Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Guofeng Luo.
Gene therapy is the ultimate therapeutic technology for diseases related to gene abnormality. However, the use of DNA alone has serious problems, such as poor stability and difficulty in entering target cells. The development of a safe and efficient gene delivery system is the cornerstone of gene therapy. Of particular interest, multifunctional peptides are rationally designed as non-viral vectors for efficient gene delivery. As components of gene delivery vectors, these peptides play critically important roles in skeleton construction, the implementation of targeting strategies, cell membrane penetration, endosome rupture, and nuclear transport.
- gene delivery vector
- gene therapy
- non-viral vector
- functional peptides
1. Peptides Act as DNA-Binding Units
Peptide-based vectors are mainly cationic peptides that are rich in basic amino acids (e.g., lysine, arginine, and histidine), which are positively charged at a normal physiological pH and can form complexes with negatively charged DNA [47][1]. Therefore, peptide-based gene delivery vectors can compress DNA, reduce their molecular size, and provide sufficient protection against nucleases. Meanwhile, the extra positive charge on the surface of the complexes can make it more accessible to the negatively charged cell membrane and promote complexes to enter the cells. For peptide-based vectors, at least 6~8 cationic residues are needed to effectively encapsulate and successfully transfect DNA, which depends on the amino acid category. For example, arginine binds DNA more strongly than lysine.
1.1. Poly-l-lysines (PLLs)
A PLL is the first cationic peptide mediating gene transfer, and it is a linear polypeptide formed by polymerizing 90~450 lysine monomers. A PLL contains a primary amine, which is positively charged after protonation [48][2]. Thus, it can be used for gene transfer via electrostatic binding to negatively charged DNA. The compress ability of PLL to DNA enhances with the increase in molecular weight, and only PLLs with a molecular weight greater than 3000 Da can compress DNA to form stable complexes. PLLs have been widely used in gene delivery because their structures are easy to modify and can introduce various functional molecules. However, the transfection efficiency of PLLs is relatively low because (1) PLLs have a certain extent of cytotoxicity. A PLL’s ability to compress DNA increases with its molecular weight, and higher-molecular-weight PLLs have good compression ability, which leads to greater cytotoxicity. (2) PLLs alone lack the ability to target specific cells and to escape from endosomes. At a physiological PH, the primary amines of PLLs are all protonated, resulting in their lack of buffering capacity in the acidic environment of endosomes after entering into cells. It is difficult for the PLL/DNA complex to escape from the endosome, which may eventually lead to DNA degradation by the nuclease. (3) The stability of the complexes is poor, and they easily form aggregates with the serum owing to the excess of positive charges [49,50,51][3][4][5].
A significant advantage of PLLs lies in their ease of chemical modification, and researchers have modified and improved the properties of PLLs in a variety of ways [52][6]. In order to reduce the cytotoxicity of PLLs, researchers began to modify them with the shielding polymer, PEG. In the work reported by Kim et al., they found that PEG modified on the amino group of a PLL could effectively reduce toxicity [53][7]. This method could reduce the binding of gene nanoparticles to serum in cells and increase the blood circulation time. Also, Kataoka et al. found that the particle size of the complex particles formed by the PLL-PEG copolymer and antisense-ODN was significantly lower than that of PLLs [54][8]. Their particle size distribution was extremely narrow,, and the resultant core–shell architecture could resist deoxyribonuclease degradation. To further reduce undesirable side effects, Zhang et al. designed ternary FK/p53/PEG-PLL(DA) complexes by introducing charge-reversible dimethylmaleic anhydride (DA) groups in addition to PEG, resulting in the formation of a detachable shielding layer (PEG-PLL(DA)) on the nanocomplex surface [38][9]. At a physiological pH of 7.4, the FK/p53/PEG-PLL(DA) complexes could extend the circulating time due to the charge shielding effect of the PEG-PLL(DA). Once it arrives at the acidic microenvironment of the tumor tissue, the PEG-PLL(DA) undergoes a charge switch for the removal of the surface shielding layer, which facilitates the subsequent tumor-cell-specific targeting. Moreover, Guo et al. covalently grafted a sugar-containing polymer (DPMAEL) onto PLLs, which not only reduced the cytotoxicity of the PLLs, but also improved the stability of the complex nanoparticles in the electrolytes and serum [55][10]. In order to improve the escape ability of the endosome and the transfection efficiency of the PLLs, Longer et al. grafted the amino group in the side chain of PLLs with the imidazole group, and the corresponding gene transfection efficiency was significantly improved [56][11]. An analysis showed that the grafted imidazole group was easily protonated in an acidic environment, and the electrostatic attraction with DNA was enhanced, which, at the same time, enhanced the buffer capacity of PLLs for efficient endo/lysosome escape. Kim et al. reported that the PLL linked with 16% histidine was a highly efficient transgenic vector with little toxicity [57][12]. Midoux et al. found that the transfection efficiency of partially histidylated PLLs increased without the assistance of chloroquine. This was related to the fact that histidylated PLLs could be further protonated at a low pH, which partially mimicked the proton sponge mechanism of PEI [58][13].
A common issue faced by polycation vectors is how to strike a balance between toxicity and transfection efficiency. The introduction of a reduction-responsive group into the carrier by molecular design is a viable strategy, such as using a disulfide-crosslinked carrier [59,60,61][14][15][16]. The disulfide bonds can be cleaved in the highly reducing environment in cells, which contributes to the release of nucleic acid molecules into the cytoplasm. As an example, Zhang et al. constructed a PEGlated reductive vector by oxidativly polycondensing CHK6HC-mPEG and CHK6HC, both of which contained the short chain of lysine segments, via disulfide bonds (Figure 21) [62][17]. The disulfide bonds in the resultant nanovectors were reductively degraded into short-chain oligopolylysine in an intracellular environment, and the cytotoxicity was greatly reduced by such reducible polycations. Moreover, the transfection efficiency of PolyHK6H-mPEG was comparable to that of PEI-mPEG, but its toxicity was much lower than that of PEI-mPEG.
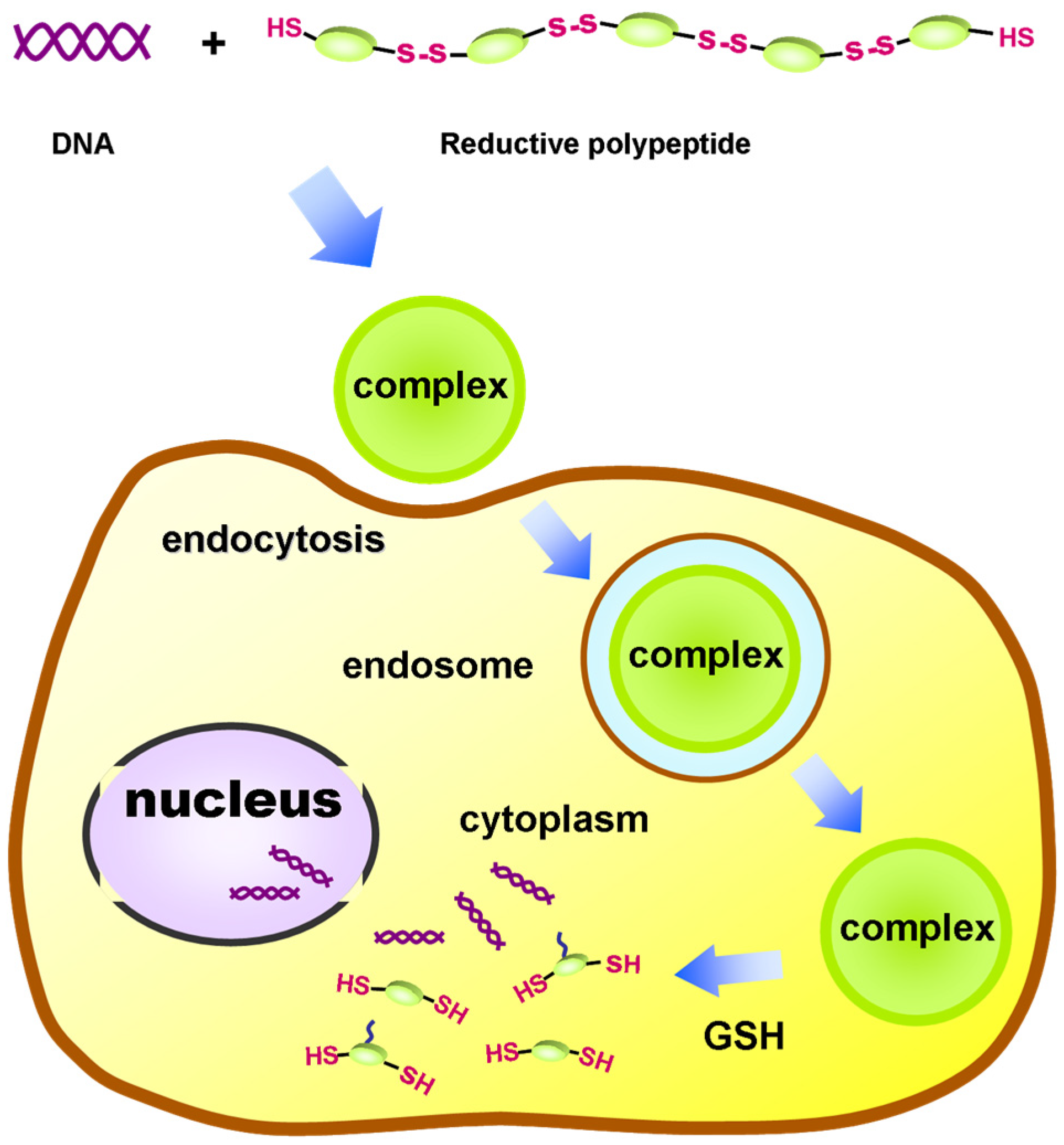
Figure 21. Schematic of the PEGlated reductive polypeptide vector used for gene delivery. Adapted with permission from the authors of [62].
Schematic of the PEGlated reductive polypeptide vector used for gene delivery. Adapted with permission from the authors of [17].
