Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Andrea Natalia Ricco.
Retrospective studies of common malignancies such as head and neck cancer often report lower incidence and/or better outcomes for patients incidentally treated with statins, the HMG-CoA reductase inhibitors commonly prescribed to reduce blood cholesterol and related cardiovascular risks. Lipophilic statins have been proposed to both sensitize to therapy and spare normal tissue, suggesting particular benefits in head and neck cancer, where treatment often incurs major toxicities. While roles for statins in prevention remain controversial, rigorous laboratory studies have confirmed the direct effects of statins on cells and tumors that enhance response to chemotherapy, radiation, targeted agents and immunotherapy.
- head and neck cancer
- statin
- cholesterol
- HMG-CoA reductase
- signal transduction
1. Introduction
Cancer remains a global health challenge, accounting for some 10 million deaths per year worldwide. Despite substantial advancements in prevention and treatment, cancer remains one of the most common causes of mortality, particularly in older adults [1]. Head and neck cancer (HNC) is a common malignancy that typically presents as a squamous cell carcinoma (HNSCC) originating in the upper aerodigestive tract [2], with over a half million new diagnoses each year worldwide [1]. HNC risk factors vary by site and may encompass tobacco and alcohol use, human papillomavirus (HPV) infection and occupational and environmental hazards [3]. Treatment options include surgery, radiation therapy, chemotherapy, immunotherapy and targeted therapies. Typically, surgery and/or radiation are combined with systemic therapies, depending on the stage and characteristics of the cancer [4]. As with many cancers, HNC diagnosis at an early stage and appropriate treatment selection are associated with complete response and high overall survival, though significant acute and long-term toxicities affect quality of life [5]. Nonetheless, many patients are diagnosed at an advanced stage and/or recur after their initial therapy, the majority of whom will eventually succumb to their disease. There is a critical, unmet need for more effective primary therapies that can be integrated into current treatment regimens without increasing adverse effects. Competitive inhibitors of the mevalonate pathway enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, commonly known as statins, may represent an opportunity to both enhance treatment efficacy and lower toxicity [6,7,8][6][7][8].
By inhibiting HMG-CoA reductase, statins can directly lower cholesterol levels as a result of decreased flux in the mevalonate pathway and reduced formation of precursors for cholesterol biosynthesis (Figure 1). However, the mevalonate pathway is also limiting for production of isoprenoids, which serve key roles in cellular metabolism and signaling such as isopentenyl diphosphate (IPP), farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP). Statins’ effects on serum cholesterol are both direct and indirect. Decreased intracellular cholesterol levels can induce upregulation of the sterol regulatory element-binding protein (SREBP). This transcription factor governs various genes involved in lipid metabolism, leading to an increased presence of low-density lipoprotein (LDL) receptors on the cell surface. This upregulation enhances the absorption of cholesterol-rich LDL particles from the bloodstream, subsequently lowering plasma LDL-cholesterol levels. Some statins also elevate high-density lipoprotein (HDL), or “good cholesterol”, potentially decreasing the risk of cardiovascular disease [9].
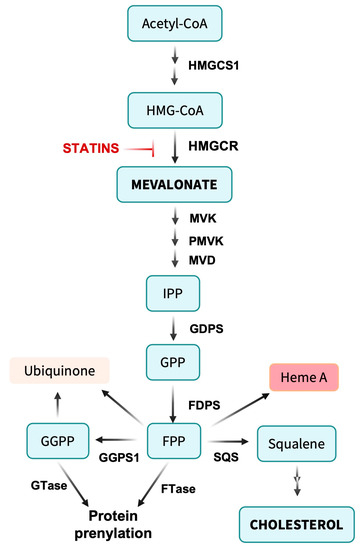
Figure 1. Overview of the mevalonate pathway. Acetyl-CoA is condensed to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). The rate-limiting step in the pathway involves the conversion of HMG-CoA to mevalonate by HMG-CoA reductase (HMGCR). Mevalonate undergoes further metabolism by mevalonate kinase (MVK), phosphomevalonate kinase (PMVK) and mevalonate decarboxylase (MVD) to yield isopentenyl pyrophosphate (IPP). IPP is converted to farnesyl pyrophosphate (FPP) by farnesyl diphosphate synthase (FDPS) and to geranyl geranyl pyrophosphate (GGPP) by geranyl geranyl diphosphate synthase (GGPPS1), providing intermediates for protein prenylation. Alternatively, two FPP molecules are linked by squalene synthase (SQS) to form the C30 isoprenoid squalene, a rate-limiting precursor for cholesterol synthesis. FPP can be directly added to biomolecules during the formation of ubiquinone (coenzyme Q10) and heme A of cytochrome c oxidase, supporting mitochondrial metabolism. Not shown are pathways from IPP to tRNA isopentenylation.
