1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease, with an estimated prevalence for industrialized countries of 1% of the population over 60 years and 3% in people older than 80 years [
1]. PD is characterized by the loss of dopaminergic neurons in substantia nigra pars compacta, which modulate the fronto–thalamo–striatal circuit, leading to a wide range of motor and non-motor symptoms (NMSs) [
2,
3]. Thus, NMS is a broad spectrum of symptoms, including mood disorders [
4], sensory and perceptual dysfunction [
5], and cognitive disturbances [
6], with visuospatial processing impairments among the NMSs having accumulated the most interest in the last years [
7,
8].
Basic visual processes are affected in PD, including reduced spatial contrast sensitivity and impaired color discrimination [
9], oculomotor control defects and diplopia [
10,
11], dry eyes disease [
12], glaucoma [
13], and visual hallucinations even in the absence of dementia [
14]. Furthermore, visual pathologies are accompanied by a poor performance in tasks requiring high-order processing capabilities, such as object mental rotation [
15], perception of space [
16], spatial maps representation, visuospatial working memory, effective navigation, and target localization [
17,
18,
19], which can be considered as preclinical markers and predictors of disease development [
20].
Impaired visual and visuospatial functions can affect a broad range of essential daily living skills, such as driving, reading, writing, or walking [
21,
22]. These problems have been reported to be increased throughout the progress of the illness, resulting in a reduction of self-efficacy and quality of life.
2. Ocular and Visual Impairments in Parkinson’s Disease
PD patients show a number of ocular and visual impairments resulting from pathological processes or consequences of medication, and usually get worse during disease progression [
24], with up to 70% of patients reporting recurrent visual complaints [
25]. Thus, a panoply of oculomotor issues have been described in PD patients [
10,
26], including poor binocular convergence, double vision (diplopia), bradykinesia and hypokinesia of ocular pursuit, impaired vertical upward and downward glaze, defective saccadic movements with longer reaction times, hypometria, and square wave jerks [
24,
27,
28,
29]. These problems have direct consequences on patients’ abilities to perform daily tasks, such as reading. writing, driving, or navigating [
5]. Furthermore, visual deficits can even be found in the prodromal stages of PD, as early as decades before the onset of motor symptoms [
30].
2.1. Retinal Changes
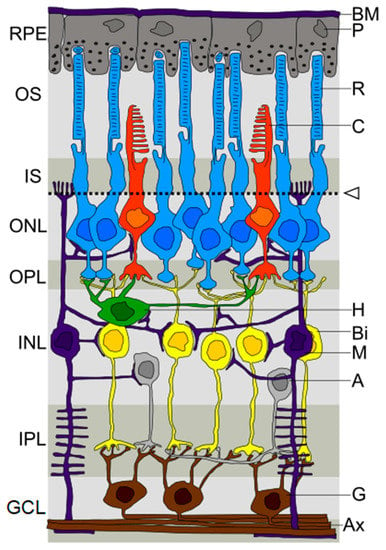
In the PD population, the main structures that make up the eyeball and the optic nerve are quantitatively and qualitatively affected. High-resolution structural imaging approaches by optical coherence tomography (OCT) of the retina show a decrease of the macular retinal thickness, macular volume, average retinal nerve fiber layer (RNFL), retinal ganglion cell layer (RGC), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), retinal pigment epithelium, and photoreceptor layer in PD patients [
31,
32,
33,
34]. However, macular thickness or volume are not always reduced [
33,
35].
Some authors have attempted to correlate retinal layer thinning with clinical scales scores. It has been reported that RNFL thickness reduction correlated to duration and severity of disease [
35,
36]. However, other authors could not confirm this alteration [
37,
38,
39] nor its relation with disease duration or severity [
40]. The RGCIPL layer thickness in the parafovea has been the parameter most frequently correlated with visual outcomes in PD patients [
40,
41,
42].
Along with structural alterations, functional changes assessed through the visual evoked response (VER) and the electroretinogram (ERG) have also been shown to correlate with PD duration and severity [
35]. It has also been proposed that electrophysiological alterations begin years before structural changes are observable [
44].