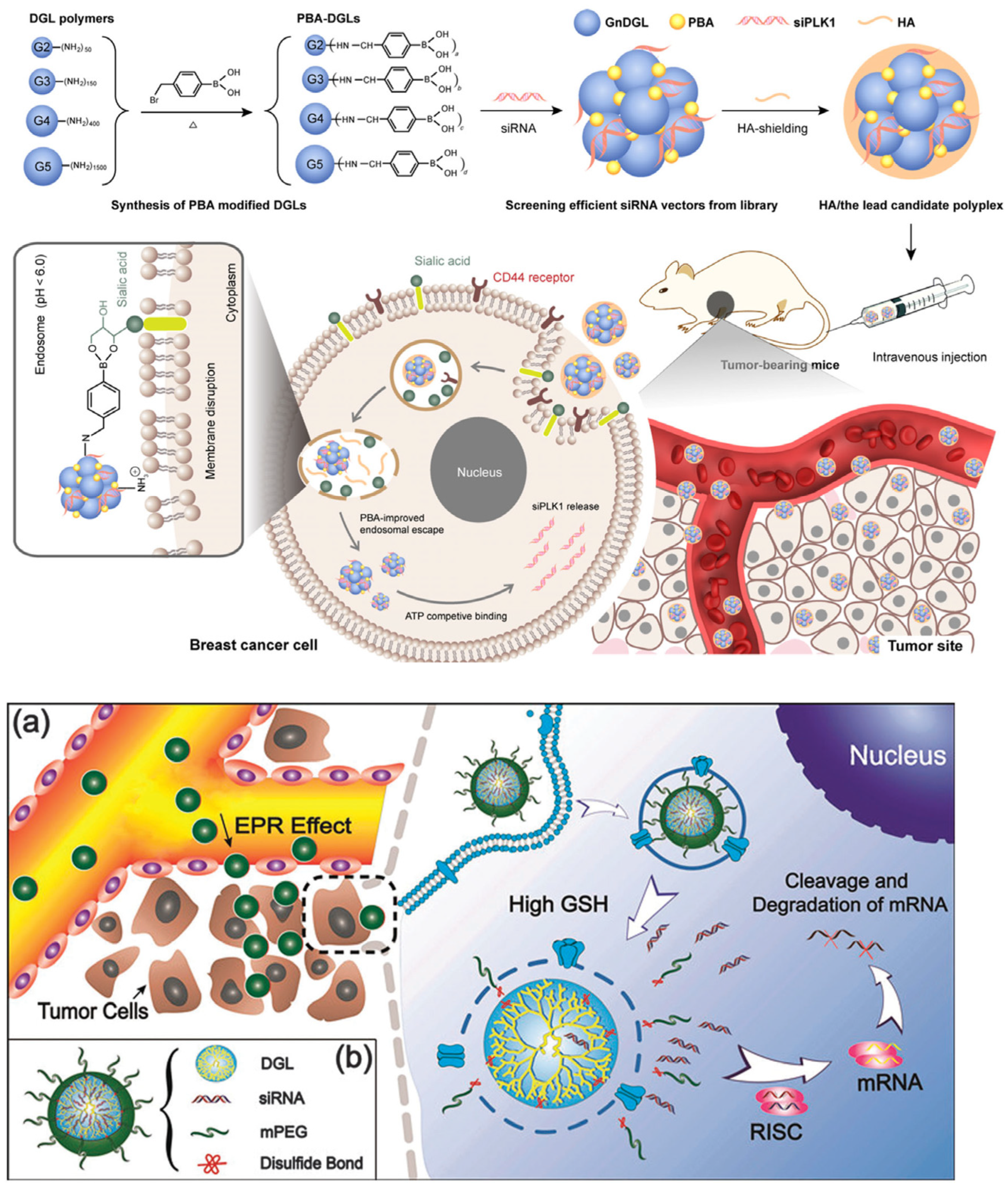
In addition, dendrigraft poly-l-lysine (DGL) polymers that are synthesized by grafting l-lysine oligomers in a repetitive manner have emerged as new kinds of dendritic poly-l-lysine (PLL) derivatives [63,64][18][19]. DGL polymers possess a dendrigraft architecture without tertiary amine groups in addition to some unique properties, including narrow polydispersity, a well-defined structure, and a high density of amine groups on the surface, rendering them suitable for gene delivery. Various DGL-based vectors have been constructed by the integration with stimuli-responsiveness or targeting groups to improve gene transfection efficiencies. For example, Liu et al. reported the modification of DGL polymers with phenylboronic acid (PBA) to enhance the endosomal escape ability (Figure 32) [65][20]. In vitro and in vivo results demonstrated that the PBA modification strategy significantly improved the transfection of siRNA in MDA-MB-231 cells and inhibited the tumor growth by effective siRAN delivery. In another work reported by Ren et al., cleavable PEG was used to modify DGL polymers, generating the resultant DGL(R)-SS-mPEG, for reducing the cytotoxicity of DGL owing to their high density of positive charges [66][21]. The resultant cleavable polymer enabled the efficient pDNA loading and is able to respond to tumor-relevant glutathione (GSH) conditions for PEG detachment, which is favorable for subsequent intracellular gene delivery. To further enhance the transfection efficiency, Huang et al. designed a multifunctional DGL-based vector by integrating a PEG and cell-penetrating peptide with DGL [67][22]. As a result, the nano-sized nanoparticles with the loading of the therapeutic gene remarkably enhanced the blood–brain barrier (BBB)-crossing efficiency, cell internalization, and gene transfection in tumor cells, resulting in a strong apoptosis on the tumor site. Such a mutifunctional DGL vector represents a potential efficient gene delivery platform for glioma therapy.
1.2. Arginine-Rich Peptides
Arginine (Arg)-rich peptides are another kind of important gene carrier backbone owing to their cationic guanidino groups for interaction with the anionic phosphate groups in DNA or RNA, resulting in the formation of polyion complexes [24][23]. These kinds of delivery systems have been widely used for the transportation of nucleic acids (e.g., plasmid DNA, antisense DNA, and siRNA) into cells [68,69][24][25]. The transfection efficiency and physicochemical properties or Arg-rich peptides are highly dependent on their lengths, sequences, secondary structures, and additional modifications. In the work reported by Tanaka et al., three kinds of Arg-rich peptides with different numbers of Args, including R9, (RRG)3, and (RRU)3, were synthesized with the additional modification of a fluorescent molecule (i.e., 6-FAM) for the delivery of pDNA (Figure 43) [70][26]. As a result, the R9/pDNA complex displayed the most efficient cellular internalization ability and transfection efficiency among the three peptides, since the unique cell-penetrating ability of R9 was remarkably reduced by the replacement of Args with Gly and Aib.
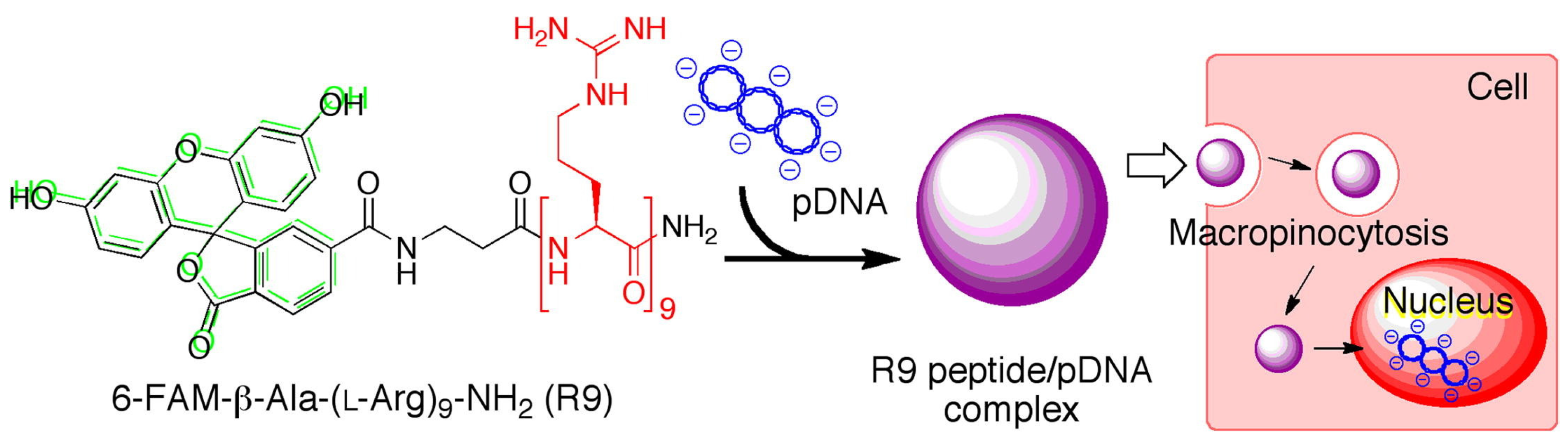
Figure 43. Schematic of the synthesis of 6-FAM-labled R9/pDNA complex for gene delivery. Adapted with permission from the authors of [70].
Schematic of the synthesis of 6-FAM-labled R9/pDNA complex for gene delivery. Adapted with permission from the authors of [26].
1.3. Poly-l-ornithine (PLO)
PLO has a positive charge under physiological conditions and can bind DNA via electrostatic attraction. Ramsay et al. showed that PLO was stronger than PLL in binding DNA under the same conditions, and the transfection efficiency of PLO was five times that of PLL when transfecting A549 cells. Similar results were obtained when transfecting COS-7 cells [71][27]. Thomas et al. found in the in vitro transfection experiment of B16 melanoma cells that the transfection efficiency was PLO > PLL > Polyargine. They also studied the transfection efficiency of co-amino acids (co-polymer(Lys:Ala)), and found that PLO >> Lys:Ala(2:l) > Lys:Ala(3:l), while Lys:Ala(1:1) had almost no transfection activity [72][28]. PLO, when combined with a DMSO, resulted in the shock-mediated efficient transfection of CHO cells. The ability to directly and effectively transfect cells in a growth-arrested state was reported for the first time. This method has also been proven to be effective for other cells including rat embryonic fibroblasts in both stable and transient transfection [73][29]. The combination of PLL and PLO can form amphiphilic colloidal vesicles, reduce toxicity, and does not rely on receptor-mediated endocytosis. Uchegbu et al. modified PLL and PLO with palmitoyl and polyethylene glycol monomethyl ether (mPEG) to obtain the amphiphilic copolymers PLP and POP [74][30]. PLP and POP could self-assemble into polymer vesicles in the presence of cholesterol. Their in vitro cytotoxicity decreased by 1–2 orders of magnitude, but their hemolysis ability was stronger than that of the PLL and PLO homopolymers. The surface of this type of polymer vesicle was close to neutral and rich in PEG, so it was beneficial to perform transfection in vivo. The polymeric vesicle–DNA complexes improved gene transfer to human tumor cell lines in comparison to the parent homopolymers despite the absence of receptor-specific ligands and lysosomotropic agents such as chloroquine. Zhuo et al. synthesized multi-arm star-shaped polyornithine PEI-P(Orn)(n) and grafted polyornithine arms onto branched PEI with a M-w of 600 via ring-opening polymerization [75][31]. In view of the excellent cell membrane penetration property of being rich in arginine, the amino side groups on polyornithine arms are partially guanidinated and transform the omithine units to arginine units. The results show that star-shaped poly(ornithine) modified by guanidine salt improved serum compatibility, reduced cytotoxicity, and improved transfection efficiency.
2. Peptides Act as Functional Moieties
2.1. Cell-Targeting Peptides
The positive charge of the cationic carrier is a double-edged sword. Although the interaction of positive and negative charge is beneficial to improve transfection efficiency, it also leads to the non-specific combination of positively charged complexes with various cells [76][32]. An ideal gene delivery vector should be able to selectively deliver targeted genes to diseased organs or cells without affecting normal tissues/cells. Otherwise, the therapeutic gene needs large doses of administration to achieve the required efficacy, which will not only cause the waste of active substances, but also increase the risk of side effects. To this end, the main strategy is to attach specific targeting ligands, such as epidermal growth factor, transferrin, folic acid, and, especially, peptides. The RES capture and PEG dilemma of gene delivery carriers are at the appropriate place. The unwanted nonspecific elimination of gene delivery vectors by reticuloendothelial cells, particularly liver sinusoidal wall cells (Kupffer cells and sinusoidal endothelial cells), is one of the most significant barriers to clinical translation, which causes a profound decrease in the delivery efficiency of gene medicines into the target tissues and raises toxicity issues [77][33]. Surface shielding by PEG onto gene delivery carriers drastically decreases the cellular uptake and endosomal escape, thus resulting in precluded transfection efficiency (PEG dilemma), although PEGylation substantially improved the colloidal stability and stealth in the harsh biological milieu [78][34]. To overcome this dilemma of PEG shielding on reduced cellular uptake, targeting ligands were strategically placed at the distal end of the PEG segments to promote specific ligand-receptor-mediated uptake [79][35].