Cholesterol plays vital roles in biological processes including cell membrane maintenance, steroid hormone synthesis, vitamin D and bile acid production and the formation of lipid rafts and caveolae that facilitate transport, signal transduction and cell polarization. In addition, statins’ impact on isoprenoid biosynthesis can produce diverse pleiotropic effects. Isoprenoids serve as lipid anchors for intracellular signaling proteins such as Ras, Rac and Rho, essential for cell growth, survival and differentiation. Modulating isoprenylation can influence cellular signaling pathways, potentially contributing to statins’ therapeutic benefits [7,8][7][8].
The cellular impacts of statins are influenced by their chemical structures. Compared to hydrophilic statins such as pravastatin and rosuvastatin that primarily suppress cholesterol biosynthesis by the liver, the lipophilic statins, such as simvastatin, lovastatin and atorvastatin, are more bioavailable in the periphery, providing the potential to impact multiple targets of mevalonate metabolism in cancer cells [10,11][10][11]. Simvastatin, a semisynthetic derivative of lovastatin, is the most commonly used statin among the greater than one quarter of U.S. adults over 40 years of age prescribed statins to reduce serum cholesterol levels and/or prevent cardiovascular disease [12]. As such, incidental use of lipophilic statins is a common finding in any large study of cancer patients.
Population-based studies have yielded provocative findings that patients on statins may display lower cancer rates and benefit from more favorable outcomes for cancer treatment, implicating lower serum cholesterol or other effects of these agents in limiting carcinogenesis and resistance [13,14,15,16][13][14][15][16]. Although specific mechanisms are still debated, there is compelling evidence that statins can sensitize HNC to radiotherapy, chemotherapy and immunotherapy while reducing adverse effects on normal tissue [6,7,8][6][7][8]. Rather than limit the benefits to patients prescribed statins for other reasons, these and other results support studies to evaluate treating HNC patients with statins alongside their cancer treatment toward improving therapeutic outcomes.
Most discussion of cholesterol focuses on the deleterious effects of excess biosynthesis and/or dietary uptake such as atherosclerosis [17]. Nonetheless, cholesterol is a critical component of cell membranes, representing about half of the lipid in plasma membranes, where its content is closely regulated to maintain membrane fluidity and other essential properties in both normal and malignant cells [18]. Further, in cholesterol-rich membranes, cholesterol may partition into rafts and form complexes with specific proteins [19]. As such, decreased cholesterol levels upon inhibition of HMG-CoA reductase by statins would be expected to compromise basic cellular functions. However, where cholesterol starvation differentially impacts cancer cells, this may provide a route to increasing the therapeutic index of cancer therapy.
