Canola, Brassica napus L., is a major oilseed crop that has various uses in the food, feed, and industrial sectors. It is one of the most widely produced and consumed oilseeds in the world because of its high oil content and favorable fatty acid composition. Canola grains and their derived products, such as canola oil, meal, flour, and bakery products, have a high potential for food applications as they offer various nutritional and functional benefits. However, they are affected by various factors during the production cycle, post-harvest processing, and storage. These factors may compromise their quality and quantity by affecting their chemical composition, physical properties, functional characteristics, and sensory attributes. Therefore, it is important to optimize the production and processing methods of canola grains and their derived products to ensure their safety, stability, and suitability for different food applications.
- Brassica napus L.
- rapeseed
- crop
- oil
- meal
1. Introduction
2. Factors Affecting the Quality of Canola Grains
The quality of canola grains is a crucial factor that affects their market value and usability for various purposes. Many factors can affect the quality of canola grains, including genetic factors, environmental factors, agronomic practices, and post-harvest factors [28][25]. Figure 1 outlines the different elements that contribute to canola grain quality and illustrates their relationships and interactions. Examining the impact of these factors is essential for optimizing the production and utilization of this valuable crop.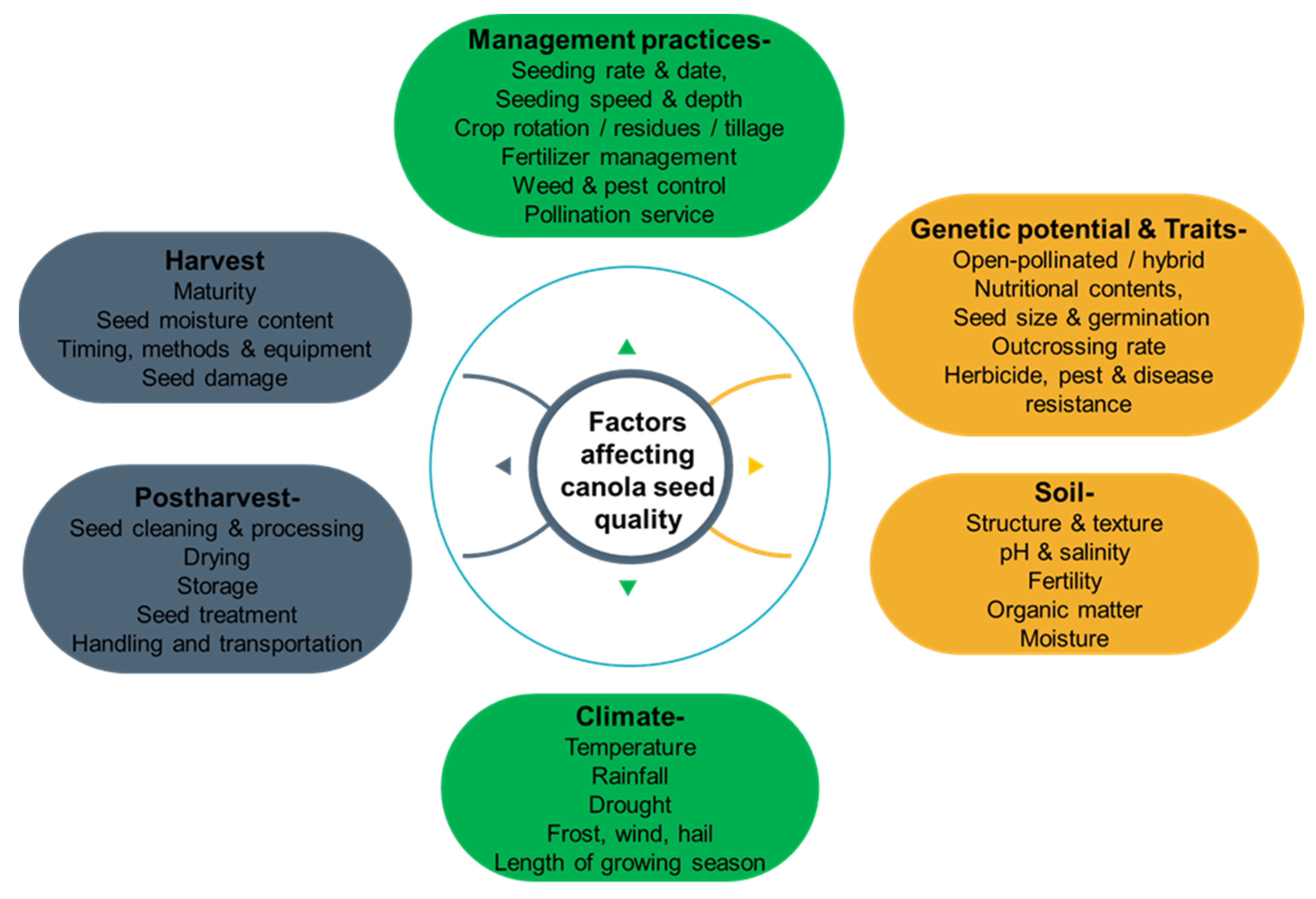
2.1. Genetics and Breeding
-
Genetic Diversity and Population Structure of Canola Germplasm
-
Genetic Architecture and Molecular Mechanisms of Quality Traits in Canola
2.2. Environmental Factors
During the crop cycle, the quality of canola grains is significantly influenced by weather conditions such as air temperature, rainfall, relative humidity, and photoperiod, especially during the flowering and seed-filling stages [62,63][52][53]. Temperature affects the oil content and fatty acid proportion of canola grains by regulating the activity and expression of enzymes involved in lipid biosynthesis [64][54]. High temperatures during the flowering stage can cause reduced seed set and lower oil content, while excessive heat during seed maturation can lead to increased green seed, altered fatty acid composition, and reduced oil quality in canola grains [64,65][54][55]. In contrast, lower temperatures can delay flowering and result in reduced yield, and during the vegetative stage, they can also affect canola growth and yield by causing frost damage, reduced seed size, increased moisture content, reduced oil content, altered fatty acid composition, and increased sinapine content [12,66,67][12][56][57]. During the reproductive phase, both very low and very high temperatures may result in flower abortion and the fall of seedpods, causing greater unevenness in the maturation of the crop and interfering with the efficiency and quality of the oil produced [68][58]. Precipitation and soil moisture availability are crucial factors for canola production because water stress can impact canola grain yield and quality by influencing various physiological processes, including photosynthesis, respiration, transpiration, nutrient uptake, and metabolism [69][59]. Drought stress can decrease canola grain yield and quality by causing oxidative stress and lipid peroxidation in canola grains, which results in higher FFA content, decreased oil content, altered fatty acid composition, increased GSL content, and reduced protein content [4,70][4][60]. Soil nutrients, particularly nitrogen (N), phosphorus (P), potassium (K), and sulfur (S), are essential for canola growth and grain quality [73,74][61][62]. Adequate soil fertility can lead to better plant growth and yield and higher oil content [75,76][63][64]. Adequate soil fertility can lead to better plant growth and yield and higher oil content [77,78][65][66]. However, excessive use of fertilizers can lead to the accumulation of nitrates in B. napus seeds, which can be harmful to human health [79][67]. Pests and diseases can affect the quality of canola grain by causing physical damage, nutrient loss, toxin accumulation, hormonal imbalance in plants, and reduced yield [80,81][68][69]. In addition, they can cause losses in oil content and quality, increase GSL content, reduce protein content and quality, and affect seed germination and vigor (Canola Council of Canada, 2020). Some of the major pests and diseases of B. napus are flea beetles, cutworms, diamondback moths, cabbage seedpod weevils, root maggots, Sclerotinia stem rot, blackleg, clubroot, and Alternaria black spot [82,83][70][71].2.3. Agronomic Practices and Management Techniques