With the rapid development of phage display technology, many peptide fragments corresponding to the functional domain of ligand molecules have been developed [76][32]. These targeted peptides can be used as ligands to intentionally deliver DNA to specific types of cells [28,80][36][37]. Arg-Gly-Asp (RGD) is an integrin-binding peptide, which can bind to many different integrins that play important roles in cell migration, cellular uptake, intercellular interaction, and signal transduction [81][38]. Therefore, many peptides with an RGD sequence have been developed to target integrin receptors. In addition to this, more and more specific targeting peptides have been found and isolated, such as tumor-homing peptides, vascular-homing peptides, and breast-tissue-homing peptides [82,83][39][40]. In order to treat neurodegenerative diseases (e.g., Parkinson’s disease or Alzheimer’s disease), some peptides that can target neuronal cells have been discovered. For example, neurotensin (NT) is a biologically active single-chain peptide that exists widely in the nervous system of mammals [84][41]. Arias-Montaño et al. used SPDP to connect NT to PLL and targeted cell lines with a high expression of NT receptors (NTRH), such as N1E-115, HT-29, etc., while in COS-7 cells lacking NTRH, there is no expression at all, which showed its strong targeting selectivity against different cell lines [85][42].
In spite of this, many cell-targeting peptides have been employed for the construction of peptide-based gene delivery systems with high specificity. For example, Kim et al. synthesized a PEG-g-PLL-based vector conjugated with the artery-wall-binding peptide (AWBP) [86][43]. Since AWBP provided targeting to the arterial wall cells, AWBP-PEG-PLL was investigated for the gene therapy of cardiovascular diseases. It was shown that the delivery of AWBP-PEG-PLL/pDNA complexes could be mediated by the specific targeting peptide (i.e., AWBP) to the bovine aorta wall cells. Moreover, NGR is another peptide motif obtained via in vivo screening, and it has been found in vivo that NGR can selectively bind to aminopeptidase N overexpressed on tumor endothelial cells [87][44]. Cristiano et al. constructed a targeted non-viral vector combining a CNGRC peptide with PEG-PLL, and this vector could specifically target and transfer genes to tumor cells with a positive CD13 expression, thus improving the PEG-PLL-mediated transfection efficiency by 12 times [88][45]. Hepatocellular carcinoma (HCC) is one of the most common types of malignant tumors worldwide. Zheng et al. fused the HCC-specific peptide SP94 (SFSIIHTPL) to the N-terminus of nine arginine residues via a four-glycine linker, achieving the HCC-specific delivery of siRNA payloads [89][46]. In addition, AP1 (an IL-4 receptor specific ligand), as a specific tumor tissue and atherosclerotic plaque-homing peptide, can selectively bind to interleukin-4 receptors (IL-4Rs) overexpressed on cells [90][47]. Park et al. constructed a non-viral gene-delivery system based on the integration of an elastin-like peptide (EL), Tat, and AP1 for cancer therapy [91][48]. The resultant ELPs could self-assemble into nanocomplexes that were stable under physiological conditions and, specifically, target to IL4-receptor-expressing tumors. This system was found to deliver plasmids harboring the siRNA to tumor cells and silence the gene of interest. Moreover, Liu et al. constructed a multifunctional peptide TAT-H6-K(C18)-YIGSR containing a targeting YIGSR sequence, which targets the laminin receptor on the surface of tumor cells [92][49]. It was found that the vector containing the YIGSR segment dramatically improved the gene transfection efficiency via LR-mediated endocytosis. In addition, Gao et al. designed a nanocarrier of GE11-PEG-PEI, which showed significantly more gene copies to EGFR-overexpressing laryngeal cancer cells in vivo than that of PEG-PEI alone. It was indicated that GE11-PEG-PEI was a suitable gene delivery vector for treating EGFR-overexpressing laryngeal cancer [93][50]. In another work, Tang et al. developed a gene delivery vector of MPC/Ad-SS-PEG, which conjugated an MC11 peptide with PEI, PEG, and adamantyl (Ad) groups [94][51]. The results of gene transfection efficiency and the tumor-targeting experiments show that the MPC/Ad-SS-PEG system may be a safe and efficient non-viral vector for FGFR-mediated targeted gene delivery for cancer gene therapy. Zhang et al. constructed T7-peptide-modified core–shell nanoparticles (T7-PEG-LPC/siRNA NPs) to deliver siRNAs [95][52]. Both in vitro and in vivo experiments demonstrated that EGFR siRNA could be specifically transported into breast cancer cells with the assistance of T7-LPC/siEGFR NPs, as a consequence of efficient receptor-mediated endocytosis for the down-regulation of EGFR expression. As a result, such a targeted delivery system could obviously inhibit the growth of breast tumors with little activation of an immune response.
2.2. Cell-Penetrating Peptides
Cell-penetrating peptides (CPPs) are a class of short amino acid sequences that are able to penetrate the cell membrane with a high efficiency [96][53]. Owing to this distinct property, CPPs have been widely used for the quick delivery of hydrophilic proteins, peptides, nucleic acids, drugs, and other foreign substances through the cell membrane into the cytoplasm or even the nucleus [97,98][54][55]. During this process, the cell membrane remains intact, and there is no significant toxic effect on the host cells. Moreover, this transmembrane effect is not restricted by the cell type. According to the structural characteristics, transmembrane peptides can be divided into two categories: one is a peptide rich in basic arginine represented by TAT (YGRKKRRQRRR), and the other is a peptide with an amphiphilic structure represented by penetratin (RQIKIWFQNRRMKWKK) [99,100][56][57]. Since some CPPs are arginine-rich cationic peptides, CPPs can be used either alone for gene transduction or as a transmembrane component in combination with other non-viral vectors.
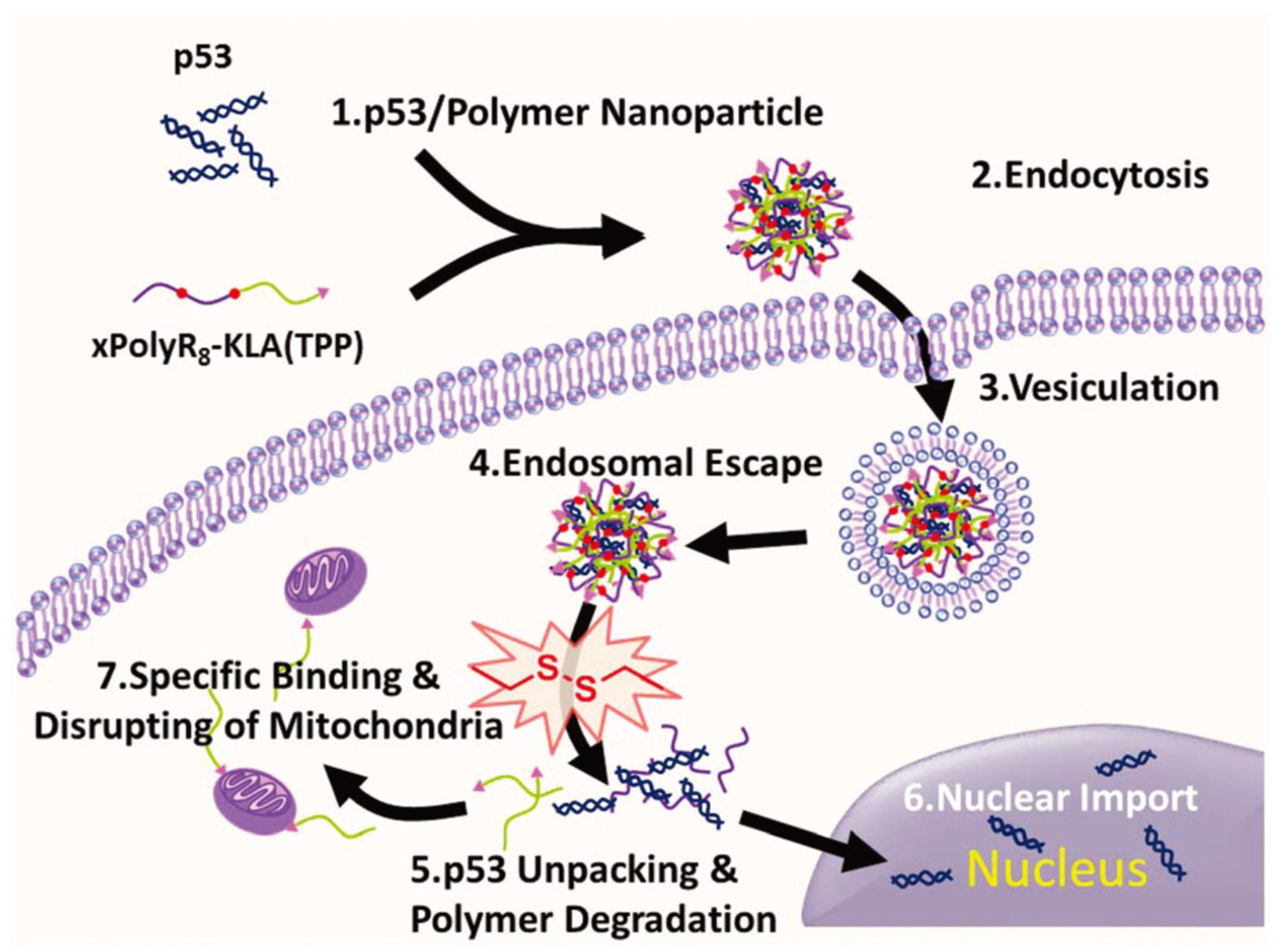
rPOA with disulfide bonds was formed via polymerization of the terminal cysteinyl-thiol groups of the short peptide. An in vivo study showed that rPOA exhibited a higher transfection efficiency than poly(ethyleneimine) (PEI) and sustained non-toxicity for 1 week. In the work reported by Zhang et al., a series of bioreducible polypeptides were synthesized, which comprise CHK6HC and CR8C in various ratios via reducible disulfide bonds [103][58]. The results suggest that increasing the amount of R8 can promote the transfection efficiency significantly. Specifically, xPolyK6-R82 exhibited the highest transfection efficiency at a w/w ratio of 50. They also synthesized a dual-functional polypeptide (xPolyR8-KLA(TPP)) via disulfide crosslinking of CR8C and C-KLA(TPP) [104][59]. With an improved cell penetrating ability, the resultant XPolyR8-KLA(TPP) enabled the efficient loading and delivering of therapeutic genes (i.e., p53) and, at the same time, could induce tumor cell apoptosis owing to the cytotoxic effect of C-KLA(TPP) (Figure 54).