Retinal degeneration may be caused by progressive dopamine depletion and α-synuclein-mediated axonal degeneration [
13]. Dopamine is a key neurotransmitter in the retina, and its depletion results in a reduced electrical response under different light conditions [
45]. IPL and GCL thinning observed in PD has been linked to dopaminergic loss in the left substantia and disease severity [
46]. Furthermore, dopamine also influences other retinal neurotransmitters involved in retinal processing, such as glutamate, GABA, and glycine, disrupting these neurotransmission pathways [
44].
Moreover, misfolded α-synuclein [
47] and phosphorilated α-synuclein [
48] have been reported in the inner retinal layer, and retinal α-synucleinopathy density scores positively correlate with brain α-synucleinopathy density scores, pathology stage, and the UPDRS-III motor sub-score [
49].
Retinal and macula nerve fiber layer thinning is also linked with minor hallucinations [
50] and disease duration and severity [
51]. Moreover, retinal layer thinning correlates with frontal and occipital cortex thickness and is linked to lower scores in the Poppelreuter-Ghent overlapping figures test and the RPMT in a visually impaired PD sample [
52]. Recently, Hannaway et al. [
53] have reported that visual dysfunction is a better predictor than retinal thickness for dementia in PD.
2.2. Glaucomatous Disturbances
There is also a higher incidence of glaucoma and glaucomatous-like visual field defects, with the majority of cases related to open-angle glaucoma [
54,
55]. However, epidemiological data are scarce, and the evidence regarding these deficits is low [
56,
57].
2.3. Pupil Reactivity
Alterations in pupil reactivity have been observed in PD patients. PD patients show a significantly lager pupil diameter, with unequal pupil size after light adaptation and longer light reflex latencies and constriction times, along with reduced contraction amplitude [
26,
58]. Some studies suggest that pupil changes could be independent from dopaminergic deficiency [
59], and dopaminergic treatment has no effect on the pupil light reflex [
60]. Alhassan et al. [
59] suggest that both parasympathetic (cholinergic) and sympathetic (adrenergic) autonomic systems are altered in PD, but the parasympathetic pathway is more affected. The parasympathetic imbalance is considered an early manifestation of PD [
61]. You et al. [
62] point out that pupillary parasympathetic dysfunction advances with the progression of PD, whereas pupillary sympathetic dysfunction changes slowly.
Pupil reactivity alterations may also reflect a sensory deficit due to impaired retinal or optic nerve function, but it has also been suggested they could be due to the degeneration of subcortical regions, such as the locus coeruleus in the brain stem [
59].
Finally, cognitively impaired PD patients show more pupil constriction deficits than those with normal cognitive functions, similarly to pupil dysfunction reported in Alzheimer’s disease [
63]. Kahya et al. [
64] have reported an increased pupillary response with increased postural demand in PD.
2.4. Eyelid and Blink Reflex
Eyelid impairments, including a reduction of blink rate, apraxia in the opening of the eyelid, blepharospasm, and ptosis of the upper and Meibomian gland disease have been reported [
26,
65,
66,
67]. Three types of eyelid movement abnormalities are notable in the experimental models of PD: blink hyperreflexia, impaired blink reflex plasticity, and reduced rate and impaired rhythm of the spontaneous blinks [
68].
Blepharospasm (BSP) is a form of focal dystonia that manifests with spasms of the eyelids, involuntary closure of the eye, and enhanced spontaneous blinking, or any combination of the previous ones [
69].
Armstrong [
70] reported that blink duration and excitability appear to be increased, which may be related to a loss of dopaminergic neurons. Furthermore, many PD patients show a reduced habituation response of the blink reflex, which may improve after treatment with levodopa or amantidine [
70], although other authors reported no positive relationship between blink rate and dopamine synthesis capacity [
71].
A reduced corneal sensitivity has been reported in PD by Reddy et al. [
72]. And, some authors have shown that the lens can show defects in PD [
12], with an increased prevalence of marked nuclear cataracts and a higher intensity of subscapular cataracts.