Agronomic practices, including seed selection, sowing date, plant density, fertilization, irrigation, disease and pest control, and harvesting, influence canola grain quality [61,77,84,85,86][65][72][73][74][75]. They can impact the yield, oil content, fatty acid composition, protein content, GSL content, and chlorophyll content of canola grains [85,87,88,89,90][74][76][77][78][79]. Cultivars differ in oil content, protein levels, disease resistance, and other traits that can affect seed quality [86][75]; thus, choosing a canola cultivar suitable for the growing region is critical. In addition, optimal seeding rates and row spacing can promote uniform plant distribution, reduce competition for resources, and improve yield and grain quality [86,91,92][75][80][81]. The sowing date of canola affects the exposure of the crop to different weather conditions during its growth cycle, which may have an impact on grain yield and quality [91][80]. Canola should be planted at the optimal time for the local climate to maximize seed yield and quality [85][74]. Irrigation can affect the soil moisture status and water stress level of canola plants, which can influence the physiological processes and biochemical reactions of canola grains [9,96][9][82]. Especially under drought-prone conditions, it can improve canola grain quality by maintaining adequate soil moisture levels, which allows for optimal seed development [97][83]. However, excessive irrigation can lead to a reduction in oil content and an increase in undesirable fatty acids [98][84]. Crop rotation with different families of plants may help in breaking disease cycles and improving soil fertility [87][76]. Seed yield and use of fertilizer were studied for six years in Alberta, Canada, in twelve treatments, including continuous cropping and rotations of canola, wheat, pea, barley, and flax [101][85]. Canola yield increased with 1- or 2-year breaks from canola. Rotations over continuous canola increased canola yield by 0.632 Mg ha−1 (19.4%) on average from 2010 to 2015. Furthermore, nitrogen saving was observed when the pea plant was included in the rotation.2.4. Harvest and Post-Harvest Management
-
Harvesting, Drying, Storage and Transportation
-
Quality Assessment Methods and Standards
3. Implications for Canola Grain-Based Foods
3.1. Canola Oil
-
Factors Affecting Canola Oil Extraction
-
Factors Affecting Canola Oil Refining
3.2. Canola Meal
The quality and nutritional value of canola meal can vary depending on several factors [19][108]. These factors include the extraction method, protein content, and the presence of anti-nutritional factors. The extraction method used to obtain canola oil from canola seeds can influence the protein content and overall quality of the canola meal. In fact, the choice of extraction method can affect the protein content and quality of canola meal. According to Khajali et Slominski [18], solvent extraction typically results in a higher protein content (38–40%) compared to mechanical pressing (34–36%). However, solvent extraction can also cause more damage to the protein structure and reduce its digestibility and amino acid availability. Moreover, solvent extraction can leave residual hexane in the meal, which can pose health risks for humans and animals. Desolventized flakes are heated during toasting to lower moisture content and enhance flavor and meal stability [150][109]. The quantity and quality of canola meals generated via desolventization and toasting can, however, be impacted by a number of factors. Temperature, time, pressure, as well as the composition and characteristics of the oilseed flakes are some of these factors. One of the most crucial elements that influence the quantity and quality of canola meal is temperature. It determines the rate and extent of solvent removal, as well as the degree of protein denaturation and Maillard reaction [18]. The type and quality of the oilseed