2. Membrane Rafts and Signal Transduction
Given the essential roles for cholesterol in maintaining tumor cell membrane functionality, strategies aimed at inhibiting cholesterol biosynthesis could restrict tumor growth and metastasis [20]. As an example, the caveolae protein caveolin-1 (CAV1) has been found to modulate the metastatic and invasive capabilities of oral squamous cell carcinoma (OSCC) cells [21]. Elevated CAV1 expression in metastatic lymph nodes correlates with poor OSCC prognosis. The link to mevalonate pathway metabolism and statins may come via a critical role for cholesterol in lipid raft-dependent functions in cancer cells and tumors [22]. Lipid rafts function as hubs for signaling proteins, selectively and dynamically regulating their recruitment or exclusion in response to intracellular and extracellular stimuli [23]. Via concentrating raft-associated proteins and maintaining stable complexes, lipid rafts facilitate signal transduction, a function mediated in part by CAV1. By disrupting rafts and caveolae, statins can have indirect effects on CAV1 and other proteins, leading to CAV1 degradation and altered cell signaling. Of particular significance, lipid raft integrity modulates survival and cell death pathways [24], suggesting a relationship between lipid rafts and therapy resistance [25]. Indeed, multiple cancer cell survival and proliferation-related signaling pathways have been linked with lipid rafts [26]. Providing a potential mechanism for the beneficial effects of statins, disrupting lipid rafts results in the inhibition of the PIK3/Akt signaling pathway, leading to radiosensitization in HNSCC [27]. A complementary effect may be to limit Akt-induced PD-L1 expression, allowing statins to potentiate anti-tumor immune response [28].3. Cholesterol’s Influence on Cancer Cell Proliferation, Survival and Therapy Resistance
Cholesterol’s role in lipid rafts and caveolae in the plasma membrane may impact cancer cell proliferation and survival via effects on signaling by receptors such as HER2 [29], EGFR [30] and CXCR4 [31], as well as transducers and effectors such as PI3K [32], SRC family kinases [25] and NOX [33], along with other regulators [34]. However, maintaining cholesterol at normal levels in other cellular membranes and subdomains impacts a wide range of pathways important to cancer cell growth, proliferation and resistance. Studies of cholesterol depletion by statins or cyclodextrins suggest that cholesterol helps maintain secretory pathway function, regulates autophagy and supports mitochondrial oxidative phosphorylation [17,35,36][17][35][36]. Other connections appear more indirect. ATAD3A, a protein associated with a wide range of physiological and pathological responses, plays a role in cholesterol metabolism [37]. Elevated ATAD3A expression has been observed in various cancers, including HNSCC [38,39,40][38][39][40]. In HNSCC, ATAD3A operates as a mitochondrial oncoprotein that stimulates disease progression via the activation of mitochondrial ERK1/2 [41,42,43][41][42][43]. Notably, the ATAD3A-ERK1/2 signaling pathway links to voltage-dependent anion channel 1 (VDAC1) [41]. VDAC1 promotes the transport of ERK1/2 to the mitochondria, vital for the formation of the ATAD3A-ERK1/2 protein complex in HNSCC cells. Thus, multiple mechanisms may link cholesterol levels to EGFR/PI3K/Akt/mTOR signaling in HNSCC and other cancers [44,45,46][44][45][46]. The calcium-activated chloride channel TMEM16A, previously ANO1, is upregulated in diverse cancers [47] and commonly overexpressed in HNSCC, which is associated with poor outcomes [48[48][49],49], establishing it as a therapeutic target. Along with binding to EGFR that may impact HNC proliferation, survival and expression of PD-L1 and thus immune evasion [47,50][47][50], TMEM16A has been implicated in resistance to conventional therapy and EGFR-targeted agents. TMEM16A upregulation can also suppress apoptosis and promote cisplatin resistance [51]. Simvastatin impairs TMEM16A channel function—potentially due to cholesterol depletion, though mevalonate-independent mechanisms may be involved—and reduces OSCC cell proliferation in a TMEM16A-dependent manner [49], suggesting statins as an alternative to TMEM16A inhibitors.4. Inflammation and Immune Response Modulation