One of the most usual oculo-visual conditions is dry eye disease, affecting up to 60% of PD patients [
12,
67]. Dry eye disease in PD can be the result of corneal hypoesthesia, decreasing blinking rate and reflex lacrimation, autonomic neuropathy leading to decreased tear secretion, increased tear osmolarity, decreased tear mucin, and lipid layer disruption secondary to meibomian gland dysfunction [
67].
2.5. Visual Acuity, Contrast Sensitivity, and Color Vision in PD
PD patients often complain of poor vision. This deficit can result from, besides other reasons, impaired visual acuity, with low-contrast acuity particularly affected [
73]. A reduction of contrast sensitivity has been reported, particularly for intermediate and high frequencies [
27] in central (foveal) and peripheral locations [
74]. Loss of color vision has also been described [
75]. Cross-sectional studies show that PD patients self-report poor eyesight [
76] and have poorer objectively measured visual function parameters [
77].
Polo et al. [
77] found that parameters corresponding to visual acuity at different contrast levels and all contrast sensitivity test results were altered in patients with PD in comparison with healthy participants, with contrast sensitivity the most affected variable. These authors also reported that patients had a tendency to protanomaly.
Polo et al. [
77] also proposed that RGC loss could be the cause of contrast and color deficiencies in PD, with dopaminergic D1 and D2 receptors playing a role in color vision and contrast sensitivity alterations [
32]. And, reduced macular thickness and volume have been associated with poor visual acuity, contrast sensitivity, and color vision [
77].
Although a retinal dopamine deficit might be related to contrast sensitivity deficits, the orientation-specific impairment points to cerebral cortex involvement [
20]. Contrast sensitivity deficits can be partially reversed with levodopa, and apomorphine has been shown to improve contrast perception at all spatial frequencies [
78].
Color discrimination deficits may be an early dopaminergic symptom in PD and a disease-specific feature [
75]. Red–green color blindness (protan–deutan axis) produces blurred vision, with reduced perception of monochromatic contours, especially for dark green, light blue, and dark red stimuli [
20]. Color vision dysfunction is observed even at early stages of the disease and progresses with the disease [
79], affecting the motor speed and core region of the body performance [
73].
Recently, dysfunction across all aspects of vision, including visual acuity, contrast sensitivity, color vision, and higher-order visual processes, have been linked with a higher risk of dementia in PD [
80].
2.6. Visual Hallucinations
Visual hallucinations (VHs) are the most common manifestation of psychosis in PD, and have been be associated with rapid cognitive decline in PD patients. Their occurrence takes place in up to 75% of PD patients [
81]. Moreover, the pathogenesis of VHs in patients with PD is not well understood. Over the course of the disease, minor hallucinations may first evolve into VHs with retained insight and, subsequently, into multimodality hallucinations with loss of insight and delusions. These usually consist of vividly perceived scenes, including people and animals. Passage hallucinations with objects passing across the peripheral visual field, extracampine hallucinations, and a sense of presence have also been described [
81].
VHs in PD have been explained as a result of dysfunction of attentional networks in combination with ambiguous visual input, which may lead to VHs when remembered images intrude into consciousness [
82]. Abnormal levels of the default mode network (DMN), a large-scale network that activates during rest, and in daydreaming and musing are observed in PD patients with VHs [
83]. According to Weil and Reeves [
81], VHs are due to over interpretation of visual input. These authors describe a reduction of white matter in posterior thalamic projections, which may play an important role for network shifting and releasing DMN inhibition [
84].
Regarding the contribution of neurotransmitter systems in VHs, it has proved difficult to disentangle, due to the overlapping functional networks involved. It is known that levodopa treatment and dopamine agonists produce VHs [
85,
86]. It has also been proposed that hypersensitization of nigrostriatal dopaminergic neurons by anti-Parkinson’s drugs contribute to VHs [
87]. The early disturbance of serotonergic and cholinergic pathways occurring in PD and glutamatergic and GABAergic changes affecting the overall balance between excitatory and inhibitory signaling may also play a role in the decoupling of the DMN [
88]. It results in the perception of priors stored in the unconscious memory and the uncontrolled emergence of intrinsic narratives produced by the DMN [
88].