flakes, as well as the required qualities of the meal, determine the proper temperature for desolventization and toasting. Anti-nutritional factors are compounds that can interfere with the digestion and absorption of nutrients in animals. Canola meal contains several anti-nutritional factors, such as GSLs, phytic acid, and tannins [17]. GSLs are sulfur-containing compounds that can degrade into toxic metabolites, such as thiocyanates and goitrin. These metabolites can impair thyroid function, reduce iodine uptake, and cause goiter in animals [151][110]. Phytic acid is a phosphorus-containing compound that can bind to minerals such as calcium, iron, zinc, and magnesium. This can reduce the bioavailability of these minerals and cause mineral deficiencies in animals [152][111]. Tannins are polyphenolic compounds that can form complexes with proteins and carbohydrates.3.3. Canola Flour and Bakery Products
-
Factors Affecting Flour Production
-
Factors Affecting Bakery Products
4. Conclusions
The quality of canola grains and their derived products, such as canola oil, meal, flour, and bakery products, is influenced by a myriad of factors throughout their production, post-harvest processing, and storage. These factors can impact the chemical composition, physical properties, functional characteristics, and sensory attributes of canola grains and their products, which in turn affect their suitability for various food applications.References
- Xiao, Z.; Pan, Y.; Wang, C.; Li, X.; Lu, Y.; Tian, Z.; Kuang, L.; Wang, X.; Dun, X.; Wang, H. Multi-Functional Development and Utilization of Rapeseed: Comprehensive Analysis of the Nutritional Value of Rapeseed Sprouts. Foods 2022, 11, 778.
- Mag, T.K. Canola Oil Processing in Canada. J. Am. Oil Chem. Soc. 1983, 60, 380–384.
- Assefa, Y.; Prasad, P.V.V.; Foster, C.; Wright, Y.; Young, S.; Bradley, P.; Stamm, M.; Ciampitti, I.A. Major Management Factors Determining Spring and Winter Canola Yield in North America. Crop Sci. 2018, 58, 2875–2880.
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Prasad, P.V.V.; Messina, C.D.; Ciampitti, I.A. Effects of Heat and Drought on Canola (Brassica napus L.) Yield, Oil, and Protein: A Meta-Analysis. Field Crops Res. 2023, 293, 108848.
- Anwar, M.M.; Ali, S.E.; Nasr, E.H. Improving the Nutritional Value of Canola Seed by Gamma Irradiation. J. Radiat. Res. Appl. Sci. 2015, 8, 328–333.
- Barthet, V.J.; Daun, J.K. 5—Seed Morphology, Composition, and Quality. In Canola; Daun, J.K., Eskin, N.A.M., Hickling, D., Eds.; AOCS Press: Hoboken, NJ, USA, 2011; pp. 119–162. ISBN 978-0-9818936-5-5.
- Guirrou, I.; El Harrak, A.; El Antari, A.; Hssaini, L.; Hanine, H.; El Fechtali, M.; Nabloussi, A. Bioactive Compounds Assessment in Six Moroccan Rapeseed (Brassica napus L.) Varieties Grown in Two Contrasting Environments. Agronomy 2023, 13, 460.
- Petrie, J.R.; Zhou, X.-R.; Leonforte, A.; McAllister, J.; Shrestha, P.; Kennedy, Y.; Belide, S.; Buzza, G.; Gororo, N.; Gao, W.; et al. Development of a Brassica napus (Canola) Crop Containing Fish Oil-Like Levels of DHA in the Seed Oil. Front. Plant Sci. 2020, 11, 727.
- Mohtashami, R.; Movahhedi Dehnavi, M.; Balouchi, H.; Faraji, H. Improving Yield, Oil Content and Water Productivity of Dryland Canola by Supplementary Irrigation and Selenium Spraying. Agric. Water Manag. 2020, 232, 106046.
- Ghazani, S.M.; Marangoni, A.G. Minor Components in Canola Oil and Effects of Refining on These Constituents: A Review. J. Am. Oil Chem. Soc. 2013, 90, 923–932.
- Johnson, G.H.; Keast, D.R.; Kris-Etherton, P.M. Dietary Modeling Shows That the Substitution of Canola Oil for Fats Commonly Used in the United States Would Increase Compliance with Dietary Recommendations for Fatty Acids. J. Am. Diet. Assoc. 2007, 107, 1726–1734.
- Sey, A.A.; Pham, T.H.; Kavanagh, V.; Kaur, S.; Cheema, M.; Galagedara, L.; Thomas, R. Canola Produced under Boreal Climatic Conditions in Newfoundland and Labrador Have a Unique Lipid Composition and Expeller Press Extraction Retained the Composition for Commercial Use. J. Adv. Res. 2020, 24, 423–434.
- Chew, S.C. Cold-Pressed Rapeseed (Brassica napus) Oil: Chemistry and Functionality. Food Res. Int. 2020, 131, 108997.
- Goyal, A.; Tanwar, B.; Sihag, M.K.; Kumar, V.; Sharma, V.; Soni, S. Rapeseed/Canola (Brassica napus) Seed. In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021; pp. 47–71. ISBN 9789811541940.
