Enhancement of the passage of water through membranes is one of the main mechanisms via which cells can maintain their homeostasis under stress conditions, and aquaporins are the main participants in this process. However, in the last few years, a number of studies have reported discrepancies between aquaporin messenger RNA (mRNA) expression and the number of aquaporin proteins synthesised in response to abiotic stress. These observations suggest the existence of post-transcriptional mechanisms which regulate plasma membrane intrinsic protein (PIP) trafficking to the plasma membrane. This indicates that the mRNA synthesis of some aquaporins could be modulated by the accumulation of the corresponding encoded protein, in relation to the turnover of the membranes.
- abiotic stress
- aquaporins
- gene expression
- post-translational modifications
1. Introduction
Aquaporins are regulated to deal with variations in the amount and availability of water, since they are channels that allow water transport across cell membranes [1]. They are regulated at multiple levels (for instance, during transcription and translation or through post-translational modifications) to modulate the trafficking, gating, or degradation/turnover [2]. This regulation is key to maintain cellular homeostasis under stress conditions, and, although our knowledge of plant aquaporins has experienced a tremendous increase over the past 20 years, different aspects of this regulation, especially in stressful conditions, are not yet known. With regard to aquaporins, the complexity of the response of plants to stress is due, on the one hand, to the multiple levels of regulation and, on the other, to the great variety of isoforms. It is important to highlight the discrepancies between messenger RNA (mRNA) and protein levels revealed in different studies over the years. A point of view that takes into account the translational modifications [3] [3] will be key to understanding and explaining these types of discrepancies. It has been and will be crucial to address this research from the in vitro angle. In vitro methods led to the discovery of the first aquaporins [4] and revealed the structure of these proteins [5], among other important aspects. Furthermore, the importance of the lipid environment and the plasma membrane (PM) as a whole remains largely unexplored as far as aquaporins are concerned. Indeed, modification of the lipids, which appears under stress [6], can induce changes in intrinsic membrane proteins [7]. Therefore, the study of aquaporins should be approached from this perspective.
2. Aquaporins under Salinity and Drought
2.1. General Features
There are different types of abiotic stress (salinity, radiation, drought, floods, extreme temperatures, heavy metals, etc.) that usually cause loss of yield in major crop plants worldwide [8,11][8][9]. Therefore, detailed studies in this field are important, and numerous works have been carried out with the aim of facing up to abiotic stresses [12][10]. Despite the fact that these stresses have different origins and, therefore, distinct effects on the soil and on plant physiology, they all, as a primary effect, alter water uptake and, thus, have a high impact on plant–water relations [13][11]. Water transport from the soil into the roots and then through the entire plant, with subsequent evaporation to the atmosphere, is crucial for the development of essential physiological activities. This transport is a constant flux, based on three different pathways: (1) apoplastic, (2) symplastic, and (3) transcellular [14][12].
Therefore, the first response of plants to stress is to maintain their water homeostasis, which is essential to deal with environmental stress and to maintain plant development [15,16][13][14]. One of the mechanisms via which cells can maintain this homeostasis under stress conditions is the enhancement of water passage through membranes, in which aquaporins have the main role [9,10][15][16]. It has been reported that these proteins play key roles in osmoregulation [17], root hydraulic conductivity (Lpr) [18], leaf hydraulic conductivity [19], and transpiration [20].
Aquaporins are transmembrane proteins belonging to the major intrinsic protein (MIP) family. They have a molecular mass between 25 and 30 kDa, assemble into tetramers, and constitute channels in the cell membranes. These channels were first characterised by their transport of water across membranes, but it has been reported that aquaporins also transport small neutral solutes (urea, silicon, boron, hydrogen peroxide) or gases (ammonia and carbon dioxide) [1]. At the structural level, these proteins have six transmembrane α-helices and five connecting loops. In the two cytoplasmic loops appear two highly conserved Asn–Pro–Ala (NPA) motifs [21]. Furthermore, four conserved residues form a typical aromatic/arginine (Ar/R) constriction that functions as the main selectivity filter. Regarding their classification, aquaporins in plants are placed in seven subfamilies according to their intracellular locations and sequence similarities: (1) plasma membrane intrinsic proteins (PIPs), (2) tonoplast intrinsic proteins (TIPs), (3) nodulin 26-like intrinsic proteins (NIPs), (4) small, basic intrinsic proteins (SIPs), (5) GlpF-like intrinsic proteins (GIPs), (6) hybrid intrinsic proteins (HIPs), and (7) uncategorised X intrinsic protein (XIPs) [22].
