Transdermal patches and various semisolid dosage forms, including hydrogels, are widely recognized delivery strategies for addressing dermal conditions. Hydrogels have been extensively investigated and reported in scientific research. Three-dimensional network architectures are developed by these hydrogels’ hydrophilic polymer chain composition that absorb and hold enormous quantities of liquid or physiological fluids. Moreover, the properties and performance of hydrogels are significantly affected by the structure of their molecules. Crosslinking agents and polymer chains are the two main constituents of hydrogels. There are both natural and artificial polymer chains in hydrogels. Natural polymers commonly used in hydrogel formulations include agarose, alginate, chitosan, collagen, gelatin, hyaluronic acid, and cellulose derivatives.
1. Types of Skin Infections (Bacterial, Fungal, and Parasitic)
Skin infections can be classified into three main types based on the causative agents: bacterial, fungal, and parasitic. Here is an overview of each type:
Bacterial infections: Bacterial skin infections are caused by various bacteria that invade and multiply within the skin. Common bacterial infections include (1) impetigo: characterized by red sores that rupture and form yellowish crusts; (2) cellulitis: a deeper infection that affects the skin and underlying tissues, causing redness, swelling, and warmth; and (3) folliculitis: inflammation of hair follicles, resulting in small red bumps or pustules.
Fungal infections: Fungal skin infections are caused by different types of fungi that thrive in warm, moist environments. Common fungal infections include athlete’s foot (Tinea pedis): affecting the feet and causing itching, redness, and cracking of the skin; ringworm (Tinea corporis): characterized by circular, red, scaly patches on the skin; jock itch (Tinea cruris): occurs in the groin area, resulting in itching, redness, and a rash; and Candidiasis: caused by the Candida fungus, typically affects areas with skin folds, such as the armpits and groin.
Parasitic infections: Parasitic skin infections are caused by parasites that infest and feed on the skin. Common parasitic infections include scabies: caused by tiny mites that burrow into the skin, leading to intense itching and a pimple-like rash; pediculosis (lice infestation): an infestation of lice on the scalp, body, or pubic area, causing itching and the presence of lice or their eggs; and cutaneous larva migrant occurs when the larvae of certain parasites penetrate the skin, resulting in itchy, winding tracks.
1.1. Molecular Structure of Hydrogels
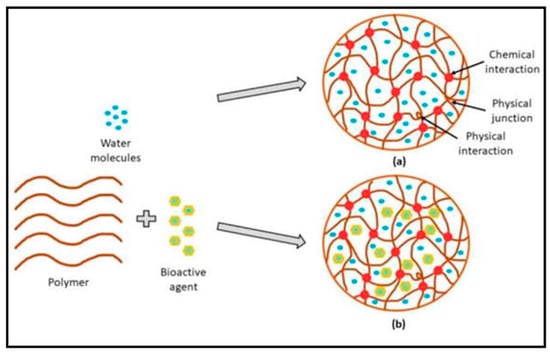
Hydrogels are a three-dimensional (3D) crosslinked network of polymer chains that absorb enormous amounts of fluid due to their hydrophilic functional groups (hydroxyl, carboxyl, amide, and amino), which adhere to the polymeric backbone
[1][26]. The term “hydrogel” was invented for the first time in 1894 by van Bemmelen. The molecular structure of hydrogels is functionally characterized by the mesh size that is crosslinked physically through hydrogen bonding and chemically via covalent bonding (
Figure 1). The hydrogel’s primary feature is its capacity to absorb large amounts of water when water molecules disperse into the structure of the hydrogel.