- Aachary, A.A.; Thiyam-Hollander, U.; Eskin, M.N.A. Canola/Rapeseed Proteins and Peptides. In Applied Food Protein Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 193–218. ISBN 978-1-118-86058-8.
- Wickramasuriya, S.S.; Yi, Y.-J.; Yoo, J.; Kang, N.K.; Heo, J.M. A Review of Canola Meal as an Alternative Feed Ingredient for Ducks. J. Anim. Sci. Technol. 2015, 57, 29.
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108 (Suppl. 2), S315–S332.
- Khajali, F.; Slominski, B.A. Factors That Affect the Nutritive Value of Canola Meal for Poultry. Poult. Sci. 2012, 91, 2564–2575.
- Renzyaeva, T.V.; Renzyaev, A.O.; Reznichenko, I.U.; Popov, A.M.; Kravchenko, S.N.; Miller, E.S. Rapeseed Processing Products as a Component of Flour-Based Food for Gerontological Purpose. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 042004.
- Bermejo-Cruz, M.; Osorio-Ruiz, A.; Rodríguez-Canto, W.; Betancur-Ancona, D.; Martínez-Ayala, A.; Chel-Guerrero, L. Antioxidant Potential of Protein Hydrolysates from Canola (Brassica napus L.) Seeds. Biocatal. Agric. Biotechnol. 2023, 50, 102687.
- Liu, Q.; Wu, L.; Pu, H.; Li, C.; Hu, Q. Profile and Distribution of Soluble and Insoluble Phenolics in Chinese Rapeseed (Brassica napus). Food Chem. 2012, 135, 616–622.
- Liersch, A.; Bocianowski, J.; Nowosad, K.; Mikołajczyk, K.; Spasibionek, S.; Wielebski, F.; Matuszczak, M.; Szała, L.; Cegielska-Taras, T.; Sosnowska, K.; et al. Effect of Genotype × Environment Interaction for Seed Traits in Winter Oilseed Rape (Brassica napus L.). Agriculture 2020, 10, 607.
- Cowling, W.A. Genetic Diversity in Australian Canola and Implications for Crop Breeding for Changing Future Environments. Field Crops Res. 2007, 104, 103–111.
- Barthet, V.J. Comparison between Canadian Canola Harvest and Export Surveys. Plants 2016, 5, 30.
- Niemann, J.; Bocianowski, J.; Wojciechowski, A. Effects of Genotype and Environment on Seed Quality Traits Variability in Interspecific Cross-Derived Brassica Lines. Euphytica 2018, 214, 193.
- Gyawali, S.; Hegedus, D.D.; Parkin, I.A.P.; Poon, J.; Higgins, E.; Horner, K.; Bekkaoui, D.; Coutu, C.; Buchwaldt, L. Genetic Diversity and Population Structure in a World Collection of Brassica napus Accessions with Emphasis on South Korea, Japan, and Pakistan. Crop Sci. 2013, 53, 1537–1545.
- Chen, S.; Nelson, M.N.; Ghamkhar, K.; Fu, T.; Cowling, W.A. Divergent Patterns of Allelic Diversity from Similar Origins: The Case of Oilseed Rape (Brassica napus L.) in China and Australia. Genome 2008, 51, 1–10.
- Chen, R.; Shimono, A.; Aono, M.; Nakajima, N.; Ohsawa, R.; Yoshioka, Y. Genetic Diversity and Population Structure of Feral Rapeseed (Brassica napus L.) in Japan. PLoS ONE 2020, 15, e0227990.
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil Content and Fatty Acids Composition in Brassica Species. Int. J. Food Prop. 2015, 18, 2145–2154.
- Fikere, M.; Barbulescu, D.M.; Malmberg, M.M.; Maharjan, P.; Salisbury, P.A.; Kant, S.; Panozzo, J.; Norton, S.; Spangenberg, G.C.; Cogan, N.O.I.; et al. Genomic Prediction and Genetic Correlation of Agronomic, Blackleg Disease, and Seed Quality Traits in Canola (Brassica napus L.). Plants 2020, 9, 719.
- Hua, S.; Chen, Z.-H.; Zhang, Y.; Yu, H.; Lin, B.; Zhang, D. Chlorophyll and Carbohydrate Metabolism in Developing Silique and Seed Are Prerequisite to Seed Oil Content of Brassica napus L. Bot. Stud. 2014, 55, 34.
- Lu, K.; Wei, L.; Li, X.; Wang, Y.; Wu, J.; Liu, M.; Zhang, C.; Chen, Z.; Xiao, Z.; Jian, H.; et al. Whole-Genome Resequencing Reveals Brassica napus Origin and Genetic Loci Involved in Its Improvement. Nat. Commun. 2019, 10, 1154.
- Nesi, N.; Delourme, R.; Brégeon, M.; Falentin, C.; Renard, M. Genetic and Molecular Approaches to Improve Nutritional Value of Brassica napus L. Seed. Comptes Rendus Biol. 2008, 331, 763–771.
- So, K.K.Y.; Duncan, R.W. Breeding Canola (Brassica napus L.) for Protein in Feed and Food. Plants 2021, 10, 2220.
- Zum Felde, T.; Baumert, A.; Strack, D.; Becker, H.C.; Möllers, C. Genetic Variation for Sinapate Ester Content in Winter Rapeseed (Brassica napus L.) and Development of NIRS Calibration Equations. Plant Breed. 2007, 126, 291–296.
