Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Hamayak Sisakian.
Heart failure with reduced ejection fraction (HFrEF) is considered a major health care problem with frequent decompensations, high hospitalization and mortality rates. In severe heart failure (HF), the symptoms are refractory to medical treatment and require advanced therapeutic strategies. Early recognition of HF sub- and decompensation is the cornerstone of the timely treatment intensification and, therefore, improvement in the prognosis. Echocardiography is the gold standard for the assessment of systolic and diastolic functions. It allows one to obtain accurate and non-invasive measurements of the ventricular function in HF.
- heart failure with reduced ejection fraction
- echocardiography
- outpatient monitoring
- left ventricular filling pressure
1. Introduction
Heart failure (HF) is considered a major health care problem with more than a million new cases every year [1]. Over the last few decades, guideline-recommended treatment has considerably improved the outcomes of heart failure with reduced ejection fraction (HFrEF) [2,3][2][3]. Despite this, the mortality and rehospitalization rates remain high [1,2,4,5][1][2][4][5]. Moreover, the number of hospitalizations for HF is expected to increase significantly in the future and may double by 2045 due to the aging and growth of the population [6,7][6][7].
HF decompensation may be provoked by trigger factors such as infection, myocardial ischemia, acute renal injury, or anemia. In the case of disease deterioration due to increasing fluid retention, subclinical congestion precedes the clinical manifestation of HF symptoms and hospitalizations for an acute HF decompensation by several days or weeks. Therefore, the identification of a vulnerable period before the symptomatic HF decompensation allows for a prompt increase in diuretic doses and treatment modification, which may prevent the upcoming hospital admission [8]. Physical examination, particularly the identification of crackles on auscultation, is the main strategy routinely used for the prediction and diagnosis of left-sided HF decompensation in outpatients [9]. However, while it allows for the identification of symptomatic patients, the asymptomatic phase of HF decompensation is usually missed.
Hospitalization negatively affects the prognosis and quality of life [10]. Moreover, it is strongly associated with a risk of cardiovascular and all-cause mortality, which increases progressively with every subsequent hospitalization [11]. Thus, the prevention of rehospitalization is one of the main goals of HF management. For an effective reduction in unexpected hospital visits, early prediction of HF decompensation and prompt treatment modification are of paramount importance. Effective ambulatory monitoring and timely treatment modification are the key strategies for the reduction in cardiovascular events and improvement in the prognosis [12]. Numerous data suggest that HF patients who have undergone an echocardiographic examination have better survival rates due to more intensive medical treatment and interventions [13].
2. Left Ventricular Decompensation: Natriuretic Peptides and Basic Echocardiography
It is evident that hemodynamic deterioration precedes the development of clinical signs and symptoms by days or weeks, thus symptomatic clinical congestion may be seen as the “tip of an iceberg” of hemodynamic compromise [14]. Due to the delay between symptom manifestation and the progressive left ventricular (LV) filling pressure increase, physical examination alone may be insufficient for the identification of patients in the vulnerable phase of asymptomatic decompensation and for the prediction of upcoming hospital readmissions in outpatients [15,16][15][16]. It is well-established that an increased venous pressure can be detected by cardiovascular ultrasound, particularly by the assessment of inferior vena cava (IVC) characteristics and the detection of interstitial pulmonary edema through B-line identification. On the other hand, intracardiac hemodynamic changes and LV filling pressure increase precede the manifestation of HF symptoms and can be diagnosed by echocardiography [12,17][12][17]. Comprehensive cardiovascular ultrasound with tissue Doppler imaging (TDI) and lung ultrasound overcome the limitations of physical examination and clinical picture-based strategies for the prediction of HF decompensation. Over the last decade, it has become apparent that natriuretic peptide-guided management of ambulatory chronic HF patients may optimize the treatment. Natriuretic peptides are important components of HF diagnostic work-up due to their high negative predictive value. However, the data on the utility of natriuretic peptides in the prevention of HF decompensations are relatively scarce. In the PARADIGM-HF trial, low levels of natriuretic peptides were associated with lower risks of cardiovascular events [18]. In the GUIDE-IT study involving 894 high risk patients with HFrEF, the most important predictor of cardiovascular death and HF hospitalizations was the baseline natriuretic peptide level [19]. The data suggest that natriuretic peptides also correlate with the risk of hospitalization and the duration of hospital stay [6]. However, with the introduction of a neprilysin inhibitor in the treatment of HFrEF, the accuracy and reliability of natriuretic peptides as prognostic markers have been questioned. By inhibiting