The FDA has approved the anti-PD-1 immune checkpoint blockade (ICB) antibodies nivolumab and pembrolizumab for cisplatin-resistant, relapsed or metastatic HNSCC patients. Clinical trials and research studies have confirmed pembrolizumab’s efficacy and safety, both as a standalone treatment and combined with chemotherapy for recurrent or metastatic HNSCC [52,53][52][53]. For patients with PD-L1-positive, relapsed, or metastatic HNSCC, pembrolizumab monotherapy is recommended as a first-line treatment [54]. Despite its potential benefits, ICB therapy faces multiple barriers including intrinsic and acquired resistance and high rates of immune-related adverse events (irAEs). There is considerable interest in combination therapies. Statins have long been appreciated for their ability to reduce inflammation [55], and part of this effect may be linked to reducing cellular cholesterol levels. Much like its effects on cancer cells, cholesterol depletion may disrupt rafts and caveolae in immune cells, dispersing receptors and transducers that mediate inflammatory signaling in innate and adaptive immune cells [56,57][56][57]. As a consequence, a concern would be that lowering cholesterol with statins might reinforce immunosuppression in the tumor microenvironment. Nonetheless, an emerging theme from recent preclinical and patient studies in multiple cancers is that statins potentiate anti-tumor immune responses and/or immunotherapy (e.g., [58,59,60,61][58][59][60][61]). Multiple cholesterol-dependent mechanisms may be involved. Nucleic acid detection in the cytoplasm, which activates the innate immune system, occurs through pattern recognition receptors (PRRs). One such PRR is the cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING) pathway [62]. Activation of cGAS by cytosolic DNA induces STING to phosphorylate and activate TBK1 and drive Type I interferon (IFN) pathway activation, which has the potential to induce an effective anti-tumor immune response [63]. As such, STING agonists are currently being evaluated in multiple contexts as cancer therapeutics [64]. Along with other effects, Type I IFN signaling may limit cholesterol synthesis, which may then further activate STING via depletion of the ER membrane cholesterol pool [65]. Simply disturbing cholesterol metabolism as with statins might be sufficient to induce this positive feedback loop. Other benefits of statins may be to reduce the suppressive influence of excess cholesterol on immune function. Class II major histocompatibility complex (MHC II) molecules are raft-associated proteins expressed on antigen presenting cells such as dendritic cells (DCs) and serve a key role in presenting processed tumor antigen peptides to effector cells, thereby eliciting anti-tumor responses [66]. Tumor cells can downregulate DC functionality by raising cholesterol levels [67]. Oxysterol secretion by tumor cells impairs DC migration to lymph nodes and reduces T cell priming [68]. Cholesterol may also contribute to T cell dysfunction directly via immune checkpoint activation and CD8+ T cell exhaustion. Membrane cholesterol serves a direct role in stabilizing PD-L1 through its interaction with cholesterol-binding CRAC motifs [69]. This suggests that reducing cholesterol might be sufficient to interrupt immune checkpoint signaling and/or potentiate immune checkpoint blockade immunotherapy. High cholesterol exposure in the tumor microenvironment is also associated with elevated PD-1 expression by infiltrating CD8+ T cells [70] and CD8+ T cell exhaustion [71]. Multiple indirect effects of statins can be ascribed to the decreased cholesterol biosynthesis. Intracellular cholesterol depletion may promote cleavage and nuclear localization of sterol regulatory element (SRE)-binding proteins (SREBPs [72]) that bind SREs, leading to compensatory expression of sterol pathway and coregulated genes. Along with inducing expression of HMGCR and other mevalonate pathway enzymes, SREBPs increase the expression of enzymes for cholesterol biosynthesis (via SREBP-2) and fatty acid and triglyceride biosynthesis (via SREBP-1) as well as the LDL receptor and diverse other proteins linked to lipid metabolism and transport. Insofar as SREBPs are cancer targets [73,74][73][74], this effect of statins may well be counterproductive beyond simply restoring mevalonate pathway activity. Along these lines, one of the SREBP-dependent proteins induced by statins is the LDL receptor negative regulatory factor PCSK9 [75,76][75][76]. PCSK9 has emerged as an alternate target for lipid lowering therapy, insofar as inhibiting PCSK9 with antibodies (alirocumab, evolocumab) or siRNA (inclisiran) leads to increased LDLR recycling rather than degradation and greater liver uptake of LDL, lowering circulating cholesterol [77]. Significantly, PCSK9 has a similar effect on MHC I, leading to its lysosomal transport and downregulation [78]. Targeting PCSK9 increased tumor cell MHC I expression, promoted CD8+ T cell tumor infiltration and cytotoxicity and potentiated the effects of PD-1/PD-L1 checkpoint blockade. Effects on CD8+ T cells may also be direct, as blocking PCSK9 can enhance T cell receptor (TCR) signaling via stabilizing LDLR [79]. While arguing for targeting PCSK9 in cancer immunotherapy [80], these considerations also raise the concern that statins have the potential to interfere with immune checkpoint blockade via activation of SREBP and upregulation of PCSK9.References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72.