- Hu, J.; Chen, B.; Zhao, J.; Zhang, F.; Xie, T.; Xu, K.; Gao, G.; Yan, G.; Li, H.; Li, L.; et al. Genomic Selection and Genetic Architecture of Agronomic Traits during Modern Rapeseed Breeding. Nat. Genet. 2022, 54, 694–704.
- Behnke, N.; Suprianto, E.; Möllers, C. A Major QTL on Chromosome C05 Significantly Reduces Acid Detergent Lignin (ADL) Content and Increases Seed Oil and Protein Content in Oilseed Rape (Brassica napus L.). Appl. Genet. 2018, 131, 2477–2492.
- Teh, L.; Möllers, C. Genetic Variation and Inheritance of Phytosterol and Oil Content in a Doubled Haploid Population Derived from the Winter Oilseed Rape Sansibar × Oase Cross. Appl. Genet. 2016, 129, 181–199.
- Zhao, J.; Becker, H.C.; Zhang, D.; Zhang, Y.; Ecke, W. Conditional QTL Mapping of Oil Content in Rapeseed with Respect to Protein Content and Traits Related to Plant Development and Grain Yield. Appl. Genet. 2006, 113, 33–38.
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890.
- McVetty, P.B.E.; Duncan, R.W. Canola, Rapeseed, and Mustard: For Biofuels and Bioproducts. In Industrial Crops: Breeding for BioEnergy and Bioproducts; Cruz, V.M.V., Dierig, D.A., Eds.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2015; pp. 133–156. ISBN 978-1-4939-1447-0.
- Spasibionek, S.; Mikołajczyk, K.; Ćwiek–Kupczyńska, H.; Piętka, T.; Krótka, K.; Matuszczak, M.; Nowakowska, J.; Michalski, K.; Bartkowiak-Broda, I. Marker Assisted Selection of New High Oleic and Low Linolenic Winter Oilseed Rape (Brassica napus L.) Inbred Lines Revealing Good Agricultural Value. PLoS ONE 2020, 15, e0233959.
- Qu, C.; Jia, L.; Fu, F.; Zhao, H.; Lu, K.; Wei, L.; Xu, X.; Liang, Y.; Li, S.; Wang, R.; et al. Genome-Wide Association Mapping and Identification of Candidate Genes for Fatty Acid Composition in Brassica napus L. Using SNP Markers. BMC Genom. 2017, 18, 232.
- Yusuf, A.O.; Richter, J.-C.; Möllers, C. Genetic Variation and QTL Analysis of Saturated Fatty Acids in Two Doubled Haploid Populations of Oilseed Rape (Brassica napus L.). Euphytica 2022, 218, 88.
- Zhu, Q.; King, G.J.; Liu, X.; Shan, N.; Borpatragohain, P.; Baten, A.; Wang, P.; Luo, S.; Zhou, Q. Identification of SNP Loci and Candidate Genes Related to Four Important Fatty Acid Composition in Brassica napus Using Genome Wide Association Study. PLoS ONE 2019, 14, e0221578.
- Li, N.; Qi, G.; Sun, X.S.; Stamm, M.J.; Wang, D. Physicochemical Properties and Adhesion Performance of Canola Protein Modified with Sodium Bisulfite. J. Am. Oil Chem. Soc. 2012, 89, 897–908.
- Knoch, D.; Abbadi, A.; Grandke, F.; Meyer, R.C.; Samans, B.; Werner, C.R.; Snowdon, R.J.; Altmann, T. Strong Temporal Dynamics of QTL Action on Plant Growth Progression Revealed through High-Throughput Phenotyping in Canola. Plant Biotechnol. J. 2020, 18, 68–82.
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of Thiocyanate and Goitrin in Human Plasma, Their Precursor Concentrations in Brassica Vegetables, and Associated Potential Risk for Hypothyroidism. Nutr. Rev. 2016, 74, 248–258.
- Jhingan, S.; Harloff, H.-J.; Abbadi, A.; Welsch, C.; Blümel, M.; Tasdemir, D.; Jung, C. Reduced Glucosinolate Content in Oilseed Rape (Brassica napus L.) by Random Mutagenesis of BnMYB28 and BnCYP79F1 Genes. Sci. Rep. 2023, 13, 2344.
- Liu, S.; Huang, H.; Yi, X.; Zhang, Y.; Yang, Q.; Zhang, C.; Fan, C.; Zhou, Y. Dissection of Genetic Architecture for Glucosinolate Accumulations in Leaves and Seeds of Brassica napus by Genome-Wide Association Study. Plant Biotechnol. J. 2020, 18, 1472–1484.
- Sontowski, R.; Gorringe, N.J.; Pencs, S.; Schedl, A.; Touw, A.J.; van Dam, N.M. Same Difference? Low and High Glucosinolate Brassica Rapa Varieties Show Similar Responses Upon Feeding by Two Specialist Root Herbivores. Front. Plant Sci. 2019, 10, 1451.
- Kirkegaard, J.A.; Lilley, J.M.; Berry, P.M.; Rondanini, D.P. Chapter 17—Canola. In Crop Physiology Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 518–549. ISBN 978-0-12-819194-1.
- Rivelli, G.M.; Gomez, N.V.; Mantese, A.I.; Miralles, D.J.; Abeledo, L.G.; Rondanini, D.P. Photothermal Quotient Describes the Combined Effects of Heat and Shade Stresses on Canola Seed Productivity. Seeds 2023, 2, 149–164.