VHs predict a range of poor outcomes, including more rapid cognitive decline and the development of dementia [
89,
90] and an increased likelihood of a move from independent living to a care home [
91].
3. Visual Cognition Impairments in Parkinson’s Disease
Visual cognition deficits have been commonly reported in PD, although there is no consensus regarding frequency, characteristics, and relationships with other variables. Nevertheless, although many authors agree that visual cognition is not the most affected domain in PD [
92,
93], the majority of studies report a significant decline in visuospatial, visuoperceptive, visuoconstructive, and visual memory functions [
94,
95,
96].
Some authors state that visual cognition deficits in PD are the consequence of central processing dysfunction rather than specific visuospatial impairments, particularly low-level perceptual deficits and executive function impairment [
97,
98]. Low-level visual dysfunction has important implications for understanding cognitive deterioration, as visual input is required for most of the standard neuropsychological tests. But, visual scenery generation and perception are simultaneously coupled with cognitive processes [
99]. Thus, it has been reported that PD patients’ performance in a wide range of neuropsychological tests involving visual cognition can be attributed to abnormalities in low-level visual functions, especially low- and high-contrast visual acuity [
96]. And, it has been suggested that lower-level vision acts as a confounder in object identification or in the time needed to interpret visual sceneries [
96].
3.1. Visuospatial Impairment
Visuospatial deficits in PD patients have commonly been assessed with the Judgement of Line Orientation test (JLO), a tool that evaluates the ability to estimate angular relationships between line segments. Several studies have reported significant decreases in JLO scores [
96,
100,
101,
102], particularly in cognitively impaired PD patients [
103]. PD patients are prone to confound oblique lines by two or more spacings [
104], and show more severe intraquadrant and horizontal lines errors [
105]. Some authors have reported that the interference of the visuospatial sketchpad (a component of working memory involved in the storage and manipulation of visual and spatial information) is relevant only in moderate to severe phases of the disease [
106]. Recently, Kawashima et al. [
107] showed that visuospatial recognition was impaired in the visuospatial o-back test, which does not involve a memory component. And, Kawabata et al. [
8] have showed deficits in position discrimination in the Visual Object and Space Perception Battery (VOSP).
Mental rotation and three-dimensional and visual transformation processes have also been reported to be impaired in PD [
15,
108]. However, there is no consensus about mental rotation abilities in PD. Thus, some authors have documented impaired mental rotation and suggest a problem of the perception of extra-personal space [
15], whereas other studies have reported spared mental rotation abilities in PD [
109]. It can be argued that each mode of mental transformation is associated with a distinct network of brain regions, and these networks are likely affected differentially by the neuropathology of PD. Amick et al. [
110] reported that PD patients showed an impaired ability to mentally rotate hands, but not objects. According to these authors, frontostriatal motor systems and the parietal lobes would play a necessary role for integrating visuospatial cognition with motor imagery during the mental rotation of hands. And, recently, Bek et al. [
111] have proposed that PD patients would present difficulties integrating visual and kinesthetic elements of motor imagery.
3.2. Visuoperceptive Impairment
The Facial Recognition Test (FRT) has been used to assess the ability to recognize faces in PD patients without involving a memory component. Some PD patients, even cognitively unimpaired ones, present more difficulties on this test than on the JLO [
103,
112,
113]. Another test, the Visual Form Discrimination Test (VFDT), has been used to evaluate visual recognition impairment in PD. Raskin et al. [
114] showed a gradual impairment of visuospatial functions, and other authors have demonstrated that non-demented PD patients fail in this test [
101,
112]. Some authors have shown that PD patients have difficulties identifying objects embedded in complex figures and are less accurate and make more mistakes in perceptual judgements on a bistable percept paradigm (BPP) [
115]. PD patients also show problems in semantical categorization of visual stimuli [
116].
Kawabata et al. [
8] investigated the features of visuoperceptual disturbances in PD using the battery VOSP. The authors found that one-third of patients exhibited impaired identification of incomplete letters and showed a reduction of functional connectivity in the primary visual network.