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42.
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers 2021, 13, 4912.
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92.
- Longo, J.; van Leeuwen, J.E.; Elbaz, M.; Branchard, E.; Penn, L.Z. Statins as Anticancer Agents in the Era of Precision Medicine. Clin. Cancer Res. 2020, 26, 5791–5800.
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241.
- Juarez, D.; Fruman, D.A. Targeting the Mevalonate Pathway in Cancer. Trends Cancer 2021, 7, 525–540.
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387.
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125.
- Beckwitt, C.H.; Shiraha, K.; Wells, A. Lipophilic statins limit cancer cell growth and survival, via involvement of Akt signaling. PLoS ONE 2018, 13, e0197422.
- Gu, Q.; Paulose-Ram, R.; Burt, V.L.; Kit, B.K. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. In NCHS Data Brief; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014; pp. 1–8.
- Thomas, T.; Loke, Y.; Beales, I.L.P. Systematic Review and Meta-analysis: Use of Statins Is Associated with a Reduced Incidence of Oesophageal Adenocarcinoma. J. Gastrointest. Cancer 2018, 49, 442–454.
- Cardwell, C.R.; Mc Menamin, Ú.; Hughes, C.M.; Murray, L.J. Statin use and survival from lung cancer: A population-based cohort study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 833–841.
- Hung, M.S.; Chen, I.C.; Lee, C.P.; Huang, R.J.; Chen, P.C.; Tsai, Y.H.; Yang, Y.H. Statin improves survival in patients with EGFR-TKI lung cancer: A nationwide population-based study. PLoS ONE 2017, 12, e0171137.
- Chan, J.S.K.; Satti, D.I.; Lee, Y.H.A.; Bin Waleed, K.; Tang, P.; Mahalwar, G.; Minhas, A.M.K.; Roever, L.; Biondi-Zoccai, G.; Leung, F.P.; et al. Association between Visit-to-Visit Lipid Variability and Incident Cancer: A Population-based Cohort Study. Curr. Probl. Cardiol. 2023, 48, 101421.
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Targeted Ther. 2022, 7, 265.
- Zhang, J.; Li, Q.; Wu, Y.; Wang, D.; Xu, L.; Zhang, Y.; Wang, S.; Wang, T.; Liu, F.; Zaky, M.Y.; et al. Cholesterol content in cell membrane maintains surface levels of ErbB2 and confers a therapeutic vulnerability in ErbB2-positive breast cancer. Cell Commun. Signal. 2019, 17, 15.
- Mollinedo, F. Raft platforms highly enriched in cholesterol: Major scaffolds for IL-6 signalling assembly with implications in inflammation and cancer. FEBS J. 2022, 289, 5891–5894.
- Chan, N.N.; Yamazaki, M.; Maruyama, S.; Abé, T.; Haga, K.; Kawaharada, M.; Izumi, K.; Kobayashi, T.; Tanuma, J.I. Cholesterol Is a Regulator of CAV1 Localization and Cell Migration in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 6035.
- Kato, K.; Miyazawa, H.; Kobayashi, H.; Noguchi, N.; Lambert, D.; Kawashiri, S. Caveolin-1 Expression at Metastatic Lymph Nodes Predicts Unfavorable Outcome in Patients with Oral Squamous Cell Carcinoma. Pathol. Oncol. Res. 2020, 26, 2105–2113.
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Investig. 2005, 115, 959–968.
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39.
- Bionda, C.; Athias, A.; Poncet, D.; Alphonse, G.; Guezguez, A.; Gambert, P.; Rodriguez-Lafrasse, C.; Ardail, D. Differential regulation of cell death in head and neck cell carcinoma through alteration of cholesterol levels in lipid rafts microdomains. Biochem. Pharmacol. 2008, 75, 761–772.
- Zeng, J.; Zhang, H.; Tan, Y.; Sun, C.; Liang, Y.; Yu, J.; Zou, H. Aggregation of lipid rafts activates c-met and c-Src in non-small cell lung cancer cells. BMC Cancer 2018, 18, 611.
- Mollinedo, F.; Gajate, C. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul. 2015, 57, 130–146.