In addition, Wang et al. designed a hybrid PEI600-Tat peptide as a gene carrier by covalently linking low-molecular-weight PEI to a bioactive Tat peptide [105][60]. It was shown that the PEI600-Tat peptide exhibited good biocompatibility and cell membrane permeability, which had a broad application prospect in gene therapy. Bienert et al. proposed an amphiphilic model peptide (MAP) with the sequence of KLALKLALKALKAALKLA, and its uptake and transfection efficiency were demonstrated to be higher than those of TAT alone [106][61]. Omar et al. developed a TAT-conjugated polyamidoamine (PAMAM) dendrimer for the transdermal delivery of DNA vaccines [107][62]. The results confirm that the TAT-conjugated PAMAM dendrimer could effectively promote DNA vaccine uptake by cells and had a stronger DNA transdermal delivery capacity than the unmodified PAMAM dendrimer. To facilitate the skin penetration of pDNA deeply into the melanoma tissues, Gao et al. constructed a gold nanoparticle (AuPT) conjugated with CPP(TAT) and cationic PEI [108][63]. The results show that the AuPT could carry pDNA through the intact stratum corneum and efficiently stimulate the intracellular uptake and nuclear targeting of pDNA in cells. Langel et al. developed a gene delivery vector, which crosslinked TP10 to a pDNA via PNA oligomers and then conjugated with PEI [109][64]. It was shown that, compared with PEI, the transfection efficiency of the conjugated TP10-PEI was several times higher, and its toxicity was lower.
2.3. Endosomal Escape Peptides
After endocytosis into cells, the vector–DNA complexes are first encapsulated in the endosome and then transported to the lysosome. If the complexes cannot escape from the endosome/lysosome into the cytoplasm as soon as possible, they will face the harsh environment of acidity (pH ≈ 4.5) and various hydrolases, which will rapidly degrade and inactivate DNA or the whole vector [110][65]. Therefore, endo/lysosomal escape has been regarded as one of the most critical bottlenecks of improving the transfection efficiency of non-viral vectors, which may greatly limit the transportation of DNA in gene therapy, resulting in low therapeutic efficiency. Therefore, whether the vector can escape from the endosomal/lysosome quickly has an important impact on the efficiency of gene therapy. Recently, the “proton sponge effect” and endosome disruptors are mainly applied to solve this challenge [111,112][66][67].
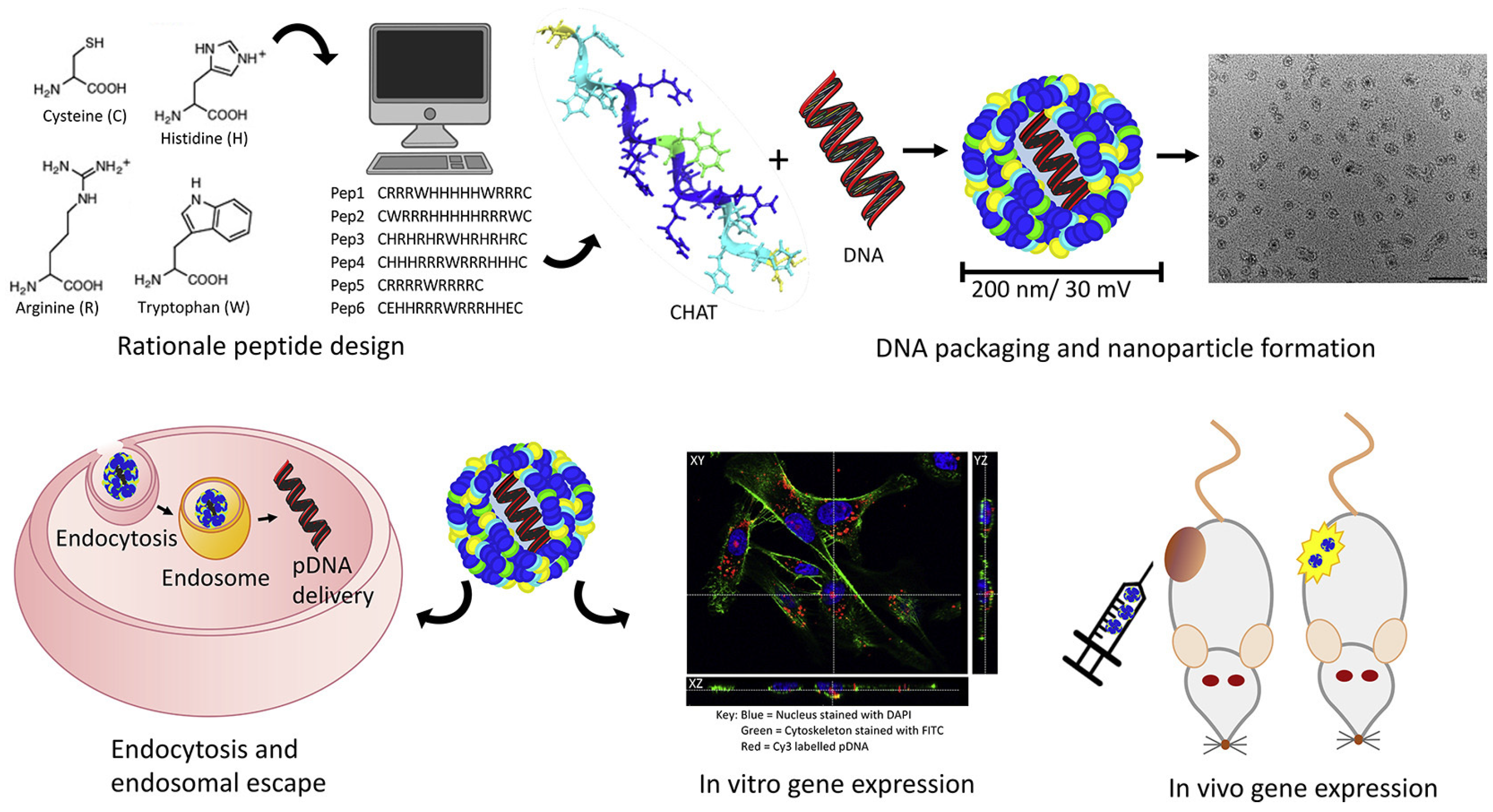
Secondary and tertiary amines on gene carriers with a “proton sponge effect” can capture a large number of protons, causing the swelling and rupture of endosomes/lysosomes. Therefore, the gene–carrier complex can quickly escape from the endosome/lysosome after endocytosis into cells. Histidine-rich peptides are a class of endosomal cleavage peptides. Histidine contains an imidazole ring that can be protonated at pH 6; thus, it has an internal endosomal/lysosomal destruction function [113][68]. Bechinger et al. found that the cationic amphipathic histidine-rich peptide LAH4 (KKALLALALHHLAHLALHLALALKKA) possesses a high pDNA delivery capacity [114][69]. Such peptides are thought to efficiently disrupt endosomal membranes. The transfection efficiency of LAH mutants depends on the number and position of histidine residues in the peptide as well as on the pH at which the in-plane to the transmembrane transition occurs. Moreover, they found that binding of the DNA complexes to the plasma membrane is mediated by heparan sulfate proteoglycans and that anionic phospholipids may be involved in the endosomal destabilization process. Moreover, histidine-rich peptides with branched structures, such as HHK4b (83mer) [115][70], [KHKH2KH2KH2KH2KHZKHK] 4-KK, and HHHHK8b [116][71], have also been used as pDNA and siRNA delivery vectors. The Tat peptide is a CPP, which can enhance cells’ uptake of drugs and protein. The application of the Tat peptide in DNA delivery is limited by the instability of the peptide–DNA complex and the inability to release DNA in the endosome. In addition, Wang et al. added different amounts of histidine to the TAT transmembrane peptide with cysteine at both ends [117][72]. They found that when 10 histidines were added into the sequence, its efficiency for transfecting pCAG-luc was the highest, which was 7000 times higher than that of TAT and equivalent to 25 kDa PEI. To greatly enhance the gene delivery efficiency, McCarthy et al. designed a linear peptide, which integrated multiple functional amino acids that play critical roles in the delivery of a functional nucleic acid (Figure 65) [118][73]. Specifically, arginine (for efficient cellular uptake and nucleic acid loading), tryptophan (to enhance the interaction with hydrophobic cell membranes), histidine (for facilitating endosomal escape), and cysteine (for controlled release and stability) were employed for the synthesis of the liner cell-penetrating peptide termed CHAT, which was subsequently complexed with pDNA to generate the nano-sized nanoparticle. As a result, peptides with all functional amino acids showed successful transfection with efficient reporter gene expression in an in vivo evaluation through intra-tumoral or intravenous delivery.
In addition, photochemical internalization (PCI), as a newly emerged technology for release of endocytosed components, has been demonstrated to be an alternative strategy for the improved cytoplasmic delivery in gene delivery [119][74]. In the work reported by Zhang et al., a pH-sensitive chimeric peptide, that is, Fmoc-12-aminododecanoic acid-H8R8-PLGVR-PEG8, was fabricated for the delivery of therapeutic genes to treat cancer (Figure 76) [120][75]. With the combination with a photosensitizer (i.e., PpIX), a dual-stage light irradiation strategy could be achieved for the efficient transportation of plasmid DNA. This chimeric peptide could target tumor cells on the basis of matrix metalloproteinase-2 (MMP-2)-triggered targeting. After cellular internalization, satisfactory endosomal escape was achieved, which was attributed to the synergistic effect of the “sponge effect” of H8 and the “PCI effect” of PpIX under short-time light irradiation, which ensured the high expression of therapeutic genes.
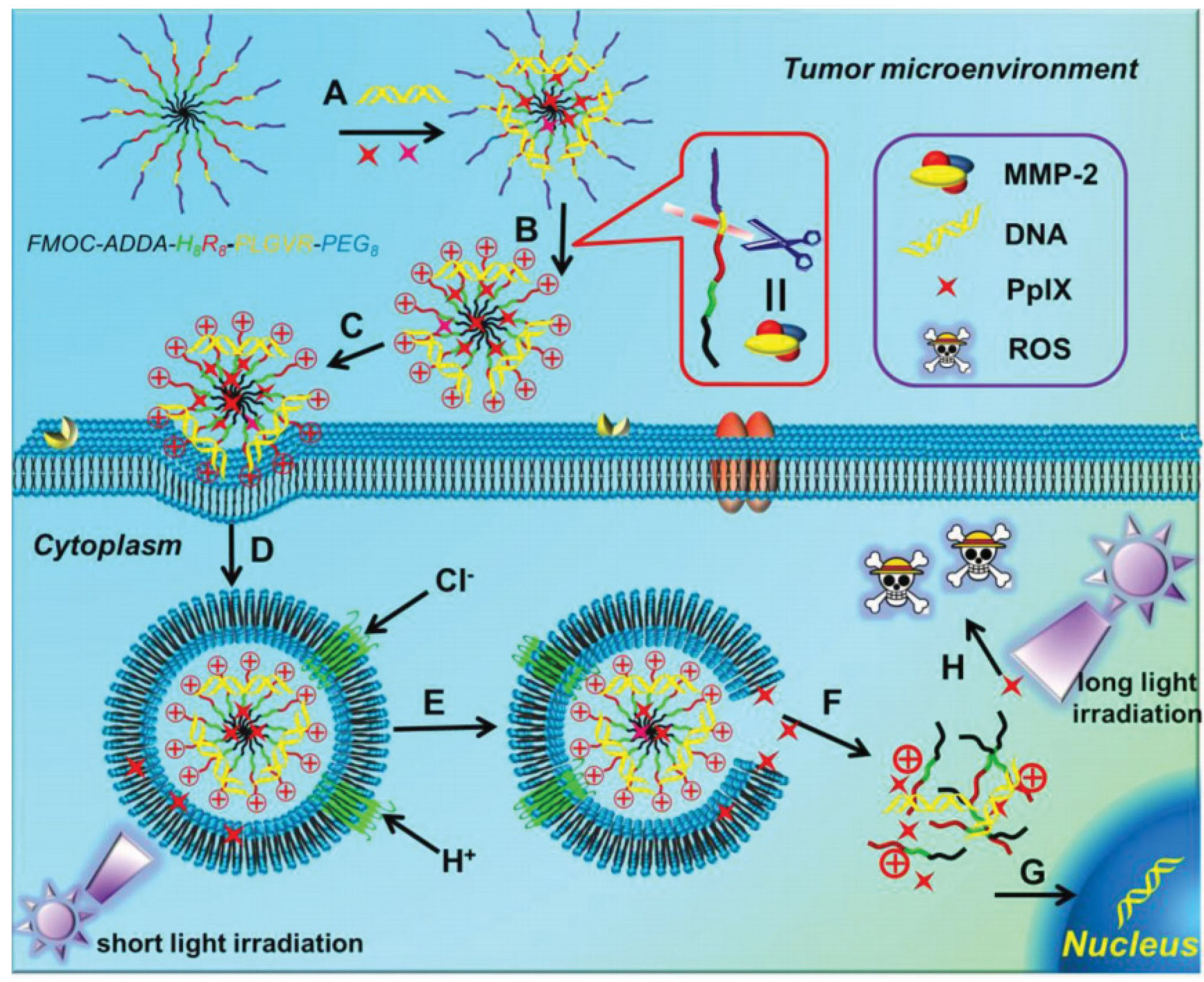
Figure 76. Schematic diagram of the chimeric peptide/PpIX/p53 system with synergistic effect of the “sponge effect” of H8 and “PCI effect” for endosomal escape. (A) Construction of the nanodelivery system; (B) MMP-2 responsive PEG detachment; (C) Cellular endocytosis; (D) Formation and acidififi cation of endosomes; (E) Endosomal escape; (F) Intracellular diffusion; (G) Nuclear translocation and gene expression; (H) Photodynamic therapy. Adapted with permission from the authors of [120][75].