Regarding the role of aquaporins in the maintenance or restoration of water homeostasis under abiotic stress, particularly drought and salinity, numerous studies have been carried out. These include research describing the behaviour of aquaporins under stress conditions from the point of view of gene expression and protein levels [23,24,25][23][24][25]. On the other hand, there are multiple investigations that include the overexpression of different aquaporins in order to elucidate their role in the response to salinity and drought stress [26,27][26][27]. Under these stress conditions, differences in the responses of aquaporins have been shown among different aquaporin homologues in diverse plants [28], among cultivars of the same plant species with different stress tolerance strategies [29], and between tissues [30]. Indeed, root transcriptomic analysis under drought stress showed both up- and downregulation of different PIP, TIP, and NIP homologues, while different homologues responded differently depending on the intensity and nature of the stress applied [10][16].
2.2. Discrepancies between the mRNA and Protein Levels of PIP Aquaporins
Aquaporins have been widely studied for years; however, there has been little investigation relating aquaporin expression and protein synthesis in the same study. There are several works that showed discrepancies between the expression of aquaporin genes and the number of aquaporin proteins present under abiotic stress conditions (Table 1) especially for PIPs. An understanding of the relationship between mRNA and protein levels is essential for integration of the physiological functions of aquaporins.
Summary of the plant responses to salinity and drought stress at the gene expression and protein accumulation levels of plasma membrane intrinsic protein (PIP) aquaporins.
| Gene Expression | Protein Amount | References |
|---|
| = | = | ||
| ↑ | [31,32] | [31][32] | |
| ↓ | [32] | ||
| ↑ | = | ||
| ↑ | |||
| ↓ | [32] | ||
| ↓ | = | [33,34,35] | [33][34][35] |
| ↑ | [24] | ||
| ↓ | [23,33,36,37] | [23][33][36][37] |
In the study carried out by Kammerloher et al. [31], in which the existence of water channels in the PM of plant cells was confirmed, these discrepancies first appeared. In this work, Arabidopsis thaliana plants were subjected to drought (0.6 M mannitol for 4 h) and changes in the PIP gene expression and protein levels were analysed. Different PIP genes (PIP1a, PIP1b, PIP1c, PIP2a and PIP2b, which correspond to AtPIP1;1, AtPIP1;2, AtPIP1;3, AtPIP2;1, and AtPIP2;2, respectively, according to Johanson et al. [38]) were analysed, and none of them were induced or repressed by the water stress. However, the amount of PIP in the PM increased twofold. At this point, there were two possible explanations for these discrepancies. On the one hand, the increase in PIP gene expression may have occurred during an earlier period of time and, 4 h after applying the stress, the mRNA level had returned to the initial levels and the amount of protein corresponding to that early overexpression response was detected. On the other hand, in A. thaliana, 13 PIP aquaporins have been described and only five were analysed individually at the mRNA level, while the protein content was determined using a nonspecific antibody. Therefore, it may have been other isoforms, apart from those analysed, that responded to the stress.
After this, other studies were carried out on A. thaliana to elucidate the regulation of aquaporins under drought stress. Jang et al. [39] [39] analysed the expression of all PIP aquaporins genes in A. thaliana under drought (applied as 0.25 M mannitol) in the long term (2 days), as well as in the short term (4 h). A general decrease in the expression of PIPs in leaves and roots after 2 days of treatment was reported, except for AtPIP1;3 AtPIP1;4, AtPIP2;1, and AtPIP2;5, whose expression increased. On the other hand, after 4 h of drought treatment, almost all the AtPIP1s, as well as AtPIP2;1 and AtPIP2;5, showed increased expression, while that of almost all the AtPIP2s and AtPIP1;5 decreased [39]. This study contrasted with that of Kammerloher et al. [31], in which the expression of none of the PIP genes analysed changed. These differences could be due to the differing intensities of the stress; in Jang et al. [39], the intensity of the stress was lower (0.25 M mannitol versus 0.6 M mannitol). However, a differential expression appeared, compared to the control conditions, in both these experiments with A. thaliana plants. Moreover, another important factor may be the advancement in the technology used, since the second study was carried out in 2004 and the first in 1994. Nevertheless, an insight into the post-transcription events is not possible because the amount of PIP in the PM was not analysed in this study [39]. A later study of aquaporins in A. thaliana under a 12 day drought stress [37] [37] revealed that the gene expression and protein levels showed a positive correlation in the aerial part. In this case, drought, applied by not watering, produced a general decrease in the mRNA level of all PIP genes, except for AtPIP1;4 and AtPIP2;5, as in the study described previously [39]. Regarding the amount of protein, determined by Western blot with nonspecific antibodies (anti-PIP1s and anti-PIP2s), a decrease in PIPs was determined after 12 days of drought stress. When the watering was restored, the mRNA level recovered, but this increase was not reflected in the amount of protein. The authors attributed these discrepancies to a general time lag between transcription and protein synthesis and/or a negative translational regulation of aquaporin transcripts [37]. The use of nonspecific antibodies to detect different aquaporins is a limitation that has appeared in many papers over the years and that could affect the interpretation of the results.