- Ladjohounlou, R.; Louati, S.; Lauret, A.; Gauthier, A.; Ardail, D.; Magne, N.; Alphonse, G.; Rodriguez-Lafrasse, C. Ceramide-Enriched Membrane Domains Contribute to Targeted and Nontargeted Effects of Radiation through Modulation of PI3K/AKT Signaling in HNSCC Cells. Int. J. Mol. Sci. 2020, 21, 7200.
- Lim, W.J.; Lee, M.; Oh, Y.; Fang, X.Q.; Lee, S.; Lim, C.H.; Park, J.; Lim, J.H. Statins Decrease Programmed Death-Ligand 1 (PD-L1) by Inhibiting AKT and β-Catenin Signaling. Cells 2021, 10, 2488.
- Chinni, S.R.; Yamamoto, H.; Dong, Z.; Sabbota, A.; Bonfil, R.D.; Cher, M.L. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol. Cancer Res. 2008, 6, 446–457.
- Irwin, M.E.; Mueller, K.L.; Bohin, N.; Ge, Y.; Boerner, J.L. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell. Physiol. 2011, 226, 2316–2328.
- Sbrissa, D.; Semaan, L.; Govindarajan, B.; Li, Y.; Caruthers, N.J.; Stemmer, P.M.; Cher, M.L.; Sethi, S.; Vaishampayan, U.; Shisheva, A.; et al. A novel cross-talk between CXCR4 and PI4KIIIα in prostate cancer cells. Oncogene 2019, 38, 332–344.
- Yang, H.; Guan, L.; Li, S.; Jiang, Y.; Xiong, N.; Li, L.; Wu, C.; Zeng, H.; Liu, Y. Mechanosensitive caveolin-1 activation-induced PI3K/Akt/mTOR signaling pathway promotes breast cancer motility, invadopodia formation and metastasis in vivo. Oncotarget 2016, 7, 16227–16247.
- Anagnostopoulou, A.; Camargo, L.L.; Rodrigues, D.; Montezano, A.C.; Touyz, R.M. Importance of cholesterol-rich microdomains in the regulation of Nox isoforms and redox signaling in human vascular smooth muscle cells. Sci. Rep. 2020, 10, 17818.
- Vona, R.; Iessi, E.; Matarrese, P. Role of Cholesterol and Lipid Rafts in Cancer Signaling: A Promising Therapeutic Opportunity? Front. Cell Dev. Biol. 2021, 9, 622908.
- Juhl, A.D.; Wüstner, D. Pathways and Mechanisms of Cellular Cholesterol Efflux—Insight From Imaging. Front. Cell Dev. Biol. 2022, 10, 834408.
- Alizadeh, J.; Kavoosi, M.; Singh, N.; Lorzadeh, S.; Ravandi, A.; Kidane, B.; Ahmed, N.; Mraiche, F.; Mowat, M.R.; Ghavami, S. Regulation of Autophagy via Carbohydrate and Lipid Metabolism in Cancer. Cancers 2023, 15, 2195.
- Lang, L.; Loveless, R.; Teng, Y. Emerging Links between Control of Mitochondrial Protein ATAD3A and Cancer. Int. J. Mol. Sci. 2020, 21, 7917.
- Gires, O.; Münz, M.; Schaffrik, M.; Kieu, C.; Rauch, J.; Ahlemann, M.; Eberle, D.; Mack, B.; Wollenberg, B.; Lang, S.; et al. Profile identification of disease-associated humoral antigens using AMIDA, a novel proteomics-based technology. Cell. Mol. Life Sci. 2004, 61, 1198–1207.
- Fang, H.Y.; Chang, C.L.; Hsu, S.H.; Huang, C.Y.; Chiang, S.F.; Chiou, S.H.; Huang, C.H.; Hsiao, Y.T.; Lin, T.Y.; Chiang, I.P.; et al. ATPase family AAA domain-containing 3A is a novel anti-apoptotic factor in lung adenocarcinoma cells. J. Cell Sci. 2010, 123, 1171–1180.