Difficulties in the perception of space and depth have also been observed in PD patients. Stereopsis impairment has been observed in some studies [
117,
118,
119]. It has been explained as a result of basic visual perception alterations, such as color vision and contrast sensitivity deficits [
118], and oculomotor behavior [
120], which appear linked to the degree of disease deterioration and motor impairment [
119].
Difficulties in the detection of motion are also observable in PD [
121,
122]. This deficit is independent of gait dysfunction and low-level vision changes, and may arise from difficulty perceptually integrating form and motion cues in posterior superior temporal sulcus [
121]. These authors reported that PD patients perform significantly worse for human motion than the object motion task.
3.3. Visuoconstructive Impairment
Visuoconstructive impairment in the block design subtest from the Wechsler Adult Intelligence Scale (WAIS) has been related to worsening of other cognitive domains, and motor and severity in PD [
123]. In the Clock Drawing Test (CDT), drawing and copy scores are significantly lower in PD, with the last correlated with high-contrast visual acuity measures [
96]. Visuoconstructive abilities have also been assessed using more complex copy tests, such as the Rey–Osterrieth Copy Figure (ROCF) [
123,
124]. Patients show impaired visual cognition, particularly judgement of line orientation and rotation [
124].
PD patients, with or without dementia, show a tendency to copy figures very close to the model, a phenomenon called “closing-in” [
125]. Initially, it has been explained as a form of constructional apraxia, and some authors have proposed patients have difficulty in the visuospatial analysis of the model and/or in holding this representation in visual working memory [
126]. Others suggest that the closing-in phenomenon would be an extreme manifestation of a default tendency of the motor system, so that the actions would be performed toward the focus of attention [
127]. De Lucia et al. [
128] have proposed that the closing-in phenomenon is related to frontal-executive impairments in PD dementia.
4. Side-of-Onset and Type of Parkinson’s Disease in Relation to Visual Symptoms
The side of motor symptom onset is an important consideration in the study of PD, as most patients initially present with symptoms on one side of the body, reflecting the loss of dopamine primarily in the contra-lateral hemisphere. The right hemisphere is more responsible than the left for many spatial abilities, and failure to distinguish patients with LPD from RPD may mean that visuospatial deficits that contribute to functional decline are missed in patients with LPD [
129].
A factor that has been shown to influence visual processing in people with PD is the body hemifield where the first motor symptoms appeared [
130] and their characteristics [
131]. Thus, Verreyt et al. [
130] reported that LPD patients more often perform worse on tasks of spatial attention and visuospatial orienting. Davidsdottir et al. [
132] examined spatial navigation and visuospatial functioning. LPD patients were generally more visually dependent than RPD patients, who in turn were more visually dependent than the control group. Moreover, egocentric midpoint estimation was dependent on visual input biases, with the deviation increasing for LPD and decreasing for RPD. Schendan et al. [
133] used a hierarchical perception task in PD, distinguishing between patients whose motor symptoms started on the left side of the body (LPDs) or the right side (RPDs). These authors observed that LPDs showed an abnormal perception of global elements, whereas RPDs perceived worse the local elements that make up an object. According to Schendan et al. [
133], the link between the link side of motor symptoms and visuospatial abilities would rely on the contralateral temporoparietal junction.
On the other side, visual deficits have also been analyzed according to the type of motor symptoms that characterize the onset of the disease, defining two phenotypes: tremor dominant-phenotype (T-D) vs. bradykinesia and rigidity dominant-phenotype (B/R-D). The Visual Activities Questionnaire showed that only the B/R-D group scored significantly worse than controls in light/dark adaptation, visual acuity, depth perception, peripheral vision, and visual processing speed, whereas B/R-D only scored worse in depth perception and light/dark adaptation compared to T-D, suggesting the influence of the type of initial symptoms on visuospatial processing [
134]. Other authors have noticed an increased risk of developing VHs in rigid-akinetic patients [
135], whereas patients with postural instability and gait difficulty performed worse than those with T-D on visuospatial measures [
131].