Endosome disruptors are generally amphoteric peptide molecules that are rich in acidic amino acids (glutamic acid and aspartic acid). In the acidic environment of the endosome, they can promote the formation of amphoteric helical structures and fuse with the endosome membrane, thereby destroying the endosome and assisting with the escape of the complexes. Ogris et al. covalently linked PEI to either the C-terminus (C-mel-PEI) or the N-terminus of melittin (N-mel-PEI). Compared with PEI, the transfection efficiency of C-mel-PEI resulted in a 160-fold increase [121][76]. The site of melittin linkage was shown to strongly influence the membrane-destabilizing activities of both conjugates and polyplexes. The study speculated that the high potency of C-mel-PEI to destabilize membranes at a neutral pH may be due to a reported destabilization mechanism proceeding through the membrane insertion of the peptide, while N-mel-PEI is supposed to induce lysis via insertion-independent pore formation according to the toroidal pore model. In order to achieve gene therapy for central nervous system diseases, Pun et al. designed a copolymer (pHgMelbHK10) for the transfection of cells in the brain, which incorporated the membrane-lytic peptide melittin [122][77]. Its N-terminus is predominantly hydrophobic, while the C-terminus is hydrophilic. Melittin is relatively water-soluble but adopts an α-helical conformation when in contact with membranes. Its cytolytic activity is based on its ability to insert into the lipid membrane and induce pore formation. The results show that the melittin-modified copolymers possessed more compact morphologies, increased the DNA binding ability, and transfected more efficiently in both neuron-like PC-12 and HeLa cells than the melittin-free copolymer, and melittin-related toxicity was observed. The in vivo results further demonstrate that an increase in the luciferase activity by about 35-fold could be produced by the application of melittin-containing polyplexes in the brain than the melittin-free polyplexes. Zimmer et al. reported that the GALA repeat and KALA repeat can effectively improve the transfection efficiency of non-viral vectors [123][78]. Specifically, a KALA peptide is an amphiphilic peptide that can form an α-helix structure at a physiological pH [124][79]. To this end, Harashima et al. reported that the modification of a KALA peptide on a pDNA-encapsulating liposomal membrane could greatly facilitate the transgene expression and immune activation in bone-marrow-derived dendritic cells (BMDCs), rendering the KALA-modified lipid nanoparticle a potential DNA vaccine for efficient anti-tumor immunotherapy [125][80]. To further explore the minimum unit of the KALA peptide and the importance of its secondary structure for these activities, they further designed and evaluated pDNA-encapsulating multi-functional envelop-type nano devices (MENDs) modified with four short KALAs [124][79]. It was found that a KALA peptide must form an α-helical structure to induce cellular uptake in BMDCs, and the KALA3 (WEAKLAKALAKALA) was the shortest segment that could form the a-helical structure, as well as elicit transgene expression and immune activation in BMDCs.
2.4. Nuclear-Localizing Peptides
To play a therapeutic role, DNA must enter the nucleus for the efficient transcription and translation of the target protein. Increasing the amount of DNA entering the nucleus can increase the expression level of the target gene. The nuclear pore complex (NPC) structure of eukaryotic cells allows for the free passage of substances with a diameter of less than 9 nm (equivalent to 40~60 ku of protein or less than 1 kb of DNA) [126][81]. Substances with a diameter of less than 25 nm can be transported into the nucleus through a passive transport process [54][8]. However, substances with a larger size require the assistance of transport molecules that can be recognized by cytoplasmic transport receptors, which are called nuclear localization signals (NLSs) [127,128][82][83].
Nuclear-targeting peptides can efficiently transport complexes in the cytoplasm into the nucleus for gene expression. Generally, NLS is a short sequence with monopartite or bipartite clusters of basic amino acids [129][84]. The import of large molecules to the nucleus mediated by NLS peptides is a two-step program related to the combination with importins and translocation through the NPC, subsequently. The most widely used NLS peptide is derived from the SV40 large T antigen with the minimal sequence PKKKRKV [130][85]. The reported binding methods include the covalent binding of NLS to DNA, the binding of DNA-PNA to NLS, and the electrostatic binding of NLS to DNA. NLS peptides can be covalently combined with DNA via chemical or photoactive groups, but this nonspecific combination may occupy the interest sites of genes and cause the prohibition of gene expression [131][86]. NLS sequences can also be ligated to specific sites in DNA via a PNA clamp, and the content of NLS peptides added per pDNA is the key to the success of nuclear entry [132][87]. Compared with the above two methods, the electrostatic binding method is much simpler in operation and has a higher success rate. Furthermore, this binding mode is reversible and does not affect the expression of pDNA. Since NLSs are usually rich in lysine and arginine, most NLSs are positively charged and can easily bind to DNA and protein via electrostatic interaction, which increases the nuclear input and enhances gene expression. However, electrostatic interaction is a physical method, and there is a possibility of DNA dissociation before the complex reaches the NPC. To this end, a crosslinking approach was reported by Chu et al. to crosslink PEI600-CyD (PC) with low-molecular-weight PEI (600 Da) by b-cyclodextrin (b-CyD), which were subsequently modified with NLS [133][88]. It was found that the introduction of an NLS peptide greatly increased the expression of transfected DNA in dividing and non-dividing cells. Zhuo et al. designed a series of gene delivery vectors containing the NLS (PKKKRKV) peptide to promote nuclear transport, octamylarginine (R8) to enhance plasmid encapsulation and cellular uptake, and stearic acid to regulate cell membrane permeability [134][89]. As a result, the prepared vector did not show significant cytotoxicity, while the maximum transfection activity (up to 80% as effective as jetPEITM) could be obtained with the assistance of these NLS-containing vectors. Furthermore, Lam et al. constructed the efficient gene delivery vectors LAH4-L1 that incorporated NLS peptides to promote nuclear importation [135][90]. Among four kinds of NLSs, SV40-derived NLS and reverse SV40-derived NLS showed the most significant improvement in DNA transfection, which resulted in an increase in gene expression by nearly 10-fold over the non-NLS-modified LAH4-L1.
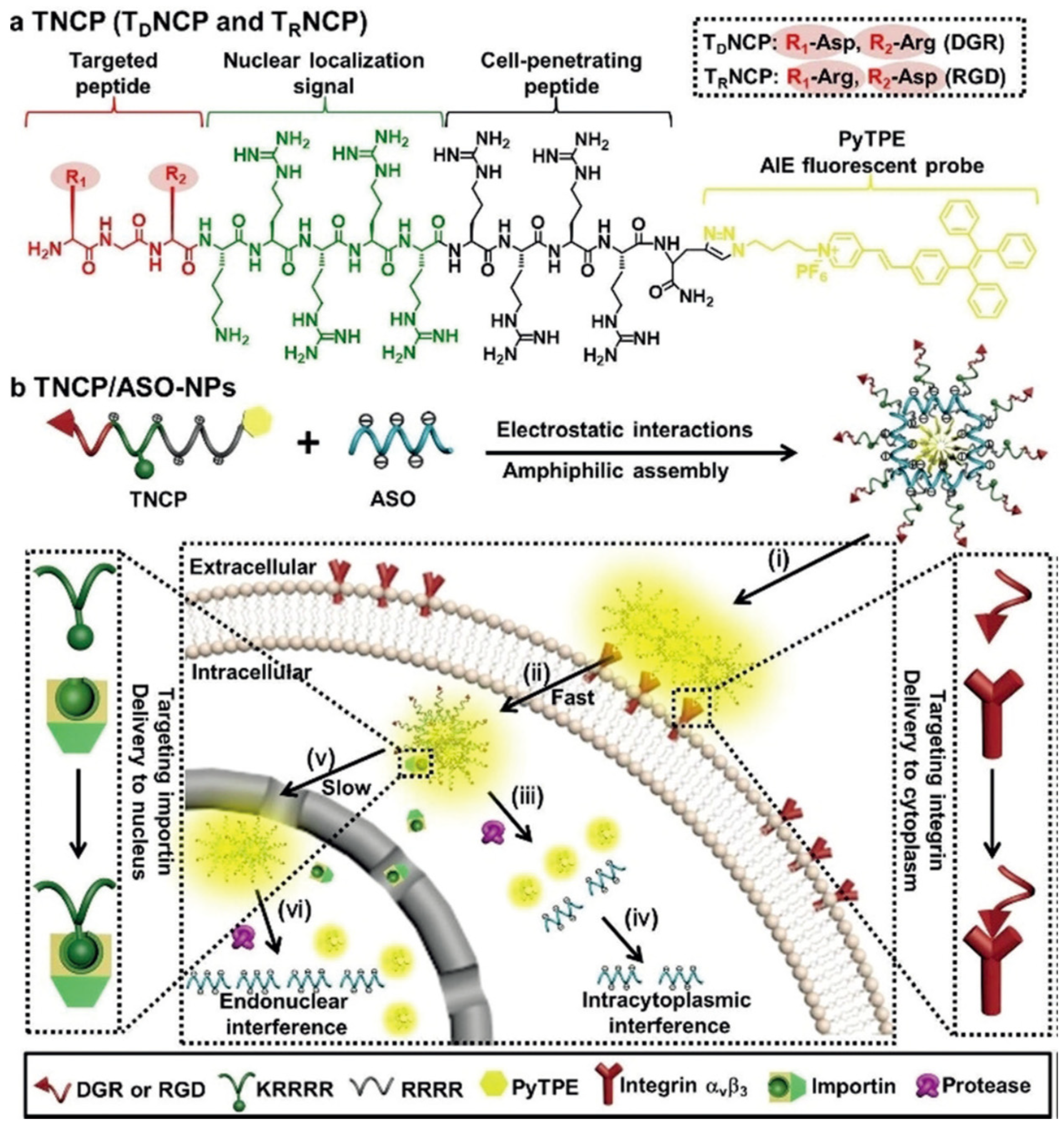
Recently, Xia et al. reported the design of a versatile gene-delivery strategy for the sequential delivery of an antisense single-stranded DNA oligonucleotide (ASO) in a targeted cell nucleus (Figure 87) [136][91]. Specifically, a multidomain designer vector, TNCP, was fabricated via the integration of a targeted peptide, NLS peptide, CPP, and an AIE fluorescent probe (PyTPE) to generate the multifunctional vector with distinct properties, including integrin targeting, cell permeability, nuclear import, as well as real-time tracking. Comparted with the delivery of siRNA that functions in the cytoplasm, the resultant TNCP/ASO complex showed effective gene transcription in a much higher efficient manner, demonstrating the nucleus-targeting delivery. In addition to the nucleocytoplasmic trafficking property, such a design provides a valuable theranostic platform for successful oligonucleotide-based therapies in further clinical applications.