- Mi, C.; Sun, C.; Yuan, Y.; Li, F.; Wang, Q.; Zhu, H.; Hua, S.; Lin, L. Effects of Low Nighttime Temperature on Fatty Acid Content in Developing Seeds from Brassica napus L. Based on RNA-Seq and Metabolome. Plants 2023, 12, 325.
- Aksouh-Harradj, N.M.; Campbell, L.C.; Mailer, R.J. Canola Response to High and Moderately High Temperature Stresses during Seed Maturation. Can. J. Plant Sci. 2006, 86, 967–980.
- McGeary, K.D.; de Koff, J.; Pokharel, B.; Link, R.; Saini, P.; Gill, T. Effect of Winter Canola Cultivar on Seed Yield, Oil, and Protein Content. Agrosyst. Geosci. Environ. 2022, 5, e20254.
- Wang, S.X.; Oomah, B.D.; McGregor, D.I.; Downey, R.K. Genetic and Seasonal Variation in the Sinapine Content of Seed from Brassica and Sinapis Species. Can. J. Plant Sci. 1998, 78, 395–400.
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. The Critical Period for Yield and Quality Determination in Canola (Brassica napus L.). Field Crops Res. 2018, 222, 180–188.
- Zeleke, K.T.; Luckett, D.J.; Cowley, R.B. The Influence of Soil Water Conditions on Canola Yields and Production in Southern Australia. Agric. Water Manag. 2014, 144, 20–32.
- Attia, Z.; Pogoda, C.S.; Reinert, S.; Kane, N.C.; Hulke, B.S. Breeding for Sustainable Oilseed Crop Yield and Quality in a Changing Climate. Appl. Genet. 2021, 134, 1817–1827.
- Cadot, S.; Bélanger, G.; Ziadi, N.; Morel, C.; Sinaj, S. Critical Plant and Soil Phosphorus for Wheat, Maize, and Rapeseed after 44 Years of P Fertilization. Nutr. Cycl. Agroecosyst. 2018, 112, 417–433.
- Grant, C.A.; Bailey, L.D. Fertility Management in Canola Production. Can. J. Plant Sci. 1993, 73, 651–670.
- Ahmad, G.; Jan, A.; Arif, M.; Jan, M.T.; Khattak, R.A. Influence of Nitrogen and Sulfur Fertilization on Quality of Canola (Brassica napus L.) under Rainfed Conditions. J. Zhejiang Univ. Sci. B 2007, 8, 731–737.
- Cheema, M.A.; Malik, M.A.; Hussain, A.; Shah, S.H.; Basra, S.M.A. Effects of Time and Rate of Nitrogen and Phosphorus Application on the Growth and the Seed and Oil Yields of Canola (Brassica napus L.). J. Agron. Crop Sci. 2001, 186, 103–110.
- Ma, B.L.; Herath, A.W.; Ma, B.L.; Herath, A.W. Timing and Rates of Nitrogen Fertiliser Application on Seed Yield, Quality and Nitrogen-Use Efficiency of Canola. Crop Pasture Sci. 2016, 67, 167–180.
- Aslam, M.M.; Farhat, F.; Siddiqui, M.A.; Yasmeen, S.; Khan, M.T.; Sial, M.A.; Khan, I.A. Exploration of Physiological and Biochemical Processes of Canola with Exogenously Applied Fertilizers and Plant Growth Regulators under Drought Stress. PLoS ONE 2021, 16, e0260960.
- Wang, Z.-H.; Li, S.-X.; Malhi, S. Effects of Fertilization and Other Agronomic Measures on Nutritional Quality of Crops. J. Sci. Food Agric. 2008, 88, 7–23.
- Van de Wouw, A.P.; Howlett, B.J. Advances in Understanding the Leptosphaeria Maculans—Brassica Pathosystem and Their Impact on Disease Management. Can. J. Plant Pathol. 2020, 42, 149–163.
- Dolatabadian, A.; Cornelsen, J.; Huang, S.; Zou, Z.; Fernando, W.G.D. Sustainability on the Farm: Breeding for Resistance and Management of Major Canola Diseases in Canada Contributing towards an IPM Approach. Can. J. Plant Pathol. 2022, 44, 157–190.
- Edde, P.A. 3—Arthropod Pests of Rapeseed (Canola) (Brassica napus L.). In Field Crop Arthropod Pests of Economic Importance; Edde, P.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 140–207. ISBN 978-0-12-818621-3.
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants 2021, 10, 1097.
- Menendez, Y.C.; Sanchez, D.H.; Snowdon, R.J.; Rondanini, D.P.; Botto, J.F. Unraveling the Impact on Agronomic Traits of the Genetic Architecture Underlying Plant-Density Responses in Canola. J. Exp. Bot. 2021, 72, 5426–5441.
- Nelson, M.N.; Nesi, N.; Barrero, J.M.; Fletcher, A.L.; Greaves, I.K.; Hughes, T.; Laperche, A.; Snowdon, R.; Rebetzke, G.J.; Kirkegaard, J.A. Chapter Two—Strategies to Improve Field Establishment of Canola: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 175, pp. 133–177.
- Page, E.R.; Meloche, S.; Moran, M.; Caldbeck, B.; Barthet, V. Effect of Seeding Date on Winter Canola (Brassica napus L.) Yield and Oil Quality in Southern Ontario. Can. J. Plant Sci. 2021, 101, 490–499.