neprilysin, sacubitril leads to an increase in B-type natriuretic peptide (BNP) and a reduction in the N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations [20]. Thus, the role of natriuretic peptides in the prediction of hospital admissions in outpatients has to be further investigated. Another concern regarding the diagnostic role of natriuretic peptides is related to morbidly obese patients, who have consistently lower natriuretic peptide levels [21,22,23][21][22][23]. Other limitations of natriuretic peptides include their level changes in atrial fibrillation and renal dysfunction. Given the limitations of natriuretic peptides, along with the complex physiology of HF, it is reasonable to consider the implementation of other parameters and markers for the improvement of HF management and the prevention of decompensation. Basic echocardiographic assessment is an important tool for the diagnosis, classification, and treatment of HF. Some authors recommend using LV ejection fraction (EF) for the assessment of prognosis in ambulatory HFrEF patients, based on the role of EF dynamic increase as a predictor marker of an improved survival and hospitalization risk in this patient population [24]. However, EF loses its prognostic role at later stages of the disease and when the values are close to the threshold [25]. As a prognostic marker for HF decompensations, EF has other important limitations. Among them, technical pitfalls, preload dependence, possible measurement errors in the presence of severe mitral regurgitation, atrial fibrillation or heart rate variations, and a weak association with functional class and exercise capacity [26,27,28][26][27][28]. The above-mentioned limitations advocate for the use of additional parameters and methods for the assessment of LV function such as TDI, longitudinal strain quantification by speckle tracking, 3-dimensional echocardiography, and cardiac magnetic resonance imaging (MRI). An increase in left atrial (LA) volume and LV filling pressure contribute to LA remodeling and HF decompensation, leading to frequent hospitalization due to the lung congestion. In clinical practice, the LA volume index is widely used to assess LA overload. A clinically significant increase is considered an LA volume index >34 mL/m2 [29]. The LA volume index is an independent predictor of cardiovascular events including atrial fibrillation as well as cardiovascular mortality [26,30][26][30]. Moreover, the LA volume index is a marker of diastolic dysfunction and, unlike EF, correlates well with the patient’s functional class and overall exercise capacity [27,31][27][31]. The LA volume index correlates with the E/e’ ratio and can serve as a useful predictor of increased LV filling pressure during exercise [32]. Echocardiography with intracardiac hemodynamic assessment provides a semiquantitative analysis of decompensation and identifies filling pressure abnormalities. LV filling pressure measurements are of special interest in patients with HFrEF because the increase in filling pressure underlines the pathophysiological mechanisms of HF deterioration and precedes the manifestation of HF signs and symptoms [12]. Currently, echocardiographic Doppler and TDI are widely used in clinical practice for the evaluation of LV systolic and diastolic functions. As a marker of diastolic dysfunction, LV filling pressure can be assessed by calculating the E/e’ ratio, where the E wave is the peak velocity of the early diastolic flow across the mitral valve, as measured by pulsed-wave Doppler, and e’ is the early diastolic velocity of the septal or lateral mitral annulus obtained by TDI (Figure 1). The accepted threshold for the normal LV end-diastolic pressure is E/e’ <8, while E/e’ >14 and peak tricuspid regurgitation (TR) velocity >2.8 m/s indicate high LV end-diastolic pressure [33]. Recent studies emphasize the importance of the routine measurements of E/e’ for the assessment of LV filling pressure, risk stratification, and prognostication in the HF patient population [33,34][33][34]. Benfari G. et al. suggested an E/e’ ratio >14 as a cut-off for the identification of high-risk patients [35]. The authors reported increased short- and long-term mortality in individuals with E/e’ >14, with a considerable worsening of prognosis in E/e’ >20 [35]. Therefore, in HFrEF, additional parameters should be considered for the assessment of LV filling pressures and atrial hypertension to predict intracardiac volume overload and subclinical deterioration.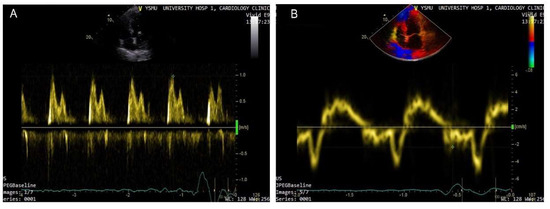
Figure 1. Doppler-based assessment of LV filling pressure. Raised filling pressure based on increased E/e’ ratio derived from the spectral Doppler of transmitral flow early peak velocity (E wave) (arrow) (A) and tissue Doppler of the mitral annular displacement early diastolic peak velocity (e’ wave) (arrow head) (B); E/e’ > 15.
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, 147–239.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 128, 2129–2200.
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016, 133, 38–360.
- Maggioni, A.P.; Dahlström, U.; Filippatos, G.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Metra, M.; et al. Heart Failure Association of ESC (HFA). EURObservational Research Programme: The Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail. 2010, 12, 1076–1084.
- Orso, F.; Fabbri, G.; Maggioni, A.P. Epidemiology of Heart Failure. Handb. Exp. Pharmacol. 2017, 243, 15–33.