Hydrogels are polymeric networks that can undergo degradation over time, and crosslinking is crucial for their structure formation and degradation control. Crosslinking involves the formation of stable connections between polymer chains, resulting in the formation of a 3D network structure. Synthetic and natural polymers can fabricate hydrogels that have 3D structures that are able to hold large quantities of water or biologic fluids. The choice of the polymer depends on the desired properties and applications of the hydrogel, which can offer precise control over the hydrogel’s properties and can be modified to enhance biocompatibility and biofunctionality
[2][27]. Moreover, synthetic hydrogels are often preferred when specific mechanical or chemical properties are required for a particular application
[3][28]. Chitosan and gelatin are frequently utilized in hydrogel compositions
[4][29]. These natural polymers offer inherent biocompatibility and biodegradability, making them suitable for various biomedical applications. Natural hydrogels can mimic the extracellular matrix, which offers a favorable environment for cell development and tissue regeneration. They are often used in wound healing, drug delivery, and tissue engineering applications. The choice between synthetic and natural polymers for hydrogel preparation depends on the desired mechanical properties, degradation behavior, biocompatibility, and specific application requirements. Synthetic and natural hydrogels have advantages and can be modified to meet the desired criteria for various biomedical and industrial applications
[5][30].
Following the type of crosslink junctions, two basic categories of hydrogels are chemically and physically bonded together. Covalent bonds between different polymer chains create permanent junctions in chemically crosslinked hydrogels. This crosslinking type imparts excellent mechanical strength to the hydrogel
[6][31]. However, it is essential to note that certain crosslinkers used in the chemical crosslinking process may have reported toxicity, requiring their extraction from the hydrogel before use to ensure safety. Physically crosslinked hydrogels, on the other hand, rely on non-covalent interactions, such as ionic contracts, hydrogen bonds, or hydrophobic interactions, to stop the dissolution of the polymer chains and keep the hydrogel structure stable
[7][32]. Physically crosslinked hydrogels offer an alternative solution to address crosslinker toxicity concerns, as the interactions can be reversibly formed and disrupted, allowing for potential recycling or reprocessing of the hydrogel
[8][33]. Both chemically and physically crosslinked hydrogels have several advantages and limitations, depending on the specific application requirements. The choice of crosslinking method depends on several factors, such as the desired mechanical properties, degradation behavior, biocompatibility, and the intended use of the hydrogel. Furthermore, research and development efforts in crosslinking techniques for hydrogels aim to enhance their mechanical properties, control degradation rates, improve biocompatibility, and explore innovative approaches that minimize the potential toxicity associated with crosslinkers
[9][34].
Figure 1.
Hydrogels’ molecular composition without bioactive (
a
) and fortified with bioactive (
b). Reproduced with permission from [10] under CC BY 3.0. ). Reproduced with permission from [35] under CC BY 3.0.
1.2. Classification of Hydrogel Products
Various characteristics of hydrogels, such as their origin or source, composition, structure, crosslinking, network charge, durability, and reaction to external stimuli, are used to categorize them
[11][36]. Some of the types are as follows:
1.2.1. Based on Sources
Synthetic Source
Synthetic hydrogels are formed by polymerizing various synthetic monomers by ionic or covalent linking. These include the use of polyacrylates and glycolates monomers. Synthetic polymers, such as polyethene glycol, are water-soluble, biocompatible, and inert for medical applications. Polyethylene glycol (PEG) has linear and branched structures with two or more hydroxy groups, which further function with others to provide crosslinking in the hydrogel
[12][37]. Polyvinyl alcohol (PVA) is a hydrophilic polyhydroxy polymer that is another example of a synthetic polymer that is widely used for creating hydrogels due to its versatile mechanical properties, adhesion, and elasticity
[13][38]. Gulafshan et al. fabricated a PVA-alginate hydrogel for accelerated wound healing
[14][39]. Other synthetically prepared hydrogels include poloxamers, poly(N-Isopropylacrylamide, and poly(propylene oxides)
[15][40].
Natural Source
Natural hydrogels are formed using natural and hybrid polymers derived from plant, animal, and marine sources. Examples of natural polymers include chitosan, a natural mucoadhesive polysaccharide derivative of chitin obtained from the deacetylation of chitin; it is reportedly used to prepare hydrogels due to negligible toxicity and improved wound healing activity
[16][41]. According to their results, Sheng et al. developed a novel photothermal chitosan incorporated hydrogel for improved wound healing
[17][42]. Use of other natural polymers includes gelatin, collagen, cellulose, and starch, due to their biodegradation and lesser therapeutic toxicities
[18][43].
