Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dimiter Avtanski and Version 2 by Catherine Yang.
Oxidative and reductive stress are deviations from the optimal conditions when the capacity of cellular redox buffer systems is exceeded. Both conditions are harmful for cellular function and viability. Antioxidant systems include small molecules, such as ascorbate, α-tocopherol, GSH, many food additives and spices, the enzymes superoxide dismutases (SOD), catalase and glutathione peroxidases (GPx), proteins peroxiredoxins, thioredoxins and others.
- aging
- NADPH oxidase
- ROS signaling
- anti-inflammatory
1. Enzymatic and Non-Enzymatic Antioxidants
Oxidative stress and its consequences are mitigated by antioxidants, either as part of the body’s natural defense mechanism or obtained from various dietary sources. Antioxidants can generally be categorized into two main groups: enzymatic and non-enzymatic. Enzymatic antioxidants, such as SOD, which catalyze the dismutation of O2•− into H2O2 and O2, catalase, which catalyzes the H2O2 hydrolyzation into H2O and O2, GPx, which catalyzes the hydrolyzation of H2O2 into H2O and O2 and the reduction of ROO• into alcohols and O2, or glutathione reductase, which catalyzes the reduction of GSSG to GSH, provide a mechanism of eliminating ROS, thus preventing cellular damage. Hydrogen peroxide, alkyl hydroperoxides and peroxynitrite are reduced by peroxiredoxins [1][75]. They are recycled by thioredoxin, which reduces the oxidized cysteine residues and is itself recycled by thioredoxin reductases. There are multiple other non-enzymatic substances with antioxidant activities, such as vitamins, namely vitamin C (ascorbic acid), vitamin E (tocopherol), and vitamin A (retinol), cofactors, namely vitamins B1, B2, B6, B12, folic acid, glutathione, minerals, namely copper, zinc, manganese and selenium, and various other compounds (carotenoids, flavonoids) absorbed from plant-based nutritional sources [2][3][76,77].
Studies have shown that natural antioxidants have the potential to improve endothelial dysfunction and reduce inflammation, which are vital contributors to the vascular complications associated with diabetes. Some antioxidants can directly scavenge ROS or modulate the signaling pathways involved in the regulation of oxidative stress. Others activate the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, which upregulates antioxidant and cytoprotective gene expression. Antioxidants can also modulate the activity of the enzymes involved in the production of vasoactive compounds, such as NOS and cyclooxygenase (COX) [4][5][6][7][78,79,80,81].
Vitamins A, C and E are three essential antioxidants that scavenge free radicals and enhance the activity of other antioxidants, thus preventing the propagation of lipid peroxidation and membrane damage [8][9][82,83]. A meta-analysis by Ashor et al. [10][84] demonstrated that supplementation with these vitamins significantly reduces arterial stiffness, which can be explained by the reduction of the effect of free radicals on the components of the vessel walls [11][85].
Plants are rich in various polyphenols with antioxidant properties that have beneficial effects on DM-associated vascular complications. For example, resveratrol, found in grapes and berries has been shown to reduce inflammation and improve insulin sensitivity and vascular dysfunction in both animal and human studies [12][13][14][86,87,88]. It acts as a potent antioxidant that inhibits the nuclear transcription factor kappa B (NF-κB) signaling pathway concomitantly with suppressing the expression of hypoxia-inducible factor 1 alpha (HIF-1α) and the vascular endothelial growth factor (VEGF), thus having pleiotropic effects on a variety of medical conditions related to inflammation, metabolic imbalance or cancer [15][16][89,90].