- Savarese, G.; Musella, F.; D’Amore, C.; Vassallo, E.; Losco, T.; Gambardella, F.; Cecere, M.; Petraglia, L.; Pagano, G.; Fimiani, L.; et al. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: A meta-analysis. JACC Heart Fail. 2014, 2, 148–158.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Murphy, N.; Shanks, M.; Alderman, P. Management of Heart Failure with Outpatient Technology. J. Nurse Pract. 2019, 15, 12–18.
- Damy, T.; Kallvikbacka-Bennett, A.; Zhang, J.; Goode, K.; Buga, L.; Hobkirk, J.; Yassin, A.; Dubois-Randé, J.L.; Hittinger, L.; Cleland, J.G.; et al. Does the physical examination still have a role in patients with suspected heart failure? Eur. J. Heart Fail. 2011, 13, 1340–1348.
- Čerlinskaitė, K.; Mebazaa, A.; Cinotti, R.; Matthay, M.; Wussler, D.N.; Gayat, E.; Juknevičius, V.; Kozhuharov, N.; Dinort, J.; Michou, E.; et al. Readmission following both cardiac and non-cardiac acute dyspnoea is associated with a striking risk of death. ESC Heart Fail. 2021, 8, 2473–2484.
- Lindmark, K.; Boman, K.; Stålhammar, J.; Olofsson, M.; Lahoz, R.; Studer, R.; Proudfoot, C.; Corda, S.; Fonseca, A.F.; Costa-Scharplatz, M.; et al. Recurrent heart failure hospitalizations increase the risk of cardiovascular and all-cause mortality in patients with heart failure in Sweden: A real-world study. ESC Heart Fail. 2021, 8, 2144–2153.
- Mangi, M.A.; Rehman, H.; Rafique, M.; Illovsky, M. Ambulatory Heart Failure Monitoring: A Systemic Review. Cureus 2017, 9, e1174.
- Tribouilloy, C.; Rusinaru, D.; Mahjoub, H.; Goissen, T.; Lévy, F.; Peltier, M. Impact of echocardiography in patients hospitalized for heart failure: A prospective observational study. Arch. Cardiovasc. Dis. 2008, 101, 465–473.
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. European Society of Cardiology; European Society of Intensive Care Medicine. Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010, 12, 423–433.
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292.
- Lampert, B.C.; Emani, S. Remote hemodynamic monitoring for ambulatory left ventricular assist device patients. J. Thorac. Dis. 2015, 7, 2165–2171.
- Dokainish, H.; Nguyen, J.S.; Bobek, J.; Goswami, R.; Lakkis, N.M. Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: An echocardiographic and invasive haemodynamic study. Eur. J. Echocardiogr. 2011, 12, 857–864.
- Zile, M.R.; Claggett, B.L.; Prescott, M.F.; McMurray, J.J.; Packer, M.; Rouleau, J.L.; Swedberg, K.; Desai, A.S.; Gong, J.; Shi, V.C.; et al. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients with Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 2425–2436.
- O’Connor, C.; Fiuzat, M.; Mulder, H.; Coles, A.; Ahmad, T.; Ezekowitz, J.A.; Adams, K.F.; Piña, I.L.; Anstrom, K.J.; Cooper, L.S.; et al. Clinical factors related to morbidity and mortality in high-risk heart failure patients: The GUIDE-IT predictive model and risk score. Eur. J. Heart Fail. 2019, 21, 770–778.
- Brunner-La Rocca, H.P.; Sanders-van Wijk, S. Natriuretic Peptides in Chronic Heart Failure. Card. Fail. Rev. 2019, 5, 44–49.
- Madamanchi, C.; Alhosaini, H.; Sumida, A.; Runge, M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: Mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 2014, 176, 611–617.
- Horwich, T.B.; Hamilton, M.A.; Fonarow, G.C. B-type natriuretic peptide levels in obese patients with advanced heart failure. J. Am. Coll. Cardiol. 2006, 47, 85–90.
- Mehra, M.R.; Uber, P.A.; Park, M.H.; Scott, R.L.; Ventura, H.O.; Harris, B.C.; Frohlich, E.D. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am. Coll. Cardiol. 2004, 43, 1590–1595.
- Breathett, K.; Allen, L.A.; Udelson, J.; Davis, G.; Bristow, M. Changes in Left Ventricular Ejection Fraction Predict Survival and Hospitalization in Heart Failure with Reduced Ejection Fraction. Circ. Heart Fail. 2016, 9, e0029620.
- Marwick, T.H. Methods used for the assessment of LV systolic function: Common currency or tower of Babel? Heart 2013, 99, 1078–1086.
- Marwick, T.H. The role of echocardiography in heart failure. J. Nucl. Med. 2015, 56 (Suppl. 4), 31S–38S.