- Chen, T.C.; Hung, Y.C.; Lin, T.Y.; Chang, H.W.; Chiang, I.P.; Chen, Y.Y.; Chow, K.C. Human papillomavirus infection and expression of ATPase family AAA domain containing 3A, a novel anti-autophagy factor, in uterine cervical cancer. Int. J. Mol. Med. 2011, 28, 689–696.
- Lang, L.; Loveless, R.; Dou, J.; Lam, T.; Chen, A.; Wang, F.; Sun, L.; Juarez, J.; Qin, Z.S.; Saba, N.F.; et al. ATAD3A mediates activation of RAS-independent mitochondrial ERK1/2 signaling, favoring head and neck cancer development. J. Exp. Clin. Cancer Res. 2022, 41, 43.
- Wortzel, I.; Seger, R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer 2011, 2, 195–209.
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566.
- Vaquero, J.; Nguyen Ho-Bouldoires, T.H.; Clapéron, A.; Fouassier, L. Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: From signaling regulation to clinical relevance. Oncogene 2017, 36, 3067–3079.
- Li, Z.; Yang, Z.; Passaniti, A.; Lapidus, R.G.; Liu, X.; Cullen, K.J.; Dan, H.C. A positive feedback loop involving EGFR/Akt/mTORC1 and IKK/NF-kB regulates head and neck squamous cell carcinoma proliferation. Oncotarget 2016, 7, 31892–31906.
- Horn, D.; Hess, J.; Freier, K.; Hoffmann, J.; Freudlsperger, C. Targeting EGFR-PI3K-AKT-mTOR signaling enhances radiosensitivity in head and neck squamous cell carcinoma. Expert Opin. Ther. Targets 2015, 19, 795–805.
- Crottès, D.; Jan, L.Y. The multifaceted role of TMEM16A in cancer. Cell Calcium 2019, 82, 102050.
- Duvvuri, U.; Shiwarski, D.J.; Xiao, D.; Bertrand, C.; Huang, X.; Edinger, R.S.; Rock, J.R.; Harfe, B.D.; Henson, B.J.; Kunzelmann, K.; et al. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012, 72, 3270–3281.
- Wang, H.; Wang, T.; Zhang, Z.; Fan, Y.; Zhang, L.; Gao, K.; Luo, S.; Xiao, Q.; Sun, C. Simvastatin inhibits oral squamous cell carcinoma by targeting TMEM16A Ca(2+)-activated chloride channel. J. Cancer Res. Clin. Oncol. 2021, 147, 1699–1711.
- Bill, A.; Gutierrez, A.; Kulkarni, S.; Kemp, C.; Bonenfant, D.; Voshol, H.; Duvvuri, U.; Gaither, L.A. ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. Oncotarget 2015, 6, 9173–9188.
- Godse, N.R.; Khan, N.; Yochum, Z.A.; Gomez-Casal, R.; Kemp, C.; Shiwarski, D.J.; Seethala, R.S.; Kulich, S.; Seshadri, M.; Burns, T.F.; et al. TMEM16A/ANO1 Inhibits Apoptosis Via Downregulation of Bim Expression. Clin. Cancer Res. 2017, 23, 7324–7332.
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965.
- Harrington, K.J.; Ferris, R.L.; Gillison, M.; Tahara, M.; Argiris, A.; Fayette, J.; Schenker, M.; Bratland, Å.; Walker, J.W.T.; Grell, P.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab vs Nivolumab Alone for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: The Phase 2 CheckMate 714 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 779–789.
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928.
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987.
- Fessler, M.B.; Parks, J.S. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 2011, 187, 1529–1535.
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24.
- Choe, E.J.; Lee, C.H.; Bae, J.H.; Park, J.M.; Park, S.S.; Baek, M.C. Atorvastatin Enhances the Efficacy of Immune Checkpoint Therapy and Suppresses the Cellular and Extracellular Vesicle PD-L1. Pharmaceutics 2022, 14, 1660.
- Selvin, T.; Berglund, M.; Lenhammar, L.; Jarvius, M.; Nygren, P.; Fryknäs, M.; Larsson, R.; Andersson, C.R. Phenotypic screening platform identifies statins as enhancers of immune cell-induced cancer cell death. BMC Cancer 2023, 23, 164.
