Acute lung injury/acute respiratory distress syndrome (ALI/ARDS), triggered by various pathogenic factors inside and outside the lungs, leads to diffuse lung injury and can result in respiratory failure and death, which are typical clinical critical emergencies. Severe acute pancreatitis (SAP), which has a poor clinical prognosis, is one of the most common diseases that induces ARDS. When SAP causes the body to produce a storm of inflammatory factors and even causes sepsis, clinicians will face a two-way choice between anti-inflammatory and anti-infection objectives while considering the damaged intestinal barrier and respiratory failure, which undoubtedly increases the difficulty of the diagnosis and treatment of SAP-ALI/ARDS.
- glucocorticoid
- severe acute pancreatitis
- acute lung injury
- acute respiratory distress syndrome
1. Introduction
2. GCs Mechanism in SAP-SIRS-ALI Treatment
22.1. Core Pathology of SAP-SIRS-ALI
2.1. Core Pathology of SAP-SIRS-ALI
Acute pancreatitis (AP) is a potential complication of abdominal surgery. Serious AP can lead to ALI/ARDS and is a common reason for AP-associated mortality. Currently, no treatment exists to slow progression. As a result of population growth, environmental changes (including changes in dietary patterns and increasing obesity and smoking rates), and improvements in diagnostic techniques, the annual global incidence of AP is increasing. In 2019, there were 34 cases of AP (95% CI: 23–49) per 100,000 in the general population and 1.16 deaths (95% CI: 0.85–1.58) per 100,000 in the general population [52][10]. This common and complex disease is also putting a major burden on national health care costs. AP-related admission costs in the United States were USD 2.2 billion in 2003 (95% CI: 2.0–2.3 billion), with a mean cost per hospital day of USD 1670 (95% CI: 1620–1720), and an average cost per hospital stay of >USD 10,000. These findings underscore the importance of identifying cost-effective treatment strategies and illustrate that the early identification and prevention of disease progression can significantly reduce hospital costs [53][11]. The mechanisms involved in the pathological process of SAP causing ALI/ARDS have been outlined by ourthe research team in a previous review. Damage to acinar cells is triggered by abnormal activation of pancreatic trypsin induced by various etiologies including glandular pathological changes such as calcium overload of alveolar cells and the disruption of pancreatic microcirculation. This promotes the destruction of mechanical, chemical, biological, and immune barriers in the intestine. Accompanied by inflammatory cell necrosis and the high production of inflammatory mediators such as IL-1β and TNF-α, this inflammatory storm increases local inflammation to SIRS, indicating that AP has begun the early stage of multi-organ dysfunction syndrome (MODS). After extensive damage to alveolar epithelial cells, capillary endothelial cells, and the blood–brain barrier caused by elevated inflammatory cell numbers and cytokine production, ALI/ARDS begins to develop [54][12]. The 1992 American College of Chest Physicians/Society of Critical Care Medicine (SCCM) Consensus Conference Committee defines this as an uncontrolled host response to infectious or non-infectious injury [55,56][13][14]. Excessive cytokine production disrupts the balance of pro- and anti-inflammatory cytokines and signals the development of SIRS. The inflammatory mediators involved in this pathological process include tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), intercellular adhesion molecule-1 (ICAM-1), platelet-activating factor (PAF), CD40L, complement component C5a, substance P, hydrogen sulfide (H2S), and chemokines [57,58,59,60,61][15][16][17][18][19]. SIRS is a double-edged sword for the body. While fighting pathogens or the body’s own necrotic tissue, SIRS can have many harmful effects including altered immune status, organ dysfunction, and a disruption in the procoagulant/anticoagulant balance [62][20]. There are two mortality peaks associated with SAP (Figure 71). The initial peak occurs 1–2 weeks following the onset of symptoms. MODS, which is induced by SIRS, is the most common cause of death at this time point, accounting for 60–80% of all fatalities. The second peak occurs approximately 2 months after onset. Most of these deaths are caused by sepsis and other infections, and the length of SIRS is directly associated with the severity of SAP and its prognosis [63][21]. Hietaranta et al. [55][13] showed that the risk of systemic complications increases as the severity of SIRS rises.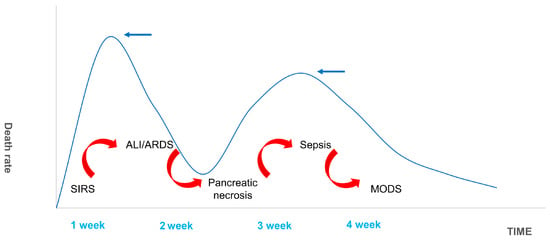
3.2. Research Progress of GC in SAP-SIRS-ALI
2.2. Research Progress of GC in SAP-SIRS-ALI
GCs are the most used drug for the treatment of ALI/ARDS caused by SAP and can effectively reduce patient mortality. After binding to GR and moving into the nucleus, GCs play a significant role in promoting an anti-inflammatory response, reducing oxygen-free radical damage, and improving microcirculation [70][28]. Kimura et al. [71][29] found that, following adrenalectomy, AP rats were more sensitive to acinar cell apoptosis due to reduced endogenous cortisol production. These animals had greater pancreatic edema, higher amylase levels, more potent inflammatory responses, and higher rates of mortality. These effects were significantly reduced following exogenous GC administration [72][30]. These studies provide evidence that GCs reduce the onset of AP [73][31].References
- Kemppainen, R.J.; Behrend, E.N. Adrenal physiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 173–186.
- Shimba, A.; Ikuta, K. Glucocorticoids Regulate Circadian Rhythm of Innate and Adaptive Immunity. Front. Immunol. 2020, 11, 2143.