- Pan, X.; Caldwell, C.D.; Falk, K.C.; Lada, R. The Effect of Cultivar, Seeding Rate and Applied Nitrogen on Brassica Carinata Seed Yield and Quality in Contrasting Environments. Can. J. Plant Sci. 2012, 92, 961–971.
- Harker, K.N.; O’Donovan, J.T.; Blackshaw, R.E.; Hall, L.M.; Willenborg, C.J.; Kutcher, H.R.; Gan, Y.; Lafond, G.P.; May, W.E.; Grant, C.A.; et al. Effect of Agronomic Inputs and Crop Rotation on Biodiesel Quality and Fatty Acid Profiles of Direct-Seeded Canola. Can. J. Plant Sci. 2013, 93, 577–588.
- May, W.E.; Hume, D.J.; Hale, B.A. Effects of Agronomic Practices on Free Fatty Acid Levels in the Oil of Ontario-Grown Spring Canola. Can. J. Plant Sci. 1994, 74, 267–274.
- Morrison, I.N.; Khan, R.; Rashid, A. Effects of Seeding Methods and Soil Crusting on Establishment of Rapeseed (Brassica napus) and Mustard (B. Juncea). Field Crops Res. 1988, 19, 27–39.
- Mocniak, L.E.; Elkin, K.R.; Dillard, S.L.; Bryant, R.B.; Soder, K.J. Building Comprehensive Glucosinolate Profiles for Brassica Varieties. Talanta 2023, 251, 123814.
- Ratajczak, K.; Sulewska, H.; Szymańska, G. New Winter Oilseed Rape Varieties—Seed Quality and Morphological Traits Depending on Sowing Date and Rate. Plant Prod. Sci. 2017, 20, 262–272.
- Harker, K.N.; Clayton, G.W.; Blackshaw, R.E.; O’Donovan, J.T.; Stevenson, F.C. Seeding Rate, Herbicide Timing and Competitive Hybrids Contribute to Integrated Weed Management in Canola (Brassica napus). Can. J. Plant Sci. 2003, 83, 433–440.
- Pavlista, A.D.; Hergert, G.W.; Margheim, J.M.; Isbell, T.A. Growth of Spring Canola (Brassica napus) under Deficit Irrigation in Western Nebraska. Ind. Crops Prod. 2016, 83, 635–640.
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259.
- Dogan, E.; Copur, O.; Kahraman, A.; Kirnak, H.; Guldur, M.E. Supplemental Irrigation Effect on Canola Yield Components under Semiarid Climatic Conditions. Agric. Water Manag. 2011, 98, 1403–1408.
- Gill, K.S. Crop Rotations Compared with Continuous Canola and Wheat for Crop Production and Fertilizer Use over 6 Yr. Can. J. Plant Sci. 2018, 98, 1139–1149.
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8.
- Stamm, M.; Roozeboom, K.; Holman, J. Harvest Management of Canola. MF3092. Kansas State University Research and Extension. Available online: https://Www.Bookstore.Ksre.Ksu.Edu/ (accessed on 22 May 2023).
- Cenkowski, S.; Sokhansanj, S.; Sosulski, F.W. Effect of Harvest Date and Swathing on Moisture Content and Chlorophyll Content of Canola Seed. Can. J. Plant Sci. 1989, 69, 925–928.
- Haile, T.A.; Shirtliffe, S.J. Effect of Harvest Timing on Dormancy Induction in Canola Seeds. Weed Sci. 2014, 62, 548–554.
- Cenkowski, S.; Sokhansanj, S.; Sosulski, F.W. Effect of Drying Temperature on Green Color and Chlorophyll Content of Canola Seed. Can. Inst. Food Sci. Technol. J. 1989, 22, 383–386.
- Canadian Grain Commission Canola and Rapeseed: Grading. Available online: https://grainscanada.gc.ca/en/grain-quality/official-grain-grading-guide/10-canola-rapeseed/grading.html (accessed on 23 April 2023).
- Vidal, N.P.; Rahimi, J.; Kroetsch, B.; Martinez, M.M. Quality and Chemical Stability of Long-Term Stored Soy, Canola, and Sunflower Cold-Pressed Cake Lipids before and after Thermomechanical Processing: A 1H NMR Study. LWT 2023, 173, 114409.
- Cai, Z.; Li, K.; Lee, W.J.; Reaney, M.T.J.; Zhang, N.; Wang, Y. Recent Progress in the Thermal Treatment of Oilseeds and Oil Oxidative Stability: A Review. Fundam. Res. 2021, 1, 767–784.
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; Khoury, A.E.; Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; et al. Mycotoxins: Factors Influencing Production and Control Strategies. AIMS Agric. Food 2021, 6, 416–447.
- Canadian Grain Commission Oilseeds Methods and Tests Used to Measure Quality. Available online: https://grainscanada.gc.ca/en/grain-research/export-quality/oilseeds/methods-tests.html (accessed on 23 April 2023).
- Rondanini, D.P.; Borrás, L.; Savin, R. Grain Qualityoilgrain Qualityin Oiloiland Cereal Cropscerealcrops. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 4550–4563. ISBN 978-1-4419-0851-3.
- Gaber, M.A.F.M.; Tujillo, F.J.; Mansour, M.P.; Juliano, P. Improving Oil Extraction from Canola Seeds by Conventional and Advanced Methods. Food Eng. Rev. 2018, 10, 198–210.
- Li, X.; Shi, J.; Scanlon, M.; Xue, S.J.; Lu, J. Effects of Pretreatments on Physicochemical and Structural Properties of Proteins Isolated from Canola Seeds after Oil Extraction by Supercritical-CO2 Process. LWT 2021, 137, 110415.