Multiple studies on the effect of curcumin, a natural polyphenolic compound derived from Curcuma longa, demonstrate pluripotent effects on oxidative stress, insulin sensitivity and cardiovascular health in people with diabetes. The mechanism of action of curcumin involves suppression of the p300/CREB-binding protein and PKC expression and modulation of multiple signaling pathways, including MAPK, JAK2/STAT3, c-Jun/AP-1, Nrf2 and Src/Akt, among others [17][18][19][91,92,93], (Figure 1). Epigallocatechin 3-gallate (EGCG), found in green tea as well as in cranberries, strawberries, blackberries, kiwis and other fruits, has insulin-mimetic actions on glucose metabolism and improves oxidative status. It has shown beneficial effects on vascular complications from diabetes, such as retinopathy, nephropathy and cardiovascular disease [20][21][22][23][24][25][94,95,96,97,98,99]. EGCG improves mitochondrial dysfunction, inhibits the formation of ROS and acts as a free radical scavenger [26][27][28][100,101,102]. The mechanism of action of EGCG includes pleiotropic activation of the phosphoinositol 3-kinase (PI3K), Akt, AMPK and eNOS signaling pathways and stimulation of the endothelial production of NO [29][30][103,104]. Human studies support the beneficial effects of EGCG on cardiovascular health. Acute supplementation with EGCG reversed endothelial dysfunction in patients with coronary artery disease [31][105], and supplementation of early atherosclerosis patients with olive oil rich in EGCG and other plant-derived polyphenols improved endothelial function [32][106].
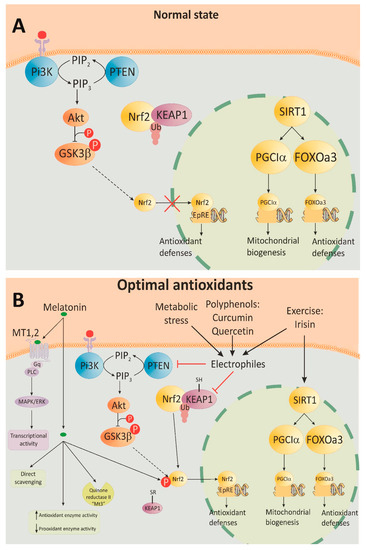
Figure 1. Nrf2 and SIRT1 signaling pathways regulation by antioxidants. (A) At optimal conditions, Nrf2 forms a KEAP1-ubiquitin complex, which downregulates Nrf2 via proteasomal degradation. Nrf2 activity is also inhibited by PTEN. This phosphatase decreases 3-phosphoinositides (PIP3) by conversion into PIP2, and the downstream Akt-GSK3β are not activated. The small amount of Nrf2 cannot be phosphorylated and translocated into the nucleus. Another mechanism of antioxidants involves SIRT1/FOXOa3 and the PGC1α pathway by expression of the antioxidant defense genes and mitochondrial biogenesis. (B) With physiological doses of antioxidants, KEAP1 is oxidized and the Nrf2 complex is destroyed, which results in an increased amount of Nrf2. PTEN is also suppressed and, thus, activates the previously described pathway with the following translocation of Nrf2 into the nucleus. Phosphorylated Nrf2 binds to the electrophile response element (EpRE) that starts the expression of the phase II antioxidant enzymes. Metabolic stress activates the Nrf2 pathway in the same manner. Moderate physical exercise stimulates both Nrf2 and SIRT1 signaling. Melatonin exerts its antioxidant effects through receptor-mediated transcriptional activity and after diffusion in the cytosol via several different pathways.
2. Diabetes Mellitus
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by high glucose levels resulting from insulin deficiency or insulin insensitivity. According to the International Diabetes Federation [33][107], today, diabetes affects more than 500 million people worldwide. One of the main consequences of chronic hyperglycemia is vascular dysfunction, characterized by a variety of abnormalities in the structure or function of the blood vessels. These complications include microvascular damage affecting the retina and the kidneys and macrovascular changes leading to atherosclerosis, coronary artery disease and stroke. Epidemiological data show that DM increases cardiovascular disease risk up to 8-fold [34][108].
It is widely accepted that DM-induced vascular dysfunction is caused, among others, by oxidative stress. Oxidative stress arises as a result of an imbalance between the generation of ROS and the capacity of the cells to detoxify and repair damage to cellular proteins, lipids and DNA, which results in cellular malfunction and death. Practically every aspect of DM (hyperglycemia, inflammation, dyslipidemia and mitochondrial dysfunction) can cause oxidative stress that can exacerbate the diabetic state, leading to vascular complications.
