Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Anne-Marie Caminade and Version 3 by Jason Zhu.
Nanoparticles are defined as matter that has at least one dimension between 1 and 100 nm, and that generally has different properties from its bulk. Phosphorus dendrimers are hyperbranched macromolecules synthesized step-by-step, bearing a phosphorus atom at each branching point. Such type of dendrimers are suitable for the synthesis of diverse types of metal nanoparticles from organometallic precursors.
- dendrimers
- nanoparticles
- hybrid materials
1. Synthesis of Gold and Silver Nanoparticles
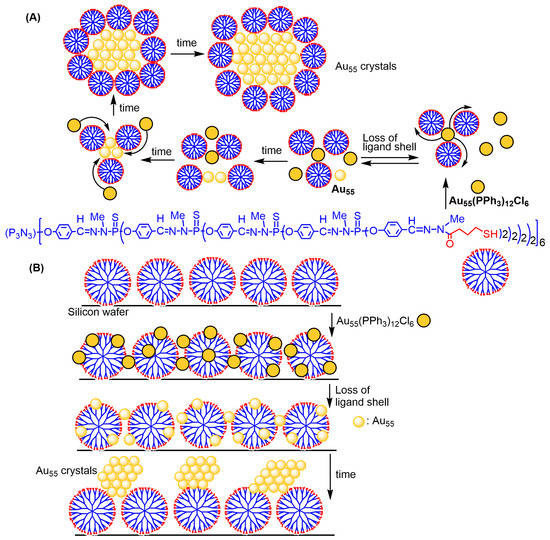
The first example of synthesis of metal nanoparticles with phosphorus dendrimers concerned a thiol-terminated fourth-generation dendrimer, equipped with 96 thiol (SH) functions, interacting with the gold cluster Au55(PPh3)12Cl6 obtained by reduction of PPh3AuCI in benzene at 50 °C by B2H6 [1][70]. The first experiments were carried out in solution in dichloromethane, and resulted for the first time in the formation of bare Au55 clusters, which self-associated in microcrystals of (Au55)∞ [2][71]. The dendrimer played two roles in this experiment: first, it removed the triphenylphosphine and chlorine ligands; then, it acted as a template for growing the crystals of Au55, as illustrated in Scheme 1A. Characterization by transmission electron microscopy (TEM), small-angle X-ray diffraction (SAXRD), wide-angle X-ray diffraction (WAXRD), X-ray absorption fine structure (EXAFS), energy-dispersive X-ray spectroscopy (EDX), and IR analyses confirmed the absence of ligands, and the preservation of the Au55 cluster structure in the crystals [2][71].
Scheme 1.
Structure of the thiol-terminated dendrimers and their application for the synthesis of Au
55
nanocrystals, either in solution (
The same thiol-terminated dendrimer was then used in an analogous experiment but carried out in the solid state. A solution of dendrimer in dichloromethane was deposited on a 9 × 9 mm silicon wafer fixed onto a spin-coater, rotating at 100 rpm, to produce a single layer of dendrimers. The wafer was then washed with dichloromethane to eliminate the unbound dendrimers. Analysis by atomic force microscopy (AFM) indicated a thickness of 1.5–2.0 nm of the almost-defect-free layer. Interaction with the gold clusters was carried out by dipping the wafer platelet for a short time into a solution of the gold clusters. After washing with dichloromethane and drying, if the wafer was kept in an atmosphere of dichloromethane for about one week, nanosized crystals of bare Au55 clusters were observed on the dendrimer surface, as illustrated in Scheme 1B. This process taking place in two-dimensional reactant arrays is analogous to the process observed in three dimensions in solution [3][72].