- Kansal, V.; Burnham, A.J.; Kinney, B.L.C.; Saba, N.F.; Paulos, C.; Lesinski, G.B.; Buchwald, Z.S.; Schmitt, N.C. Statin drugs enhance responses to immune checkpoint blockade in head and neck cancer models. J. Immunother. Cancer 2023, 11, e005940.
- Chiang, C.H.; Chen, Y.J.; See, X.Y.; Chang, Y.C.; Wang, S.S.; Peng, C.Y.; Horng, C.S.; Hsia, Y.P.; Chiang, C.H.; Peng, C.M.; et al. Efficacy of lipophilic statins on outcomes of patients treated with immune checkpoint inhibitors. Oncology, 2023; online ahead of print.
- Hu, T.; Pan, M.; Yin, Y.; Wang, C.; Cui, Y.; Wang, Q. The Regulatory Network of Cyclic GMP-AMP Synthase-Stimulator of Interferon Genes Pathway in Viral Evasion. Front. Microbiol. 2021, 12, 790714.
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118.
- Flood, B.A.; Higgs, E.F.; Li, S.; Luke, J.J.; Gajewski, T.F. STING pathway agonism as a cancer therapeutic. Immunol. Rev. 2019, 290, 24–38.
- York, A.G.; Williams, K.J.; Argus, J.P.; Zhou, Q.D.; Brar, G.; Vergnes, L.; Gray, E.E.; Zhen, A.; Wu, N.C.; Yamada, D.H.; et al. Limiting Cholesterol Biosynthetic Flux Spontaneously Engages Type I IFN Signaling. Cell 2015, 163, 1716–1729.
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312.
- Wylie, B.; Macri, C.; Mintern, J.D.; Waithman, J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers 2019, 11, 521.
- Villablanca, E.J.; Raccosta, L.; Zhou, D.; Fontana, R.; Maggioni, D.; Negro, A.; Sanvito, F.; Ponzoni, M.; Valentinis, B.; Bregni, M.; et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 2010, 16, 98–105.
- Wang, Q.; Cao, Y.; Shen, L.; Xiao, T.; Cao, R.; Wei, S.; Tang, M.; Du, L.; Wu, H.; Wu, B.; et al. Regulation of PD-L1 through direct binding of cholesterol to CRAC motifs. Sci. Adv. 2022, 8, eabq4722.
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655.
- Ma, X.; Bi, E.; Lu, Y.; Su, P.; Huang, C.; Liu, L.; Wang, Q.; Yang, M.; Kalady, M.F.; Qian, J.; et al. Cholesterol Induces CD8(+) T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019, 30, 143–156.e5.
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131.
- Xue, L.; Qi, H.; Zhang, H.; Ding, L.; Huang, Q.; Zhao, D.; Wu, B.J.; Li, X. Targeting SREBP-2-Regulated Mevalonate Metabolism for Cancer Therapy. Front. Oncol. 2020, 10, 1510.
- Zhao, Q.; Lin, X.; Wang, G. Targeting SREBP-1-Mediated Lipogenesis as Potential Strategies for Cancer. Front. Oncol. 2022, 12, 952371.
- Dubuc, G.; Chamberland, A.; Wassef, H.; Davignon, J.; Seidah, N.G.; Bernier, L.; Prat, A. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1454–1459.
- Rashid, S.; Curtis, D.E.; Garuti, R.; Anderson, N.N.; Bashmakov, Y.; Ho, Y.K.; Hammer, R.E.; Moon, Y.A.; Horton, J.D. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA 2005, 102, 5374–5379.
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The Evolving Future of PCSK9 Inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329.
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C.Y. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020, 588, 693–698.
- Yuan, J.; Cai, T.; Zheng, X.; Ren, Y.; Qi, J.; Lu, X.; Chen, H.; Lin, H.; Chen, Z.; Liu, M.; et al. Potentiating CD8(+) T cell antitumor activity by inhibiting PCSK9 to promote LDLR-mediated TCR recycling and signaling. Protein Cell 2021, 12, 240–260.
- Seidah, N.G.; Prat, A. The Multifaceted Biology of PCSK9. Endocr. Rev. 2022, 43, 558–582.
More