3. Chimeric Peptide for Gene Delivery
Peptides with different functionalities can be integrated into a single system, generating the so-called chimeric peptide [89,137][46][92]. These peptides combine the beneficial properties of different kinds of peptides with distinct biological features, which have gained much attention in the delivery of gene therapeutics [138][93]. To achieve successful gene transportation with a high expression efficiency, the peptide sequence needs to be designed with enough positive charge to achieve the effective compression function of DNA. Therefore, peptides usually need to contain a certain amount of positively charged amino acids (e.g., lysine, arginine, and histidine). As mentioned above, considering the versatility of peptide-based vectors for gene delivery, cell-targeting peptides, CPPs, endosome escape peptides, and NLS peptides can be combined for gene transfer. Generally, there are two ways of covalently binding different peptides: amino acid condensation and disulfide bond crosslinking. For example, cysteine can be introduced at the end of peptide chains with different functions, and then these functional peptides can be polymerized together via oxidation between cysteines to form reducible disulfide bonds. In the work reported by Liang et al., a new chimeric peptide (PL-1, KTLLPTPGGGGRRRRRRRRR) was developed, in which plectin-1-targeted PDAC (pancreatic ductal adenocarcinoma)-specific and arginine-rich RNA-binding motifs were integrated [139][94]. The prepared multifunctional PL-1 could condense miRNA (miR-212), resulting in the formation of self-assemble supramolecular nanoparticles, which exhibited good stability in serum condition. Moreover, these nanoparticles showed a much-enhanced delivery efficiency in transporting miR-212 into PDAC cells specifically. Through the co-delivery of the chemotherapeutic drug of doxorubicin, the therapeutic effect in inducing PDAC cell apoptosis and autophagy was dramatically enhanced by PL-1/miR-212 nanoparticles both in vitro and in vivo.
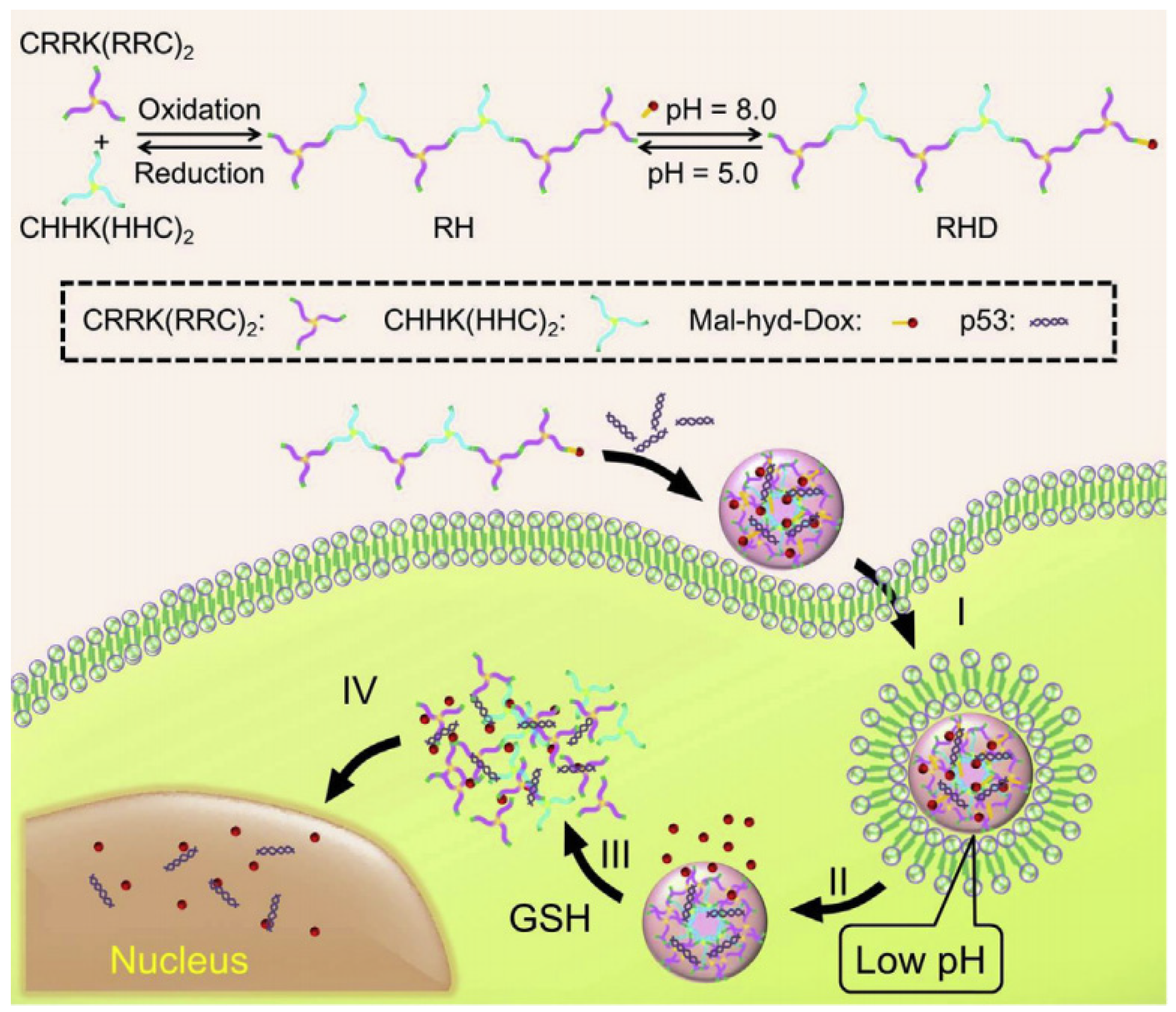
In addition, Zhang et al. combined CPP with NLS and synthesized the peptide sequence of TAT-PKKKRKV for the gene therapy of limb ischemia disease [140][95]. Both in vivo and in vitro experiments demonstrated that TAT-PKKKRKV was a highly efficient and low-toxic gene delivery vector, which could effectively promote the expression of the protein in the ischemic hind limbs of rats, thereby improving the ability of local angiogenesis. In order to solve the barriers faced by non-viral vectors in gene delivery, such as cell membrane penetration and endosome escape and entry into the nucleus, Liu et al. designed a series of multifunctional cationic peptides for gene delivery by combining CPP(TAT), endosome escape α-helix peptide (LLKK)3, and stearic acid through the sulfhydryl crosslinking of cysteine [141][96]. Two of them showed a low toxicity and high transfection efficiency. Among them, C18-C(LLKK)3C-TAT was used to compress and transport PGL-3 plasmid, and the efficiency of luciferase expression was equivalent to that of commercial liposome Lipofectamine 2000 in 293T cells and NIH-3T3 cells. In another work, Zhang et al. fabricated a highly branched and reducible polypeptide (RH), which was synthesized via the oxidation-induced crosslinking of two three-armed peptides (i.e., (CRR)2KRRC and (CHH)2KHHC), to obtain a dual-responsive gene/drug co-delivery system (Figure 98) [142][97]. Specifically, the antitumor drug DOX was covalently conjugated with the highly branched polypeptide RH via pH-sensitive hydrazone bonds, resulting in the formation of RHD, which were subsequently complexed with p53. The resultant RHD/p53 nanosystem could efficiently transport DOX and p53 to tumor cells and possess the ability of endosome escape owing to the protonation by (CHH)2KHHC. The pH-triggered DOX release and GSH-triggered p53 release showed a much-enhanced effect in tumor cell killing, representing their great potential for synergetic tumor treatment with a much-reduced dose of chemotherapeutic drugs.
References
- Zhang, W.; Chen, Q.; Wu, F.; Dai, J.; Ding, D.; Wu, J.; Lou, X.; Xia, F. Peptide-based nanomaterials for gene therapy. Nanoscale Adv. 2021, 3, 302–310.
- Zhao, K.; Li, D.; Cheng, G.; Zhang, B.; Han, J.; Chen, J.; Wang, B.; Li, M.; Xiao, T.; Zhang, J.; et al. Targeted Delivery Prodigiosin to Choriocarcinoma by Peptide-Guided Dendrigraft Poly-l-lysines Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5458.
- Männistö, M.; Vanderkerken, S.; Toncheva, V.; Elomaa, M.; Ruponen, M.; Schacht, E.; Urtti, A. Structure–activity relationships of poly(l-lysines): Effects of pegylation and molecular shape on physicochemical and biological properties in gene delivery. J. Control. Release 2002, 83, 169–182.
- Golda, A.; Pelisek, J.; Klocke, R.; Engelmann, M.G.; Rolland, P.-H.; Mekkaoui, C.; Nikol, S. Small Poly-l-lysines improve cationic lipid-mediated gene transfer in vascular cells in vitro and in vivo. J. Vasc. Res. 2007, 44, 273–282.
- Clements, B.A.; Incani, V.; Kucharski, C.; Lavasanifar, A.; Ritchie, B.; Uludağ, H. A comparative evaluation of poly-l-lysine-palmitic acid and Lipofectamine ™ 2000 for plasmid delivery to bone marrow stromal cells. Biomaterials 2007, 28, 4693–4704.
- Klink, D.; Yu, Q.-C.; Glick, M.C.; Scanlin, T. Lactosylated poly-l-lysine targets a potential lactose receptor in cystic fibrosis and non-cystic fibrosis airway epithelial cells. Mol. Ther. 2003, 7, 73–80.
- Choi, Y.H.; Liu, F.; Kim, J.-S.; Choi, Y.K.; Jong Sang, P.; Kim, S.W. Polyethylene glycol-grafted poly-l-lysine as polymeric gene carrier. J. Control. Release 1998, 54, 39–48.
- Harada, A.; Togawa, H.; Kataoka, K. Physicochemical properties and nuclease resistance of antisense-oligodeoxynucleotides entrapped in the core of polyion complex micelles composed of poly(ethylene glycol)–poly(l-Lysine) block copolymers. Eur. J. Pharm. Sci. 2001, 13, 35–42.