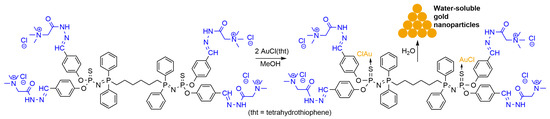
A small water-soluble phosphorus-containing dendrimer, functionalized with four Girard’s reagents T (acethydrazide trimethylammonium chloride) and two P=N-P=S linkages, was especially synthesized for obtaining gold crystals from AuCl(tht) (tht = tetrahydrothiophene). AuCl(tht) was synthesized by the reaction of tht with HAuCl4 in water/methanol [4][73]. This dendrimer was especially engineered for reacting with AuCl(tht). Indeed, it was previously shown that the sulfur atom included in P=N-P=S linkages is specifically suitable for the complexation of AuCl, whereas the other P=S groups included in the dendrimers, but not in P=N-P=S linkages, do not react [5][74]. In addition, a reaction was rapidly observed at room temperature in water between Girard’s reagent T and AuCl(tht), leading to a black precipitate, showing that this reagent is indeed able to reduce gold, but not in the form of nanoparticles. With the hydrazone bond being slightly in equilibrium with the aldehyde and hydrazine forms, the dendrimer functionalized with this reagent should release a small quantity of Girard’s reagent T in water. This dendrimer acted first as a complexing agent for AuCl. The complex was stable in many types of solvents, but when adding water, the small quantity of released Girard’s reagent T acted as a mild reducer, and the structure of the dendrimer was finally a template for the formation of gold nanocrystals (Scheme 2). Shape-controlled Au nanoparticles in the form of triangles and associated triangles were obtained [6][75].
Besides gold, silver nanoparticles were also obtained from phosphorus dendrimers. In the first step, a first-generation dendrimer functionalized with aldehydes was grafted to silica nanoparticles (mean diameter 12 nm), bearing amine surface functions issued from aminopropyltriethoxysilane (APTES) [7][76]. In the second step, polyethylene glycol (ca., 11 CH2CH2O units) having an amine at one end was reacted with the remaining aldehyde functions of the grafted dendrimers. To stabilize the imine functions, a hydrophosphorylation with dimethyl phosphite was carried out, producing a secondary amine and a dimethyl phosphite in place of all the imine groups. The reaction was in particular characterized by IR, which showed the disappearance of the aldehyde functions. The addition of silver acetate in a water suspension of the modified silica induced the darkening of the suspension, inducing the formation of silver nanoparticles on the surface of silica, both with and without a reducing agent (NaBH4) (Scheme 3) [8][77]. A typical plasmon absorption band centered between 394 and 416 nm was observed only when using the reducing agent. Evolution with time produced various silver oxide nanoparticles (AgO, Ag2O, Ag3O…). The antibacterial properties of silver NPs are well known [9][10][78,79]; thus, the properties of these materials were estimated by determining the minimal inhibitory concentration (MIC) and the minimal bactericidal concentration (MBC) on four typical bacterial strains (Gram+: S. aureus and E. hirae; Gram−: E. coli and P. aeruginosa). The silver-loaded silica NPs exhibited bacteriostatic activities against all bacterial strains in the 25–500 ppm range (silver equivalents), as shown in Table 1 [8][77].
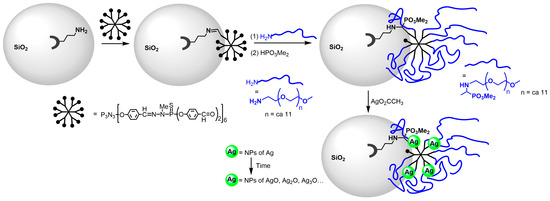
Table 1.
MIC and MBC of silica NPs functionalized with pegylated dendrimers hosting silver NPs determined on 4 typical bacterial strains.
| Materials | MIC | 1 | /MBC | 1 | of | S. aureus | CIP 4.83 (Gram+) |
MIC | 1 | /MBC | 1 | of | E. hirae | CIP 58.55 (Gram+) |
MIC | 1 | /MBC | 1 | of | E. coli | CIP 103571 (Gram−) |
MIC | 1 | /MBC | 1 | of | P. aeruginosa | CIP 104116 (Gram−) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | 0 | @SidendriPEG | 500/>500 | 250/>500 | 250/500–1000 | 250/>500 | ||||||||||||||||||||||
| Ag | 0 | @SidendriPEG | 50–62.5/>500 | 25–31.25/>500 | 50–62.5/250 | 25–31.25/250 |
1
in ppm (silver equivalents).