Even though the link between diabetes and oxidative stress is complicated and has many parts, there are several ways that diabetes can damage blood vessels [35][109]. On the one hand, hyperglycemic conditions in DM can cause an increase in the polyol pathway flux that can lead to the formation of ROS and cause a decrease in NO availability. Aldose reductase is part of the polyol pathway, which converts glucose into sorbitol. Disruption of this pathway leads to a buildup of sorbitol in the cells, resulting in oxidative stress, inflammation and increased adhesion of monocytes and macrophages to the endothelial cells [34][108]. Persistent hyperglycemia also causes activation of the NF-κB signaling pathway and an increase in NO production, which react with the superoxide anion radicals to form reactive peroxynitrites [8][82]. On the other hand, high glucose levels activate PKC isoforms and proinflammatory cytokine and prostaglandin expression, thus causing oxidative stress, inflammation, endothelial dysfunction, plaque formation and atherosclerosis. Additionally, hyperglycemia leads to protein and lipid modulation and the formation of advanced glycation end-products, concomitantly with the activation of the hexosamine pathway flux, which can further intensify ROS production and inflammation [35][36][37][109,110,111]. Dyslipidemia, defined by elevated levels of circulating triglycerides and low-density lipoprotein (LDL) cholesterol, induces oxidative stress via the activation of NOX [38][112]. Moreover, mitochondrial dysfunction in DM inhibits electron transport chain activity and enhances the uncoupling of oxidative phosphorylation, which can further upregulate ROS generation.
3. Antiallergic Potential of Curcumin and Tetrahydrocurcumin: Structural Features, Signaling and Supplementary Properties
Orally taken curcumin (CUR) is converted into tetrahydrocurcumin (THC) or its conjugated forms [39][40][113,114]. Different animal studies have shown that CUR possesses anti-inflammatory and antiallergic properties [41][115]. These effects are usually associated with suppression of the production of prostaglandins (PGs), leukotrienes (LTs) [41][42][115,116], NO, [43][117] and cytokines (IL-16, IL-5, and TNF-α), as well as the inhibition of histamine release from mast cells [44][45][118,119].
Suzuki et al. (2005) hypothesized that numerous pharmacological actions of CUR are based on its antioxidant properties [46][120]. Thus, by using an in vitro approach, they have determined the antiallergic and antioxidant activities of various CUR derivative compounds and further investigated the relationships between these two activities. Specifically, they studied CUR, THC and some of their glycosides, showing the inhibition of histamine release from a commonly used histamine-releasing cell line (RBL-2H3) induced by concanavalin A or calcimycin (A23187). The obtained results confirmed that various CUR analogs can act in the process of degranulation, after the entry of Ca2+ into the mast cells, thereby causing the inhibition of the histamine release [46][120]. Moreover, the same authors showed that CUR inhibited histamine release with the same intensity as THC. In the literature, however, CUR was more potent than THC in inhibiting PGE2 generation or NO production [47][48][49][121,122,123]. Conversely, THC was more potent than CUR in the induction of antioxidant responses [39][50][113,124]. It is also well-known that ROS are necessary for the induction of inflammation. Macrophage-generated ROS can induce the production of PGE2, NO and cytokines (IL-1α, IL-6, and TNF-α), which initiate inflammation [51][52][53][125,126,127]. TNF-α induces ROS production, which triggers inflammatory conditions and endothelial disfunction, and can change VSMC from the contractile into the secretory phenotype [54][128]. Further, the free radicals released from the metabolites of unsaturated fatty acids also induced histamine release in rat mast cells [55][56][57][129,130,131]. CUR treatment has been shown to inhibit ROS release from macrophages [58][132] and reduce histamine release from mast cells [59][60][7,8]. These findings indicate that the antiallergic properties of CUR are closely related to its free radical scavenging properties. One of the most important non-antioxidant dependent mechanisms related to the antiallergic effects of CUR is the inhibition of PKC, phospholipase A2 and phospholipase C, [42][61][62][116,133,134], as well as COX and 5-lipoxygenase (5-LO) [41][63][64][115,135,136].