- Wilcke, J.R.; Davis, L.E. Review of glucocorticoid pharmacology. Vet. Clin. N. Am. Small Anim. Pract. 1982, 12, 3–17.
- Meduri, G.U.; Annane, D.; Confalonieri, M.; Chrousos, G.P.; Rochwerg, B.; Busby, A.; Ruaro, B.; Meibohm, B. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med. 2020, 46, 2284–2296.
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276.
- Dubashynskaya, N.V.; Bokatyi, A.N.; Skorik, Y.A. Dexamethasone Conjugates: Synthetic Approaches and Medical Prospects. Biomedicines 2021, 9, 341.
- Salton, F.; Confalonieri, P.; Meduri, G.U.; Santus, P.; Harari, S.; Scala, R.; Lanini, S.; Vertui, V.; Oggionni, T.; Caminati, A.; et al. Prolonged Low-Dose Methylprednisolone in Patients with Severe COVID-19 Pneumonia. Open Forum Infect. Dis. 2020, 7, ofaa421.
- Xuan, N.; Zhang, X.; Hu, W.; Chen, G.; Wang, Y.; Zhang, S.; Cui, W.; Zhang, G. Effects of the working experience, educational background, professional titles, and hospital grades of intensive care unit doctors on clinical glucocorticoid use in acute respiratory distress syndrome. Medicine 2022, 101, e29021.
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545.
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184.
- Fagenholz, P.J.; Fernández-del Castillo, C.; Harris, N.S.; Pelletier, A.J.; Camargo, C.A., Jr. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas 2007, 35, 302–307.
- Ge, P.; Luo, Y.; Okoye, C.S.; Chen, H.; Liu, J.; Zhang, G.; Xu, C.; Chen, H. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: A troublesome trio for acute pancreatitis. Biomed. Pharmacother 2020, 132, 110770.
- Hietaranta, A.; Kemppainen, E.; Puolakkainen, P.; Sainio, V.; Haapiainen, R.; Peuravuori, H.; Kivilaakso, E.; Nevalainen, T. Extracellular phospholipases A2 in relation to systemic inflammatory response syndrome (SIRS) and systemic complications in severe acute pancreatitis. Pancreas 1999, 18, 385–391.
- Rangel-Frausto, M.S.; Pittet, D.; Costigan, M.; Hwang, T.; Davis, C.S.; Wenzel, R.P. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995, 273, 117–123.
- Bhatia, M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr. Drug Targets-Inflamm. Allergy 2002, 1, 343–351.
- Bhatia, M. Inflammatory response on the pancreatic acinar cell injury. Scand. J. Surg. 2005, 94, 97–102.
- Bhatia, M.; Brady, M.; Shokuhi, S.; Christmas, S.; Neoptolemos, J.P.; Slavin, J. Inflammatory mediators in acute pancreatitis. J. Pathol. 2000, 190, 117–125.
- Bhatia, M.; Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004, 202, 145–156.
- Bhatia, M.; Wong, F.L.; Cao, Y.; Lau, H.Y.; Huang, J.; Puneet, P.; Chevali, L. Pathophysiology of acute pancreatitis. Pancreatology 2005, 5, 132–144.
- Adib-Conquy, M.; Cavaillon, J.M. Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 2009, 101, 36–47.
- Xiong, J.; Zhu, S.; Zhou, Y.; Wu, H.; Wang, C. Regulation of omega-3 fish oil emulsion on the SIRS during the initial stage of severe acute pancreatitis. J. Huazhong Univ. Sci. Technol. 2009, 29, 35–38.
- Ward, N.S.; Casserly, B.; Ayala, A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin. Chest Med. 2008, 29, 617–625.
- Bone, R.C. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit. Care Med. 1996, 24, 1125–1128.
- Gunjaca, I.; Zunic, J.; Gunjaca, M.; Kovac, Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation 2012, 35, 758–763.
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006, 177, 1967–1974.
- Zhang, X.P.; Zhang, L.; Chen, L.J.; Cheng, Q.H.; Wang, J.M.; Cai, W.; Shen, H.P.; Cai, J. Influence of dexamethasone on inflammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J. Gastroenterol. 2007, 13, 548–556.
- Zhang, X.P.; Zhang, L.; Wang, Y.; Cheng, Q.H.; Wang, J.M.; Cai, W.; Shen, H.P.; Cai, J. Study of the protective effects of dexamethasone on multiple organ injury in rats with severe acute pancreatitis. Jop 2007, 8, 400–412.
- Zhao, Y.; Xiong, R.P.; Chen, X.; Li, P.; Ning, Y.L.; Yang, N.; Peng, Y.; Jiang, Y.L.; Zhou, Y.G. Hsp90 regulation affects the treatment of glucocorticoid for pancreatitis-induced lung injury. Mol. Cell. Biochem. 2018, 440, 189–197.
- Kimura, K.; Shimosegawa, T.; Sasano, H.; Abe, R.; Satoh, A.; Masamune, A.; Koizumi, M.; Nagura, H.; Toyota, T. Endogenous glucocorticoids decrease the acinar cell sensitivity to apoptosis during cerulein pancreatitis in rats. Gastroenterology 1998, 114, 372–381.
- Abe, R.; Shimosegawa, T.; Kimura, K.; Abe, T.; Kashimura, J.; Koizumi, M.; Toyota, T. The role of endogenous glucocorticoids in rat experimental models of acute pancreatitis. Gastroenterology 1995, 109, 933–943.
- Muller, C.A.; Vogeser, M.; Belyaev, O.; Gloor, B.; Strobel, O.; Weyhe, D.; Werner, J.; Borgstrom, A.; Buchler, M.W.; Uhl, W. Role of endogenous glucocorticoid metabolism in human acute pancreatitis. Crit. Care Med. 2006, 34, 1060–1066.