2. Synthesis of Palladium, Platinum, and Ruthenium Nanoparticles
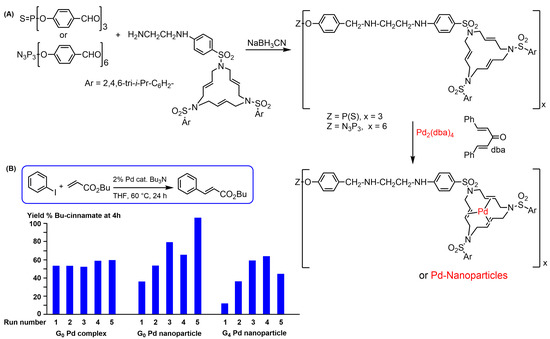
Triaza triolefinic 15-membered macrocycles functionalized with a diamine (Scheme 3) were reacted with the aldehyde terminal functions of generations 0, 1, and 4 of phosphorhydrazone dendrimers, followed by the reduction of the imine bonds with NaBH3CN to obtain stable compounds. Reaction with the palladium complex Pd2(dba)4 (dba = dibenzylidene acetone) afforded discrete complexes when using a strict stoichiometry of reagents (one Pd per macrocycle), or Pd nanoparticles when using an excess (Scheme 3A) [11][80]. Both the discrete complexes and the Pd NPs stabilized by the dendrimers were used as catalysts in Mizoroki–Heck reactions, monitored by 1H NMR [12][81]. Both types of catalysts could be recovered and reused several times. The catalytic efficiency of the discrete complexes did not change with the number of reuses (four times), whereas the nanoparticles became more and more efficient with the number of reuses, particularly when stabilized with the zeroth-generation dendrimer (Scheme 3B). This result can be explained by a decrease in the size of the nanoparticles with recycling, as they were less stabilized by the small zeroth-generation dendrimer than by the large fourth-generation dendrimer [11][80].
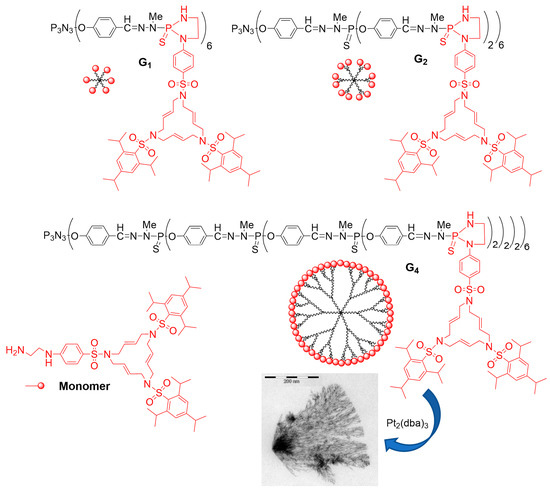
The same type of macrocycles was also grafted to the P(S)Cl2 terminal functions of phosphorhydrazone dendrimers (Figure 1, R = Cl). Two Cl were needed for grafting one macrocycle, by the formation of a five-membered phosphadiaza heterocycle. A monomer and generations 1, 2, and 4 were synthesized, and they were reacted with the platinum complex Pt2(dba)3 [13][82]. Platinum nanoparticles were obtained in all cases, which organized in dendritic networks when using the dendrimers. The mean length of the dendritic branches composed of Pt NPs surprisingly increased with the generation of the dendrimers, with the longest branches being obtained with generation 4, obtained using high-resolution transmission electron microscopy (HRTEM). No network was observed when using the monomer. It was proposed that large dendrimers having numerous macrocycles as terminal groups should wrap Pt NPs more efficiently and produce longer ribbons than the smaller ones [14][15][83,84].
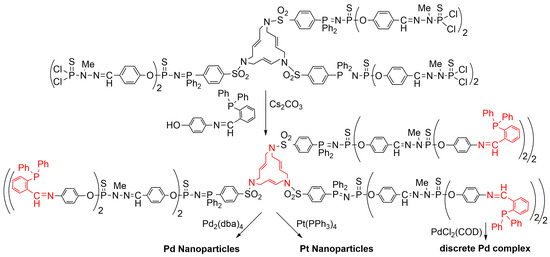
Another family of phosphorhydrazone dendrimers was built from the same type of 15-membered triazatriolefinic macrocycle, which was used as the core instead of as terminal functions [16][85]. The dendrimers were built up to generation 3 and were functionalized on the P(S)Cl2 terminal functions with a phenol bearing an iminophosphine, at the level of the second and third generations (Scheme 11). The phosphines on the surface were suitable for the complexation of palladium dichloride PdCl2 from PdCl2(COD) (COD: cyclooctadiene), as shown previously [17][86], but not the macrocycle at the core. Indeed, these macrocycles are suitable for the complexation of Pd0, but not PdII [18][87]. However, the single macrocycle at the core of these dendrimers was suitable for the complexation of Pd0, and for the elaboration of palladium nanoparticles from Pd2(dba)4 and of platinum nanoparticles from Pt(PPh3)4, using an excess of metal in both cases. It was shown in these latter cases that the phosphines on the surface were not involved in the complexation of the nanoparticles [16][85].