3.1. Structurally Associated Antiallergic Properties of CUR and THC
Recent data clearly show that when administered orally, CUR retains its antiallergic activity, despite its extensive metabolism of THC. Structurally, it contains two methoxy groups, two phenolic hydroxy groups and three conjugated double bonds (Figure 2). Current studies have shown that the potency to inhibit the release of histamine does not depend on the reduction of the number of conjugated double bonds, but rather on the high potency of THC. On the other hand, Futagami et al., (1996) report equivalent inhibitory activity of dimethoxy-CUR and CUR to histamine release [45][119]. However, phenolic glycoside analogs of CUR and THC show weaker potency in inhibiting histamine release, while their tetraacetate or octaacetate derivatives have a negligible inhibitory effect on histamine release [47][121]. Based on this, it has been established that the phenolic hydroxy groups of CUR and THC play a key role in inhibiting histamine release. The antioxidant activity of CUR and its monoglycoside is significant, but it is not so for the diglycoside and bis-dimethoxy analog of CUR [46][120]. THC shows similar results. Such results highlight the important role of phenolic hydroxy and methoxy analogs of CUR in the development of antioxidant capacity [46][65][66][120,137,138]. This is related to the fact that the antiallergic activity of CUR is in part due to its antioxidant activity. However, it should be noted that some antioxidant-impotent analogs [28][40][46][66][102,114,120,138] show distinct antiallergic activity. On the other hand, the compounds possessing tetraacetate in their chemical structures (Figure 2) [41][49][115,123], are not characterized by inhibitory effects on the release of histamine, despite their ability to prevent the production of free radicals [46][65][66][120,137,138]. Compounds 3 and 11, compared to compounds 4 and 12, whose structures lack tetraacetate, have higher molecular weight and lower solubility in water. This likely affects their passage through the membrane, resulting in very low antiallergic effects in cells.
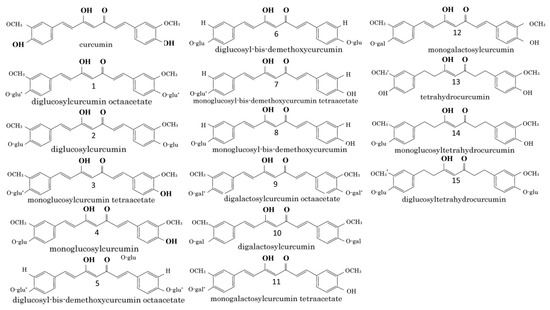
Figure 2.
Chemical structures of curcumin related compounds. Abbreviations: glu: glucose; gal: galactose; *: tetraacetate or octaacetate.
3.2. THC Associated STAT6-Dependent and STAT6-Independent Signaling in Airway Allergic Reactions
Allergic asthma is a chronic inflammatory disease broadly defined by increased inflammatory infiltrates, mucus production, bronchoconstriction and airway hyperreactivity [67][139]. A pronounced Th2 response, as a consequence of eosinophilic tissue infiltration, is the primary inflammatory phenotype in allergic asthma. The first line of protection mainly includes the use of bronchodilators and inhaled or oral corticosteroids, but their use is limited due to numerous side effects [66][138]. It seems that there have been great advances in the treatment of asthma (such as the anti-IL-5/IL-13 antibody) [67][68][69][139,140,141], but antibody-based treatments also include certain limitations, as: (1) the identification of potentially responsive patients based on these biomarkers is required, (2) adverse effects may occur even after discontinuation of therapy, and (3) treatment with monoclonal antibodies is still very expensive. Hence, the need to develop new drugs and strategies to treat asthma is enormous. We, and others, have shown that different CUR analogs can modulate airway disorders, such as bronchopulmonary dysplasia, chronic obstructive pulmonary disease, asthma and pulmonary fibrosis, through the induction of multiple mechanisms, which are related to its anti-inflammatory, antioxidant and antibacterial properties [70][71][72][73][74][142,143,144,145,146]. The approach in the synthesis of new structural analogs of CUR proved to be one of the most elegant in improving the low bioavailability of CUR [75][147].