Conflicting data regarding the transcriptional response of PIP genes and the abundance of PIPs have also been reported in studies carried out with A. thaliana under salt stress. It is noteworthy that, as with the drought studies, some discrepancies between studies were also found in work on aquaporins under salinity. Although it is difficult to compare studies due to differences in the length and intensity of the stress, some general considerations can be stated. In [39], a study with A. thaliana was conducted under salinity (150 mM NaCl for 24 h). In the aerial part, the expression of most PIP aquaporins did not change—except for AtPIP2;2 and AtPIP2;3, whose expression increased, and AtPIP2;6, whose expression decreased. In the roots, something different occurred; the aquaporins whose expression changed were principally PIP1s, specifically AtPIP1;1, AtPIP1;2, and AtPIP1;3 (for which it increased) and AtPIP1;5 (for which it decreased). Among the root PIP2 aquaporins, only the expression of AtPIP2;7 changed, showing an increase. Unfortunately, no protein measurements were performed in this study. There are no other studies in which the aerial part was analysed in these terms and there are no other works where the results obtained were similar to those described here. For example, in a study conducted in A. thaliana grown under conditions of salinity similar to those used in the study described above (100 mM NaCl for 24 h), a general decrease in PIP genes expression was shown [23]; only the expression of AtPIP2;3, AtPIP2;6, and AtPIP2;8 did not change. It is difficult to find an explanation for this and, hence, it is necessary to focus on the differences between the two studies. In the first study described, 150 mM NaCl was applied to 20 day old plants, whereas 100 mM NaCl was applied to 14 day old plants in the second study. In the work carried out by Boursiac et al. [23], the amount of protein was also analysed 24 h after the treatment commenced, and the results showed a correlation between the mRNA levels and the amounts of PIP aquaporins in the PM, as both decreased. However, when the amounts of PIP aquaporins in the PM were analysed after long-term stress application (100 mM NaCl for 10 days), the amounts of PIP1 were similar to the control, while, for PIP2 aquaporins, the decrease continued [40]. In this study, the expression levels were not determined and, therefore, it is difficult to correctly interpret what is happening. Studies carried out in the short term (1 h and 4 h) with 10 and 100 mM NaCl showed a decrease in the expression of most PIP aquaporin genes, with the exception of AtPIP2;6, which in almost all cases showed a different behaviour [41,42][41][42]. There are not many published works in which a complete analysis of both the levels of expression of PIP genes and the abundance of PIP aquaporins was performed in relation to different intensities and durations of a stress. Hence, more specific experiments are needed to determine how the aquaporin levels in a model plant such as A. thaliana respond to salinity and, thereafter, to extend this knowledge to other species. Recently, Pou et al. [34] [34] published a complete study of AtPIP2;7 in A. thaliana with the objective of clarifying the conflicting data from other studies, such as those described above, of at least one of the PIP aquaporins. In this work, AtPIP2;7 was characterised at the gene and protein levels under salt stress (2 and 4 h at 150 mM NaCl). Salt stress triggered AtPIP2;7 transcriptional repressions, but the amount of protein did not change after 4 h of treatment.
The results of the experiments carried out on A. thaliana show a downregulation of the expression of most aquaporins under drought or salinity stress, to limit water loss and induce stomatal closure [23,39,43][23][39][43]. Additionally, these experiments are a window into the complex system of regulation of aquaporins in plants subjected to water-related stress. Figure 1 shows a graphical summary of the response to water stress regarding the expression levels of PIP genes and the abundance of PIP in the PM in A. thaliana, which has been the major model plant in the past three decades [44]. Furthermore, it is important to point out that most of the work in this sense was conducted in the root, because it is the first organ to come into contact with the stress.