- Chen, S.; Rong, L.; Lei, Q.; Cao, P.-X.; Qin, S.-Y.; Zheng, D.-W.; Jia, H.-Z.; Zhu, J.-Y.; Cheng, S.-X.; Zhuo, R.-X.; et al. A surface charge-switchable and folate modified system for co-delivery of proapoptosis peptide and p53 plasmid in cancer therapy. Biomaterials 2016, 77, 149–163.
- Zhou, D.; Li, C.; Hu, Y.; Zhou, H.; Chen, J.; Zhang, Z.; Guo, T. Glycopolymer modification on physicochemical and biological properties of poly(l-lysine) for gene delivery. Int. J. Biol. Macromol. 2012, 50, 965–973.
- Putnam, D.; Gentry, C.A.; Pack, D.W.; Langer, R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc. Natl. Acad. Sci. USA 2001, 98, 1200–1205.
- Bikram, M.; Ahn, C.-H.; Chae, S.Y.; Lee, M.; Yockman, J.W.; Kim, S.W. Biodegradable poly(ethylene glycol)-co-poly(l-lysine)-g-histidine multiblock copolymers for nonviral gene delivery. Macromolecules 2004, 37, 1903–1916.
- Fajac, I.; Allo, J.-C.; Souil, E.; Merten, M.; Pichon, C.; Figarella, C.; Monsigny, M.; Briand, P.; Midoux, P. Histidylated polylysine as a synthetic vector for gene transfer into immortalized cystic fibrosis airway surface and airway gland serous cells. J. Gene Med. 2000, 2, 368–378.
- Choi, S.; Lee, K.-D. Enhanced gene delivery using disulfide-crosslinked low molecular weight polyethylenimine with listeriolysin o-polyethylenimine disulfide conjugate. J. Control. Release 2008, 131, 70–76.
- Oupický, D.; Carlisle, R.C.; Seymour, L.W. Triggered intracellular activation of disulfide crosslinked polyelectrolyte gene delivery complexes with extended systemic circulation in vivo. Gene Ther. 2001, 8, 713–724.
- Sun, W.; Davis, P.B. Reducible DNA nanoparticles enhance in vitro gene transfer via an extracellular mechanism. J. Control. Release 2010, 146, 118–127.
- Yang, J.; Wang, H.-Y.; Yi, W.-J.; Gong, Y.-H.; Zhou, X.; Zhuo, R.-X.; Zhang, X.-Z. PEGylated peptide based reductive polycations as efficient nonviral gene vectors. Adv. Healthc. Mater. 2013, 2, 481–489.
- Liu, Y.; He, X.; Kuang, Y.; An, S.; Wang, C.; Guo, Y.; Ma, H.; Lou, J.; Jiang, C. A bacteria deriving peptide modified dendrigraft poly-l-lysines (dgl) self-assembling nanoplatform for targeted gene delivery. Mol. Pharm. 2014, 11, 3330–3341.
- Hofman, J.; Buncek, M.; Haluza, R.; Streinz, L.; Ledvina, M.; Cigler, P. In vitro transfection mediated by dendrigraft poly(l-lysines): The effect of structure and molecule size. Macromol. Biosci. 2013, 13, 167–176.
- Ye, L.; Liu, H.; Fei, X.; Ma, D.; He, X.; Tang, Q.; Zhao, X.; Zou, H.; Chen, X.; Kong, X.; et al. Enhanced endosomal escape of dendrigraft poly-L-lysine polymers for the efficient gene therapy of breast cancer. Nano Res. 2021, 15, 1135–1144.
- Tang, M.; Dong, H.; Li, Y.; Ren, T. Harnessing the PEG-cleavable strategy to balance cytotoxicity, intracellular release and the therapeutic effect of dendrigraft poly-l-lysine for cancer gene therapy. J. Mater. Chem. B 2016, 4, 1284–1295.
- Yao, H.; Wang, K.; Wang, Y.; Wang, S.; Li, J.; Lou, J.; Ye, L.; Yan, X.; Lu, W.; Huang, R. Enhanced blood–brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system. Biomaterials 2015, 37, 345–352.
- Saccardo, P.; Villaverde, A.; Gonzalez-Montalban, N. Peptide-mediated DNA condensation for non-viral gene therapy. Biotechnol. Adv. 2009, 27, 432–438.
- Nakase, I.; Akita, H.; Kogure, K.; Gräslund, A.; Langel, Ü.; Harashima, H.; Futaki, S. Efficient Intracellular Delivery of Nucleic Acid Pharmaceuticals Using Cell-Penetrating Peptides. Acc. Chem. Res. 2011, 45, 1132–1139.
- Kato, T.; Oba, M.; Nishida, K.; Tanaka, M. Cell-penetrating helical peptides having l-arginines and five-membered ring α,α-disubstituted α-amino acids. Bioconjug. Chem. 2014, 25, 1761–1768.
- Oba, M.; Demizu, Y.; Yamashita, H.; Kurihara, M.; Tanaka, M. Plasmid DNA delivery using fluorescein-labeled arginine-rich peptides. Bioorg. Med. Chem. 2015, 23, 4911–4918.
- Ramsay, E.; Hadgraft, J.; Birchall, J.; Gumbleton, M. Examination of the biophysical interaction between plasmid DNA and the polycations, polylysine and polyornithine, as a basis for their differential gene transfection in-vitro. Int. J. Pharm. 2000, 210, 97–107.
- Thomas, B.J.; Lucas, P.; Moss, S.H. Transfection of melanoma cells using DNA-polylysine complexes: Polymer composition affects expression of reporter gene. Eur. J. Pharm. Sei. 1996, 4, 70.
- Dong, Y.; Skoultchi, A.I.; Pollardl, J.W. Efficient DNA transfection of quiescent mammalian cells using poly-L-ornithine. Nucleic Acids Res. 1993, 21, 771–772.
- Brown, M.D.; Schatzlein, A.; Brownlie, A.; Jack, V.; Wang, W.; Tetley, L.; Gray, A.I.; Uchegbu, I.F. Preliminary characterization of novel amino acid based polymeric vesicles as gene and drug delivery agents. Bioconjug. Chem. 2000, 11, 880–891.
- Cai, M.; Zhang, Z.; Su, X.; Dong, H.; Zhong, Z.; Zhuo, R. Guanidinated multi-arm star polyornithines with a polyethylenimine core for gene delivery. Polymer 2014, 55, 4634–4640.
- Palchetti, S.; Digiacomo, L.; Giulimondi, F.; Pozzi, D.; Peruzzi, G.; Ferri, G.; Amenitsch, H.; Cardarelli, F.; Mahmoudi, M.; Caracciolo, G. A mechanistic explanation of the inhibitory role of the protein corona on liposomal gene expression. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183159.
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 2020, 6, eabb8133.
- Mishra, S.; Webster, P.; Davis, M.E. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004, 83, 97–111.
- Dirisala, A.; Osada, K.; Chen, Q.; Tockary, T.A.; Machitani, K.; Osawa, S.; Liu, X.; Ishii, T.; Miyata, K.; Oba, M.; et al. Optimized rod length of polyplex micelles for maximizing transfection efficiency and their performance in systemic gene therapy against stroma-rich pancreatic tumors. Biomaterials 2014, 35, 5359–5368.
- Kang, Z.; Meng, Q.; Liu, K. Peptide-based gene delivery vectors. J. Mater. Chem. B 2019, 7, 1824–1841.
- Stanbridge, L.J.; Dussupt, V.; Maitland, N.J. Baculoviruses as vectors for gene therapy against human prostate cancer. J. Biomed. Biotechnol. 2003, 2003, 79–91.
- Yu, C.-h.; Law, J.B.K.; Suryana, M.; Low, H.Y.; Sheetz, M.P. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc. Natl. Acad. Sci. USA 2011, 108, 20585–20590.
- Ruoslahti, E.; Duza, T.; Zhang, L. Vascular homing peptides with cell-penetrating properties. Curr. Pharm. Des. 2005, 11, 3655–3660.
- Essler, M.; Ruoslahti, E. Molecular specialization of breast vasculature: A breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc. Natl. Acad. Sci. USA 2002, 99, 2252–2257.
- Tyler-McMahon, B.M.; Boules, M.; Richelson, E. Neurotensin: Peptide for the next millennium. Regul. Pept. 2000, 93, 125–136.
- Martinez-Fong, D.; Navarro-Quiroga, I.; Ochoa, I.; Alvarez-Maya, I.; Meraz, M.A.; Luna, J.; Arias-Montaño, J.-A. Neurotensin-SPDP-poly-l-lysine conjugate: A nonviral vector for targeted gene delivery to neural cells. Mol. Brain Res. 1999, 69, 249–262.
- Nah, J.-W.; Yu, L.; Han, S.-o.; Ahn, C.-H.; Kim, S.W. Artery wall binding peptide-poly(ethylene glycol)-grafted-poly(l-lysine)-based gene delivery to artery wall cells. J. Control. Release 2002, 78, 273–284.
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727.
- Moffatt, S.; Wiehle, S.; Cristiano, R.J. Tumor-specific gene delivery mediated by a novel peptide-polyethylenimine-DNA polyplex targeting aminopeptidase N/CD13. Hum. Gene Ther. 2005, 16, 57–67.
- Wang, H.; Chen, W.; Xie, H.; Wei, X.; Yin, S.; Zhou, L.; Xu, X.; Zheng, S. Biocompatible, chimeric peptide-condensed supramolecular nanoparticles for tumor cell-specific siRNA delivery and gene silencing. Chem. Commun. 2014, 50, 7806–7809.
- Hong, H.-y.; Lee, H.Y.; Kwak, W.; Yoo, J.; Na, M.-H.; So, I.S.; Kwon, T.-H.; Park, H.-S.; Huh, S.; Oh, G.T.; et al. Phage display selection of peptides that home to atherosclerotic plaques: IL-4 receptor as a candidate target in atherosclerosis. J. Cell Mol. Med. 2008, 12, 2003–2014.
- Yi, A.; Sim, D.; Lee, Y.-J.; Sarangthem, V.; Park, R.-W. Development of elastin-like polypeptide for targeted specific gene delivery in vivo. J. Nanobiotechnol. 2020, 18, 15.
- Meng, Z.; Kang, Z.; Sun, C.; Yang, S.; Zhao, B.; Feng, S.; Meng, Q.; Liu, K. Enhanced gene transfection efficiency by use of peptide vectors containing laminin receptor-targeting sequence YIGSR. Nanoscale 2018, 10, 1215–1227.
- Ren, H.; Zhou, L.; Liu, M.; Lu, W.; Gao, C. Peptide GE11–Polyethylene Glycol–Polyethylenimine for targeted gene delivery in laryngeal cancer. Med. Oncol. 2015, 32, 185.
- Ping, Y.; Hu, Q.; Tang, G.; Li, J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials 2013, 34, 6482–6494.
- Yu, M.-Z.; Pang, W.-H.; Yang, T.; Wang, J.-C.; Wei, L.; Qiu, C.; Wu, Y.-F.; Liu, W.-Z.; Wei, W.; Guo, X.-Y.; et al. Systemic delivery of siRNA by T7 peptide modified core-shell nanoparticles for targeted therapy of breast cancer. Eur. J. Pharm. Sci. 2016, 92, 39–48.