- Dunford, N.T.; Temelli, F. Extraction Conditions and Moisture Content of Canola Flakes as Related to Lipid Composition of Supercritical CO2 Extracts. J. Food Sci. 1997, 62, 155–159.
- Thiyam-Holländer, U.; Eskin, N.A.M.; Matthäus, B. (Eds.) Canola and Rapeseed: Production, Processing, Food Quality, and Nutrition; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-429-08691-5.
- Sampaio, K.A.; Ayala, J.V.; Van Hoed, V.; Monteiro, S.; Ceriani, R.; Verhé, R.; Meirelles, A.J.A. Impact of Crude Oil Quality on the Refining Conditions and Composition of Nutraceuticals in Refined Palm Oil. J. Food Sci. 2017, 82, 1842–1850.
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209.
- Gaber, M.A.F.M.; Juliano, P.; Mansour, M.P.; Shrestha, P.; Taylor, C.; Smith, R.; Trujillo, F.J. Improvement of the Canola Oil Degumming Process by Applying a Megasonic Treatment. Ind. Crops Prod. 2020, 158, 112992.
- Ma, Y.; Shi, L.; Liu, Y.; Lu, Q. Effects of Neutralization, Decoloration, and Deodorization on Polycyclic Aromatic Hydrocarbons during Laboratory-Scale Oil Refining Process. J. Chem. 2017, 2017, e7824761.
- Gharby, S.; Harhar, H.; Mamouni, R.; Matthäus, B.; Addi, E.H.A.; Charrouf, Z. Chemical Characterization and Kinetic Parameter Determination under Rancimat Test Conditions of Four Monovarietal Virgin Olive Oils Grown in Morocco. OCL 2016, 23, A401.
- Monte, M.L.; Monte, M.L.; Pohndorf, R.S.; Crexi, V.T.; Pinto, L.A.A. Bleaching with Blends of Bleaching Earth and Activated Carbon Reduces Color and Oxidation Products of Carp Oil. Eur. J. Lipid Sci. Technol. 2015, 117, 829–836.
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, e6627013.
- Bell, J.M. Factors Affecting the Nutritional Value of Canola Meal: A Review. Can. J. Anim. Sci. 1993, 73, 689–697.
- Daun, J.K.; Eskin, M.N.A.; Hickling, D. (Eds.) Canola: Chemistry, Production, Processing, and Utilization, 1st ed.; Academic Press and AOCS Press: Urbana, IL, USA, 2011; ISBN 978-0-9818936-5-5.
- Mawson, R.; Heaney, R.K.; Zdunczyk, Z.; Kozlowska, H. Rapeseed meal-glucosinolates and their antinutritional effects Part 3. Animal growth and performance. Nahrung 1994, 38, 167–177.
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684.
- Hansen, J.Ø.; Skrede, A.; Mydland, L.T.; Øverland, M. Fractionation of Rapeseed Meal by Milling, Sieving and Air Classification—Effect on Crude Protein, Amino Acids and Fiber Content and Digestibility. Anim. Feed Sci. Technol. 2017, 230, 143–153.
- Liu, C.; Liu, L.; Li, L.; Hao, C.; Zheng, X.; Bian, K.; Zhang, J.; Wang, X. Effects of Different Milling Processes on Whole Wheat Flour Quality and Performance in Steamed Bread Making. LWT Food Sci. Technol. 2015, 62, 310–318.
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea Starch: Composition, Structure and Properties—A Review. Starch-Stärke 2002, 54, 217–234.
- Aider, M.; Barbana, C. Canola Proteins: Composition, Extraction, Functional Properties, Bioactivity, Applications as a Food Ingredient and Allergenicity—A Practical and Critical Review. Trends Food Sci. Technol. 2011, 22, 21–39.
- Nicolosi, A.; Laganà, V.R.; Di Gregorio, D. Habits, Health and Environment in the Purchase of Bakery Products: Consumption Preferences and Sustainable Inclinations before and during COVID-19. Foods 2023, 12, 1661.
- Barragán-Martínez, L.P.; Román-Guerrero, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Impact of Fat Replacement by a Hybrid Gel (Canola Oil/Candelilla Wax Oleogel and Gelatinized Corn Starch Hydrogel) on Dough Viscoelasticity, Color, Texture, Structure, and Starch Digestibility of Sugar-Snap Cookies. Int. J. Gastron. Food Sci. 2022, 29, 100563.
- Salama, H.H.; Hashim, A.F. A Functional Spreadable Canola and Milk Proteins Oleogels as a Healthy System for Candy Gummies. Sci. Rep. 2022, 12, 12619.
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food Industry By-Products Used as Functional Ingredients of Bakery Products. Trends Food Sci. Technol. 2017, 67, 106–128.
- Jang, A.; Bae, W.; Hwang, H.-S.; Lee, H.G.; Lee, S. Evaluation of Canola Oil Oleogels with Candelilla Wax as an Alternative to Shortening in Baked Goods. Food Chem. 2015, 187, 525–529.
- Hesso, N.; Loisel, C.; Chevallier, S.; Le-Bail, A.; Queveau, D.; Pontoire, B.; Le-Bail, P. Monitoring Cake Baking by Studying Different Ingredient Interactions: From a Model System to a Real System. Food Hydrocoll. 2015, 51, 7–15.
