu, Y.; Du, L.B. Codelivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019, 84, 367–377.
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer—I. curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000, 3, 84–93.
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol. Urol. 2000, 4, 1–6.
- Dorai, T.; Cao, Y.C.; Dorai, B.; Buttyan, R.; Katz, A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001, 47, 293–303.
- Davis, J.N.; Muqim, N.; Bhuiyan, M.; Kucuk, O.; Pienta, K.J.; Sarkar, F.H. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int. J. Oncol. 2000, 16, 1091–1098.
- Chittasupho, C.; Anuchapreeda, S.; Sarisuta, N. CXCR4 targeted dendrimer for anti-cancer drug delivery and breast cancer cell migration inhibition. Eur. J. Pharm. Biopharm. 2017, 119, 310–321.
- Wang, M.; Li, Y.; HuangFu, M.; Xiao, Y.; Zhang, T.; Han, M.; Xu, D.; Li, F.; Ling, D.; Jin, Y.; et al. Pluronic-attached polyamidoamine dendrimer conjugates overcome drug resistance in breast cancer. Nanomedicine 2016, 11, 2917–2934.
- Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic polyesters for medical imaging and theranostic applications. Eur. J. Pharm. Biopharm. 2015, 97, 350–370.
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138.
- Granada-Ramirez, D.A.; Arias-Ceron, J.S.; Rodriguez-Fragoso, P.; Vazquez-Hernandez, F.; Luna-Arias, J.P.; Herrera-Perez, J.L.; Mendoza-Alvarez, J.G. Quantum dots for biomedical applications. In Nanobiomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 411–436.
- Zhao, M.-X.; Zeng, E.-Z. Application of functional quantum dot nanoparticles as fluorescence probes in cell labeling and tumor diagnostic imaging. Nanoscale Res. Lett. 2015, 10, 171.
- Ahar, M.J. A Review on Aptamer-Conjugated Quantum Dot Nanosystems for Cancer Imaging and Theranostic. J. Nanomed. Res. 2017, 5, 1–9.
- Huang, H.K.; Yan, J.; Liu, P.; Zhao, B.Y.; Cao, Y.; Zhang, X.F. A novel cancer nanotheranostics system based on quantum dots encapsulated by a polymer-prodrug with controlled release behavior. Aust. J. Chem. 2017, 70, 1302–1311.
- AbdElhamid, A.S.; Helmy, M.W.; Ebrahim, S.M.; Bahey-El-Din, M.; Zayed, D.G.; Dein, E.A.Z.E.; El-Gizawy, S.; Elzoghby, A.O. Layer-by-layer gelatin/chondroitin quantum dots-based nanotheranostics: Combined rapamycin/celecoxib delivery and cancer imaging. Nanomedicine 2018, 13, 1707–1730.
- Jiang, W.; Chen, J.; Gong, C.; Wang, Y.; Gao, Y.; Yuan, Y. Intravenous delivery of enzalutamide based on high drug loading multifunctional graphene oxide nanoparticles for castration-resistant prostate cancer therapy. J. Nanobiotechnol. 2020, 18, 50.
- Gokarna, A.; Jin, L.-H.; Hwang, J.S.; Cho, Y.-H.; Lim, Y.T.; Chung, B.H.; Youn, S.H.; Choi, D.S.; Lim, J.H. Quantum dot-based protein micro- and nanoarrays for detection of prostate cancer biomarkers. Proteomics 2008, 8, 1809–1818.
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287.
More