- Pastore, M.C.; Mandoli, G.E.; Aboumarie, H.S.; Santoro, C.; Bandera, F.; D’Andrea, A.; Benfari, G.; Esposito, R.; Evola, V.; Sorrentino, R.; et al. Basic and advanced echocardiography in advanced heart failure: An overview. Heart Fail. Rev. 2020, 25, 937–948.
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11.
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310.
- Di Tullio, M.R.; Qian, M.; Thompson, J.L.P.; Labovitz, A.J.; Mann, D.L.; Sacco, R.L.; Pullicino, P.M.; Freudenberger, R.S.; Teerlink, J.R.; Graham, S.; et al. Left atrial volume and cardiovascular outcomes in systolic heart failure: Effect of antithrombotic treatment. ESC Heart Fail. 2018, 5, 800–808.
- Romano, G.; Magro, S.; Agnese, V.; Mina, C.; Di Gesaro, G.; Falletta, C.; Pasta, S.; Raffa, G.; Baravoglia, C.M.H.; Novo, G.; et al. Echocardiography to estimate high filling pressure in patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2020, 7, 2268–2277.
- Hammoudi, N.; Achkar, M.; Laveau, F.; Boubrit, L.; Djebbar, M.; Allali, Y.; Komajda, M.; Isnard, R. Left atrial volume predicts abnormal exercise left ventricular filling pressure. Eur. J. Heart Fail. 2014, 16, 1089–1095.
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314.
- Cameli, M.; Pastore, M.C.; Mandoli, G.E.; Nistor, D.; Lisi, E.; Tok, Ö.Ö.; Cavigli, L.; Romano, A.; Mondillo, S. Prognosis and Risk Stratification of Patients with Advanced Heart Failure (from PROBE). Am. J. Cardiol. 2019, 124, 55–62.
- Benfari, G.; Miller, W.L.; Antoine, C.; Rossi, A.; Lin, G.; Oh, J.K.; Roger, V.L.; Thapa, P.; Enriquez-Sarano, M. Diastolic Determinants of Excess Mortality in Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2019, 7, 808–817.
- Ommen, S.R.; Nishimura, R.A.; Appleton, C.P.; Miller, F.A.; Oh, J.K.; Redfield, M.M.; Tajik, A.J. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000, 102, 1788–1794.
- Park, J.H.; Marwick, T.H. Use and Limitations of E/e’ to Assess Left Ventricular Filling Pressure by Echocardiography. J. Cardiovasc. Ultrasound 2011, 19, 169–173.
- Lancellotti, P.; Galderisi, M.; Edvardsen, T.; Donal, E.; Goliasch, G.; Cardim, N.; Magne, J.; Laginha, S.; Hagendorff, A.; Haland, T.F.; et al. Echo-Doppler estimation of left ventricular filling pressure: Results of the multicentre EACVI Euro-Filling study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 961–968.
- Jones, R.; Varian, F.; Alabed, S.; Morris, P.; Rothman, A.; Swift, A.J.; Lewis, N.; Kyriacou, A.; Wild, J.M.; Al-Mohammad, A.; et al. Meta-analysis of echocardiographic quantification of left ventricular filling pressure. ESC Heart Fail. 2021, 8, 566–576.
- Obokata, M.; Kane, G.C.; Reddy, Y.N.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure with Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838.
- Hummel, Y.M.; Liu, L.C.Y.; Lam, C.S.P.; Fonseca-Munoz, D.F.; Damman, K.; Rienstra, M.; van der Meer, P.; Rosenkranz, S.; van Veldhuisen, D.J.; Voors, A.A.; et al. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: A study utilizing simultaneous echocardiography and invasive measurements. Eur. J. Heart Fail. 2017, 19, 1651–1660.
- Marini, C.; Fragasso, G.; Italia, L.; Sisakian, H.; Tufaro, V.; Ingallina, G.; Stella, S.; Ancona, F.; Loiacono, F.; Innelli, P.; et al. Lung ultrasound-guided therapy reduces acute decompensation events in chronic heart failure. Heart 2020, 106, 1934–1939.
- Cuthbert, J.J.; Pellicori, P.; Flockton, R.; Kallvikbacka-Bennett, A.; Khan, J.; Rigby, A.S.; Girerd, N.; Zannad, F.; Cleland, J.G.F.; Clark, A.L. The prevalence and clinical associations of ultrasound measures of congestion in patients at risk of developing heart failure. Eur. J. Heart Fail. 2021, 23, 1831–1840.
- Ballo, P.; Guarini, G.; Simioniuc, A.; Gistri, T.; Fontanive, P.; Di Bello, V.; Dini, F.L.; Marzilli, M. Prognostic value of pulsed tissue Doppler imaging for the assessment of left ventricular systolic function in patients with nonischemic dilated cardiomyopathy. Echocardiography 2012, 29, 291–297.
More