1.2.2. Based on Crosslinking
Crosslinked hydrogels are divided into chemical-mediated hydrogels and physical hydrogels based on the preparation method. Chemical hydrogels have more permanent linking than physical hydrogels
[19][44].
Chemically Crosslinked Hydrogels
As permanent crosslinking is involved in this type of polymer, these are preferred over physical ones. Crosslinked agents are grafted onto the polymer backbone. It involves reactions such as polymer–polymer conjugation, enzyme-catalyzed reactions, and ionic reactions. The development of chemically crosslinked hydrogels with free radical polymerization between ß-cyclodextrin and favipiravir for sustained release delivery was reported
[20][45]. The chemically crosslinked hydrogels formed with higher mechanical strength due to covalent bonding. Karoyo et al. reported the formation of a beta-cyclodextrin hydrogel by chemical crosslinking with hexamethyl diisocyanate
[21][46].
Physically Crosslinked Hydrogels
These are self-assembled hydrogels produced by the interactions between molecules of hydrophobic groups, hydrogen bonds, and the electrostatic attraction of molecules
[22][47]. Gabriela et al. fabricated porous chitosan-based hydrogels by physical crosslinking; NaOH was used as a crosslinking agent, and with the help of X-ray photoelectron spectroscopy, the physical properties were analyzed, and biocompatibility studies were tested
[23][48].
1.2.3. Classification Based on Response to Stimuli
Various external stimuli influence a hydrogel’s ability to expand and deflate. In reaction to specific physical or chemical stimuli, they experience an extent of collapse or shift in phase. Depending on how polymers respond to stimuli, hydrogels may be sensitive to changes in temperature, magnetic and electric fields, solvent composition, pH, sound, or molecular species
[24][49].
2. Chitosan Hydrogels Are Currently in Clinical Trials, and Patents
Chitosan hydrogels have emerged as a promising biomaterial in recent years, leading to their investigation in clinical trials for various medical applications. These trials have evaluated chitosan hydrogels’ safety, efficacy, and feasibility in diverse areas, including regenerative medicine and drug delivery. In regenerative medicine, chitosan hydrogels have great potential as scaffolds for tissue engineering and wound healing
[25][147]. The unique properties of chitosan, such as biocompatibility, biodegradability, and antimicrobial activity, make it an attractive material for promoting tissue regeneration and facilitating the healing process. Clinical trials continue to assess chitosan hydrogels’ effectiveness in promoting the repair and regeneration of damaged tissues, such as cartilage, bone, skin, and nerve tissues. Chitosan hydrogels are also being investigated in clinical trials for drug delivery applications
[26][148]. The versatile nature of chitosan allows the incorporation of various therapeutic agents, such as drugs, growth factors, and genes, within the hydrogel matrix. This enables the controlled and sustained release of the therapeutic payload, enhancing treatment outcomes and reducing potential side effects. Clinical trials are exploring the potential of chitosan hydrogels as local drug delivery systems for conditions such as cancer, chronic wounds, and ocular diseases
[27][149]. Through these ongoing clinical trials, researchers aim to gather valuable data on the safety, efficacy, and optimal usage of chitosan hydrogels in real-world medical settings. The results obtained from these trials will provide insights into the potential of chitosan hydrogels as innovative solutions for addressing unmet medical needs, paving the way for their future clinical applications and commercialization. Several concerns with chitosan-based hydrogels must be addressed. Chitosan, for example, has a low solubility, and hydrogels have poor mechanical properties, limiting their usage in medical devices
[28][150]. Polysaccharides have been employed in wound healing dressings for tissue regeneration due to their low toxicity and positive compatibility profile. However, due to the lack of protein structure, natural polysaccharides have very poor biostability and difficulties creating a “matrix” to cover the damaged tissue during wound healing, encouraging wound contraction and scar formation
[29][151]. Furthermore, the development of temperature- or pH-responsive and functionalized biomaterials, such as chitosan-based hydrogels, necessitate the use of toxic chemicals, which are both expensive and dangerous (
Table 1 and
Table 2).
Table 2.
Examples of chitosan-based hydrogels in clinical trials/marketed products.