CD4+ T cells are critical components of the adaptive immune response. They play an important role in the recruitment and activation of other immune cells, dampening the current immunological responses and the maintenance of immunologic memory. Following activation of the T cell receptors and co-stimulation by antigen-presenting cells (APC), I CD4+ T cells develop into one of multiple T helper cell subtype lineages. These subgroups have distinct transcription factors, cell surface proteins and secreted molecule combinations. T helper type 2 (Th2) cells defend the host against intestinal helminths and external microorganisms, while also supporting B cell-dependent humoral responses. Recent studies showed that the signal transducer and activator of transcription 6 (STAT6)-dependent IL-4/IL-4Rα/Jak1-STAT6 and STAT6-independent Jagged1/Jagged2-Notch1/Notch2 signaling pathways play a key role in the inflammatory processes during allergic reactions in the airways [76][77][148,149], (Figure 3). A study by Chen et al. (2018), showed that THC treatment reduced the expression of the interleukin receptor 4α (IL-4Rα) and transcription factor GATA3, and the phosphorylation of Janus kinase 1 (Jak1) and STAT6 in local leukocytes [76][148]. The effect of THC was more pronounced in the inhibition of the STAT6-independent pathway, Jagged1, Jagged2 and the activated forms of Notch 1 (NICD1) and Notch 2 (NICD2) [76][148]. The same group of authors is of the opinion that the reduction of Th2 cells after THC treatment is partially caused by the inhibition of GATA3 through the STAT6-dependent and STAT6-independent signaling pathways. Interestingly, they showed that treatment with THC or CUR suppressed the Th17 and Tc17 cell subsets, but did not modulate either the Th1 or regulatory T-cell (Treg) responses [76][148]. It should be taken into account that Th17 cells have high plasticity and flexibility during inflammatory processes, i.e., they differentiate and induce transition into other T-cell phenotypes through cytokine expression (such as intermediate Th1/Th17 cells and Tregs) [77][78][79][80][149,150,151,152].
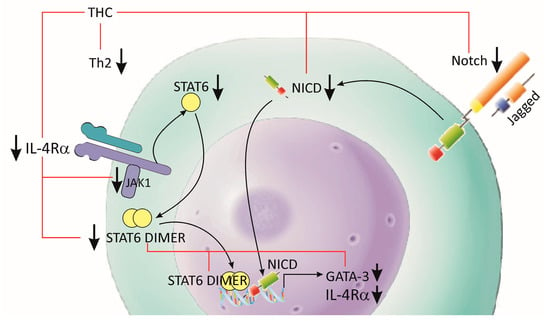
Figure 3. Effects of THC on Th2 differentiation. Allergic inflammation and asthma are characterized by pathological Th2 cell activation. THC treatment reduced the expression of IL-4Rα and GATA3, and the phosphorylation of Jak1 and STAT6 in Th2. The inhibitory effect of THC was additionally supported by the downregulation of Jagged1/Jagged2 and NICD1/NICD2 signaling.
The studies presented above confirmed for the first time that the bioavailability of THC is significantly higher in mice treated with THC than in those treated with CUR. It has been unequivocally shown that THC is more effective than CUR in alleviating the symptoms associated with the allergic reaction. THC has also been shown to inhibit the growth of Th2 cell lines, as well as the production of Th2 cytokines IL-4 and IL-5. The inhibitory effects of THC on IL-5 levels have a key role in the prevention of eosinophilic infiltration [81][153]. Numerous studies have also shown more pronounced antioxidant effects of THC in comparison with CUR [72][73][74][144,145,146]. Taken together, these results are all in favor of the fact that THC is far superior to CUR in the treatment of allergic asthma.
3.3. THC Potentiation of the Therapeutic Effects of Corticosteroids in a Mouse Model of Allergic Asthma
In their study involving asthmatic mice, Wu et al. (2020) showed that THC supplementation has similar effects to treatment with the corticosteroid dexason (DEX) in the regulation of the inflammatory processes of the airway, as well as in the regulation of the pathological changes in the lung [82][154]. In addition, the same group found that THC enhanced the therapeutic effects of DEX compared to monotherapy (THC alone or DEX alone), manifested, among others, by lower mucus production and a weaker Th2 and Th17 response. Hence, it appears that supplementation with THC may have a potential application in clinical use.
Besides the numerous limitations to glucocorticoid therapy, minimizing potential side effects involves various approaches, with the safest being demonstrated in the combination therapy of DEX with nutrients, which (1) enhances the therapeutic effects of DEX, (2) reduces the required dosage of DEX, and (3) prevents or at least alleviates the side effects caused by DEX.
The obtained results from recent studies indicate that THC is better tolerated compared to DEX, which is probably due to the good response in humans to CUR even at high doses of up to 12 g/day [83][155], while toxicity studies in rats showed no harmful effects of THC up to 400 mg/kg/day. Thus, THC can be considered an alternative therapy for allergic asthma.