- Borrelli, A.; Tornesello, A.; Tornesello, M.; Buonaguro, F. Cell penetrating peptides as molecular carriers for anti-cancer agents. Molecules 2018, 23, 295.
- Habault, J.; Poyet, J.-L. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules 2019, 24, 927.
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, Ü. Cell-penetrating peptides: Design, synthesis, and applications. ACS Nano 2014, 8, 1972–1994.
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206.
- Howl, J.; Nicholl, I.D.; Jones, S. The many futures for cell-penetrating peptides: How soon is now? Biochem. Soc. Trans. 2007, 35, 767–769.
- Chen, S.; Han, K.; Yang, J.; Lei, Q.; Zhuo, R.-X.; Zhang, X.-Z. Bioreducible polypeptide containing cell-penetrating sequence for efficient gene delivery. Pharm. Res. 2013, 30, 1968–1978.
- Chen, S.; Rong, L.; Jia, H.-Z.; Qin, S.-Y.; Zeng, X.; Zhuo, R.-X.; Zhang, X.-Z. Co-delivery of proapoptotic peptide and p53 DNA by reduction-sensitive polypeptides for cancer therapy. Biomater. Sci. 2015, 3, 753–763.
- Alexis, F.; Lo, S.L.; Wang, S. Covalent attachment of low molecular weight poly(ethylene imine) improves tat peptide mediated gene delivery. Adv. Mater. 2006, 18, 2174–2178.
- Oehlke, J.; Scheller, A.; Wiesner, B.; Krause, E.; Beyermann, M.; Klauschenz, E.; Melzig, M.; Bienert, M. Cellular uptake of an α-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Biophys. Acta 1998, 1414, 127–139.
- Bahadoran, A.; Moeini, H.; Bejo, M.H.; Hussein, M.Z.; Omar, A.R. Development of Tat-conjugated dendrimer for transdermal dna vaccine delivery. J. Pharm. Pharm. Sci. 2016, 19, 325–338.
- Niu, J.; Chu, Y.; Huang, Y.-F.; Chong, Y.-S.; Jiang, Z.-H.; Mao, Z.-W.; Peng, L.-H.; Gao, J.-Q. Transdermal gene delivery by functional peptide-conjugated cationic gold nanoparticle reverses the progression and metastasis of cutaneous melanoma. ACS Appl. Mater. Interfaces 2017, 9, 9388–9401.
- Kilk, K.; El-Andaloussi, S.; Järver, P.; Meikas, A.; Valkna, A.; Bartfai, T.; Kogerman, P.; Metsis, M.; Langel, Ü. Evaluation of transportan 10 in PEI mediated plasmid delivery assay. J. Control. Release 2005, 103, 511–523.
- Degors, I.M.S.; Wang, C.; Rehman, Z.U.; Zuhorn, I.S. Carriers break barriers in drug delivery: Endocytosis and endosomal escape of gene delivery vectors. Acc. Chem. Res. 2019, 52, 1750–1760.
- Wojnilowicz, M.; Glab, A.; Bertucci, A.; Caruso, F.; Cavalieri, F. Super-resolution imaging of proton sponge-triggered rupture of endosomes and cytosolic release of small interfering RNA. ACS Nano 2018, 13, 187–202.
- Brock, D.J.; Kondow-McConaghy, H.M.; Hager, E.C.; Pellois, J.-P. Endosomal escape and cytosolic penetration of macromolecules mediated by synthetic delivery agents. Bioconjug. Chem. 2018, 30, 293–304.
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228.
- Kichler, A.; Mason, A.J.; Bechinger, B. Cationic amphipathic histidine-rich peptides for gene delivery. Biochim. Biophys. Acta 2006, 1758, 301–307.
- Chen, Q.R. Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res. 2002, 30, 1338–1345.
- Leng, Q. Modified branched peptides with a histidine-rich tail enhance in vitro gene transfection. Nucleic Acids Res. 2005, 33, e40.
- Lo, S.L.; Wang, S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials 2008, 29, 2408–2414.
- McErlean, E.M.; Ziminska, M.; McCrudden, C.M.; McBride, J.W.; Loughran, S.P.; Cole, G.; Mulholland, E.J.; Kett, V.; Buckley, N.E.; Robson, T.; et al. Rational design and characterisation of a linear cell penetrating peptide for non-viral gene delivery. J. Control. Release 2021, 330, 1288–1299.
- Selbo, P.K.; Weyergang, A.; Høgset, A.; Norum, O.-J.; Berstad, M.B.; Vikdal, M.; Berg, K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control. Release 2010, 148, 2–12.
- Han, K.; Lei, Q.; Jia, H.-Z.; Wang, S.-B.; Yin, W.-N.; Chen, W.-H.; Cheng, S.-X.; Zhang, X.-Z. A tumor targeted chimeric peptide for synergistic endosomal escape and therapy by dual-stage light manipulation. Adv. Funct. Mater. 2015, 25, 1248–1257.
- Boeckle, S.; Wagner, E.; Ogris, M. C- versus N-terminally linked melittin-polyethylenimine conjugates: The site of linkage strongly influences activity of DNA polyplexes. J. Gene Med. 2005, 7, 1335–1347.
- Schellinger, J.G.; Pahang, J.A.; Johnson, R.N.; Chu, D.S.H.; Sellers, D.L.; Maris, D.O.; Convertine, A.J.; Stayton, P.S.; Horner, P.J.; Pun, S.H. Melittin-grafted HPMA-oligolysine based copolymers for gene delivery. Biomaterials 2013, 34, 2318–2326.
- Lochmann, D.; Jauk, E.; Zimmer, A. Drug delivery of oligonucleotides by peptides. Eur. J. Pharm. Biopharm. 2004, 58, 237–251.
- Miura, N.; Tange, K.; Nakai, Y.; Yoshioka, H.; Harashima, H.; Akita, H. Identification and evaluation of the minimum unit of a KALA peptide required for gene delivery and immune activation. J. Pharm. Sci. 2017, 10, 3113–3119.
- Miura, N.; Shaheen, S.M.; Akita, H.; Nakamura, T.; Harashima, H. A KALA-modified lipid nanoparticle containing CpG-free plasmid DNA as a potential DNA vaccine carrier for antigen presentation and as an immune-stimulative adjuvant. Nucleic Acids Res. 2015, 43, 1317–1331.
- Alber, F.; Dokudovskaya, S.; Veenhoff, L.M.; Zhang, W.; Kipper, J.; Devos, D.; Suprapto, A.; Karni-Schmidt, O.; Williams, R.; Chait, B.T.; et al. The molecular architecture of the nuclear pore complex. Nature 2007, 450, 695–701.
- Zanta, M.A.; Belguise-Valladier, P.; Behr, J.-P. Gene delivery: A single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl. Acad. Sci. USA 1999, 96, 91–96.
- van der Aa, M.A.E.M.; Mastrobattista, E.; Oosting, R.S.; Hennink, W.E.; Koning, G.A.; Crommelin, D.J.A. The nuclear pore complex: The gateway to successful nonviral gene delivery. Pharm. Res. 2006, 23, 447–459.
- Fontes, M.R.M.; Teh, T.; Kobe, B. Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-α11Edited by K. Nagai. J. Mol. Biol. 2000, 297, 1183–1194.
- Yu, J.; Xie, X.; Zheng, M.; Yu, L.; Zhang, L.; Zhao, J.; Jiang, D.; Che, X. Fabrication and characterization of nuclear localization signal-conjugated glycol chitosan micelles for improving the nuclear delivery of doxorubicin. Int. J. Nanomed. 2012, 7, 5079–5090.
- van der Aa, M.A.E.M.; Koning, G.A.; d’Oliveira, C.; Oosting, R.S.; Wilschut, K.J.; Hennink, W.E.; Crommelin, D.J.A. An NLS peptide covalently linked to linear DNA does not enhance transfection efficiency of cationic polymer based gene delivery systems. J. Gene Med. 2005, 7, 208–217.
- Escriou, V.; Carrière, M.; Scherman, D.; Wils, P. NLS bioconjugates for targeting therapeutic genes to the nucleus. Adv. Drug Deliv. Rev. 2003, 55, 295–306.
- Hu, Q.; Wang, J.; Shen, J.; Liu, M.; Jin, X.; Tang, G.; Chu, P.K. Intracellular pathways and nuclear localization signal peptide-mediated gene transfection by cationic polymeric nanovectors. Biomaterials 2012, 33, 1135–1145.
- Wang, H.-Y.; Chen, J.-X.; Sun, Y.-X.; Deng, J.-Z.; Li, C.; Zhang, X.-Z.; Zhuo, R.-X. Construction of cell penetrating peptide vectors with N-terminal stearylated nuclear localization signal for targeted delivery of DNA into the cell nuclei. J. Control. Release 2011, 155, 26–33.
- Xu, Y.; Liang, W.; Qiu, Y.; Cespi, M.; Palmieri, G.F.; Mason, A.J.; Lam, J.K.W. Incorporation of a nuclear localization signal in pH responsive LAH4-L1 peptide enhances transfection and nuclear uptake of plasmid DNA. Mol. Pharm. 2016, 13, 3141–3152.
- Cheng, Y.; Sun, C.; Liu, R.; Yang, J.; Dai, J.; Zhai, T.; Lou, X.; Xia, F. A Multifunctional peptide-conjugated aiegen for efficient and sequential targeted gene delivery into the nucleus. Angew. Chem. Int. Ed. 2019, 58, 5049–5053.
- Wu, Y.; Tang, Y.; Xie, S.; Zheng, X.; Zhang, S.; Mao, J.; Wang, B.; Hou, Y.; Hu, L.; Chai, K.; et al. Chimeric peptide supramolecular nanoparticles for plectin-1 targeted miRNA-9 delivery in pancreatic cancer. Theranostics 2020, 10, 1151–1165.
- Zhang, C.; Wu, W.; Li, R.Q.; Qiu, W.X.; Zhuang, Z.N.; Cheng, S.X.; Zhang, X.Z. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv. Funct. Mater. 2018, 28, 1804492.
- Chen, W.; Zhou, Y.; Zhi, X.; Ma, T.; Liu, H.; Chen, B.W.; Zheng, X.; Xie, S.; Zhao, B.; Feng, X.; et al. Delivery of miR-212 by chimeric peptide-condensed supramolecular nanoparticles enhances the sensitivity of pancreatic ductal adenocarcinoma to doxorubicin. Biomaterials 2019, 192, 590–600.
- Qu, W.; Qin, S.-Y.; Ren, S.; Jiang, X.-J.; Zhuo, R.-X.; Zhang, X.-Z. Peptide-based vector of VEGF plasmid for efficient gene delivery in vitro and vessel formation in vivo. Bioconjug. Chem. 2013, 24, 960–967.
- Luan, L.; Meng, Q.; Xu, L.; Meng, Z.; Yan, H.; Liu, K. Peptide amphiphiles with multifunctional fragments promoting cellular uptake and endosomal escape as efficient gene vectors. J. Mater. Chem. B 2015, 3, 1068–1078.
- Chen, S.; Lei, Q.; Li, S.-Y.; Qin, S.-Y.; Jia, H.-Z.; Cheng, Y.-J.; Zhang, X.-Z. Fabrication of dual responsive co-delivery system based on three-armed peptides for tumor therapy. Biomaterials 2016, 92, 25–35.
More