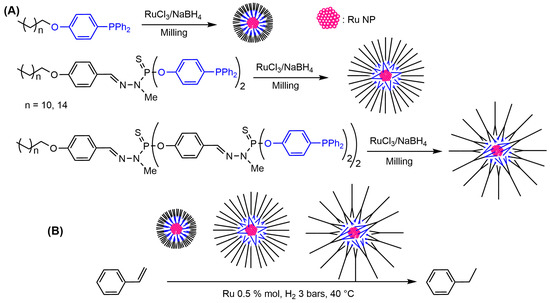
Besides palladium and platinum, ruthenium nanoparticles were also elaborated. In that case, dendrons having one, two, or four triphenylphosphine functions on their surface were used for milling under air with a mixture of ruthenium trichloride RuCl3 and sodium borohydride NaBH4 as reducing agent. Ruthenium nanoparticles (Ru NPs) having a diameter in the 2 to 3 nm range and protected by the dendrons were obtained in this way. It should be noted that the largest dendrons afforded the less hindered nanoparticles. Indeed, a smaller number of large dendrons was grafted per Ru NP, compared to the large number of small dendrons, as illustrated in Scheme 4A. These Ru NPs were air-stable upon storage, and all of them efficiently catalyzed hydrogenation of styrene. The best catalytic results were obtained with the largest dendron, which facilitates access of the reagents to the less protected Ru NPs (Scheme 12B) [19][88].
Scheme 4.
(
A
) Structure of dendrons having phosphines on their surface, and their use for obtaining Ru nanoparticles. (
3. Synthesis of Titanium Nanoparticles
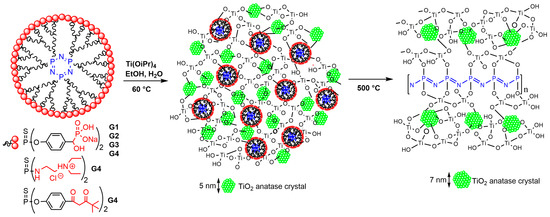
The possibility of phosphorus dendrimers to react with metal alkoxides was demonstrated early with the elaboration of hybrid materials, from dendrimers with carboxylic acid end groups and either titanium oxo clusters [20][89] or cerium or titanium alkoxides [21][90]. On the other hand, a dendron functionalized with phosphonates was able to strongly interact with the surface of nanocrystalline mesoporous titania thin film [22][91]. Thus, dendrimers of generations 1 to 4, functionalized with phosphonic acids on the surface, were reacted with titanium tetraisopropoxide Ti(OiPr)4. Stable porous hybrid materials based on a titanium oxo network entrapping the dendrimers were obtained, which surprisingly included nanocrystals of anatase TiO2 (ca., 5 nm). Furthermore, the anatase structure was stable up to 800 °C and did not transform to the brookite or rutile phase, commonly observed at this temperature range [23][92]. Anatase, rutile, and brookite are three crystalline forms of TiO2. The anatase-to-rutile transformation occurs in the temperature range of 700–1000 °C, whereas brookite is converted into rutile by heating to temperatures above 700 °C [24][93]. Other fourth-generation phosphorus dendrimers equipped with ammoniums or diketone terminal functions were also reacted with Ti(OiPr)4 at mild temperature (60 °C) and also afforded materials including anatase nanocrystals (4.8 to 5.2 nm in size). Small-angle X-ray diffraction studies (SAXD) indicated that the material was organized as an ordered mesoporous network. Heating the materials at 500 °C induced the ring opening of the cyclotriphosphazene core of the dendrimers, and the cleavage of the dendrimer branches, to afford a linear polyphosphazene polymer bridged mineral phase, as illustrated in Scheme 13 [25][94]. The dendritic structure provides a confined medium for the low-temperature crystallization of TiO2, and the heteroatoms of the core and the branches (P, N, S) passivate the surface of the anatase nanocrystals. The phase transformation is generally initiated at the anatase surface; thus, its passivation prevents its transformation. Finally, the ring opening polymerization of the cyclophosphazene core restricts the anatase growth and affords thermally stable, interpenetrating mesoporous polyphosphazene–anatase networks [24][93]. Later on, the materials obtained after calcination at 500 °C were used as catalysts in photocatalytic water splitting for hydrogen production [26][95]. A recent paper reported the hemolytic activity and cytotoxicity of related dendrimer-TiO2 materials [27][96].