Shen et al. (2018) revealed the immuno-modulatory properties of THC against asthma depended on the inhibition of the Th2 response due to the downregulation of the IL-4Rα-Jak1-STAT6 and Jagged1/Jagged2–Notch1/Notch2 pathways [83][155]. Considering that DEX treatment can induce side effects [84][85][156,157], such as deterioration of the articular cartilage [86][158], the alleviation of vascular dysfunction and blood pressure [87][159], these findings suggest a potential protective role of THC against DEX-induced side effects, and that a combination therapy involving THC and DEX may be safer than DEX alone in the treatment of allergic asthma. Whether a higher dose of THC can further enhance the therapeutic effects of DEX and reduce the side effects remains unknown, and more pharmacological experiments should be conducted in the next phase.
In general, the hydroxyl groups of CUR may play a significant role in exerting both antioxidant and antiallergic activities. At the same time, some of the CUR analogs do not possess antiallergic activity despite their antioxidant characteristics.
THC exhibits a more dominant anti-inflammatory efficacy than CUR, which qualifies it as a bioactive product that overcomes the limitations of CUR. Oral administration of THC alleviates airway inflammation by reducing symptoms like eosinophilic infiltration, the generation of Th2-associated cytokines and Th2 and Th17 responses, as well as the suppression of the Th2 signaling pathways IL-4/L-4Rα/Jak1-STAT6 and Jagged1/Jagged2–Notch 1/Notch2.
Different pharmacological activities of the various CUR analogs are now under investigation in further studies, where it is expected that light will be shed on the antiallergic mechanisms. Thus, the therapeutic benefits of THC in the treatment of allergic airway reactions are promising and will fuel further studies to determine its clinical significance. In this direction, combined therapy with THC and DEX showed superior therapeutic implications, such as higher therapeutic effects than monotherapy and reduced use of glucocorticoids to avoid the related side effects.
4. Flavonoids
Flavonoids are a group of natural phytophenolic compounds, based on 15 carbon skeletons consisting of two benzene rings connected via a heterocyclic pyran ring. There are variations in the level of oxidation and the substitutions of the benzene rings classified into the following subgroups: flavones, flavonols, flavanonols, flavanones, isoflavones, flavan-3-ols, anthocyanidins, chalcones and aurones [88][160]. Natural sources of flavonoids are multiple plant seeds, stems, leaves, fruits and flowers, where these compounds are responsible for the attraction of pollinators, and protect from UV rays, freezing and pathogens [89][161]. Besides the well-known functions of flavonoids in plants, it turns out that these compounds play an important role in inflammation, allergies and oxidative stress in animals and humans. They are able to inhibit the mitogen-activated protein kinases (MAPKs) and NF-kB, the key modulators in the expression of several pro-inflammatory genes [90][162]. Some flavonoids suppress the activity of Th2 cells via the transcription factors GATA-3 and STAT-6 [91][163]. Flavonoids from ginger, ginkgo biloba and artichoke inhibit phosphodiesterases, which is another mechanism influencing inflammation and chronic allergies [92][164]. Sudachitin is a specific flavonoid in some citruses that has been shown to suppress lipopolysaccharide-induced inflammation in mouse macrophage-like RAW264 cells and decrease the levels of TNF-α and nitrates produced in these cells [93][165]. Lots of research data present the direct effects of flavonoids on different immune cells by hindering their proliferative and adhesive properties, and by reducing histamine, prostaglandins and cytokines secretion and the production of IgE antibodies [94][166]. In addition, they act as potent scavengers of free radicals generated during the inflammation process [95][96][97][167,168,169].
Quercetin, a flavonoid found in various fruits, vegetables and grains, has been shown to normalize glucose levels, inhibit inflammation and oxidative stress, and improve endothelial function. Quercetin suppresses PKC, blocks calcium channels and modulates cytokine expression [13][98][87,170]. Silymarin is an extract from the plant Silybum marianum, which contains a combination of flavonolignans, such as silidianin, silibinin, silicristin and isosilibinin. It is used for renoprotection against oxidative stress and inflammation in different in vitro and in vivo animal and human models of chronic kidney disease and diabetic nephropathy [99][171]. Luteolin, a flavonoid that is abundant in carrots, onion, celery, apples and chamomile, demonstrated hepatoprotective activity in Pb-intoxicated rats due to the upregulation of Nrf2 expression and the murine double minute 2 (Mdm2) gene, together with the suppression of p53 expression [100][172].
The antioxidant and anti-inflammatory properties of flavonoids make them valuable dietary compounds in the prevention of cardiovascular diseases. Flavonoids act as antagonists of thromboxane A2 receptors, protect the collagen in blood vessels against oxidative stress, block the rise of intracellular calcium and prevent platelet aggregation through multiple pathways [101][173]. Many flavonoids induce vasodilatation through stimulation of NO production and, thus, increase the plasma NO level. Hesperetin can activate the voltage-gated ion channels in the vascular smooth muscles and, thus, have an antihypertensive effect through a hyperpolarization-dependent decrease in the calcium influx [102][174].
Many other naturally derived compounds with antioxidative properties have been utilized in traditional medicine or for culinary purposes for centuries. These compounds have been extensively studied, chemically modified and formulated as clinically approved medications. It is estimated that approximately half of the currently used medications originate from plants [103][175]. One notable example is metformin, one of the most prescribed drugs for treating type 2 DM (T2DM), which is synthesized through a chemical modification of guanidine found in Galega officina. Another class of medications inspired by nature is the group of thiazolidinediones.
5. Overdoses of Polyphenols
Supplements containing polyphenols have antioxidant or pro-oxidant properties, depending on the dose level and the biological environment [104][105][106][176,177,178]. At normal physiological conditions, the thioredoxin and glutathione systems are the first line of defense against excessive polyphenol-induced stress. Toxic levels of polyphenols strongly activate the Nrf2 system in selenium-deficient conditions. The side effects of excessive polyphenol consumption are a result of their auto-oxidation and ROS (H2O2 and superoxide anion) production. Furthermore, copper and iron ions promote the oxidation of polyphenols and, thus, amplify the production of ROS [107][108][179,180]. They are also transformed into quinones and semiquinones, which covalently bound to the free thiol group of cysteine residues in proteins, leading to the formation of quinoproteins [109][181]. Toxic and lethal doses can be reached when consumed in isolated form as dietary supplements rather than as plant food. These negative effects of antioxidants have been assigned to the term “antioxidative stress” [110][182]. Vitamins C and E, SOD, GSH and beta-carotene have a potentially harmful effect due to antioxidative stress. The most common ROS and NO are signaling molecules, which regulate transcription factors and the activity of any SH-containing molecule (GSH, PKC, Ca2+-ATPase, collagenase and tyrosine kinases). For this reason, the complete elimination of ROS compromises normal cell signaling and function [111][183].
Antioxidants activate numerous enzymes for antioxidative defense through SIRT2/FOXO, NF-κB and Nrf2/ARE signal pathways (Figure 4). The same mechanisms are activated by moderate, intermittent stress factors, such as exercise and energy restrictions. Much data confirm the relationship between energy restriction and stimulation of the antioxidant defense, which increases peroxidative stress resistance [112][184]. Moderate physical exercise enhances mitochondrial activity and ROS production. The latter initiates an adaptive stress response, which results in improved health. On the other hand, simultaneous antioxidant supplementation and physical activity can reduce its positive effect on health [113][185].
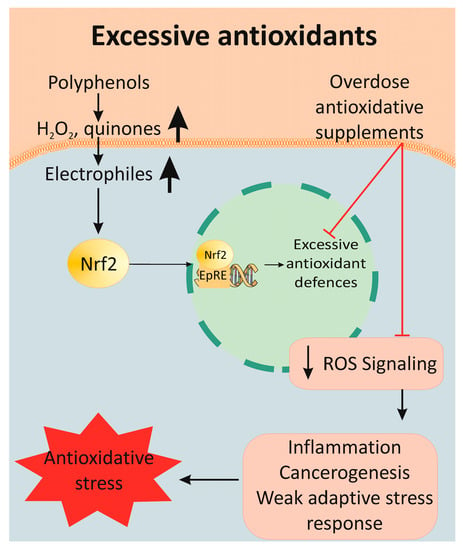
Figure 4. Mechanism of antioxidative stress after supplementation with polyphenolic overdoses. Many antioxidants lead to the overexpression of antioxidant enzymes, which dramatically decreases the ROS signaling pathway. As a result, the induction of an inflammatory response, carcinogenesis and instability in the cell redox balance are observed, all of which are collectively determined as antioxidative stress.