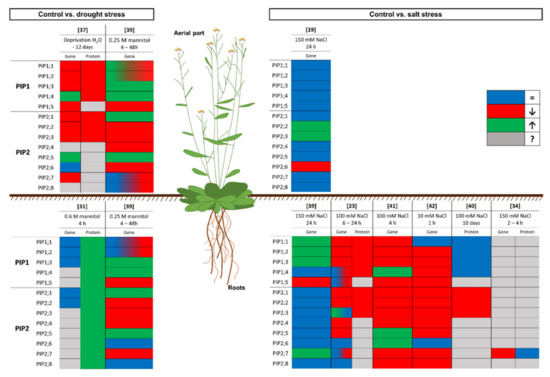
Graphical summary of the response of the model plant
to drought and salinity stress at the gene expression and protein levels. The box colour indicates the response of the plant compared to control conditions: the gene expression or protein amount does not change (blue), decreases (red), or increases (green); or unknown data (grey). Numbers in brackets refer to the related bibliography.
In species other than A. thaliana, there have been numerous studies where dissimilarity between the changes in mRNA and those in the protein levels for PIP aquaporins was reported. In Zea mays under drought stress, lasting 4 days (short-term drought) or 12 days (sustained drought), discrepancies between the mRNA and protein levels were reported [33]. In fact, ZmPIP1;2 and ZmPIP2;4 expression decreased after 4 days but the ZmPIP1;2 and ZmPIP2;4 protein levels were not reduced. However, a decline in the protein amount was detected after 12 days. Therefore, there was a correlation but only in the long term. On the other hand, this correlation was not found for ZmPIP2;1 and ZmPIP2;2 because the expression level was reduced after 4 days, but the protein level did not change after 4 or 12 days. This could be related to regulation at the functional activity level, through gating regulation to decrease water permeability, before the reduction of the level of expression was effective at the cellular level.
Suga et al. [32], in an experiment conducted with radish under drought (0.3 M mannitol for 6 h) and salinity (150 mM NaCl for 6 h), showed some discrepancies between the PIP mRNA and PIP protein levels. RsPIP2;1 increased 1 h after the mannitol treatment and then decreased gradually; however, this increase was not reflected in the amount of RsPIP2;1 protein, which gradually decreased after the treatment. At first glance, this result could be explained by a low translation rate and/or a rapid turnover of PM or protein degradation. However, the same work showed a different behaviour of RsPIP2;1 in radish under short-term (6 h) salt stress, since the expression of RsPIP2;1 did not change, but the protein amount increased [32]. This pattern is similar to that reported by Kammerloher et al. [31] [31] and described above for A. thaliana under drought stress; the mRNA levels did not change, but the protein increased. This could be due to accelerated trafficking, to redistribute aquaporins from reservoirs located in other organelles to the PM without the need to increase gene expression. For the same isoform (HvPIP2;1) in barley subjected to salt stress (150 mM NaCl) for 2 days, its expression decreased and was not correlated with the amount of protein, since that did not change [35]. The results reported in these studies show a regulation of aquaporins only at the protein level, since the increase in the short term was not due to mRNA translation and could have been due to post-translational modification or a redistribution of aquaporins in the cells. On the other hand, in the longer term, the mRNA level decreased, possibly due to inhibition by the redistributed aquaporins.
Additionally, other kinds of discrepancies have been reported. Muries et al. [24] [24] showed that, in broccoli plants grown with 80 mM NaCl for 15 days, the expression levels of PIP1 and PIP2 decreased while the amounts of PIP1 and PIP2 protein in the PM increased. Furthermore, this increase in the protein amount was reported previously by López-Pérez et al. [6]. Although, in this study, some information could have been masked due to the use of nonspecific primers and antibodies, the explanation could be that the accumulation of the encoded protein inhibited the mRNA synthesis [45]. Furthermore, the importance of the stress intensity in the appearance or not of discrepancies between mRNA and protein levels was patent in work carried out in barley with HvPIP2;1 [35,36][35][36]. In this work, 150 mM and 200 mM NaCl applied for 2 days decreased the HvPIP2;1 expression, but the protein amount only decreased with the higher concentration of NaCl.
References
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358.
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618.
- Santoni, V. Plant Aquaporin Posttranslational Regulation. In Plant Aquaporins: From Transport to Signaling; Chaumont, F., Tyerman, S.D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 83–105. ISBN 978-3-319-49395-4.
- Preston, G.M.; Agre, P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114.
- Karlsson, M.; Fotiadis, D.; Sjövall, S.; Johansson, I.; Hedfalk, K.; Engel, A.; Kjellbom, P. Reconstitution of water channel function of an aquaporin overexpressed and purified from Pichia pastoris. FEBS Lett. 2003, 537, 68–72.
- López-Pérez, L.; Martínez-Ballesta, M.d.C.; Maurel, C.; Carvajal, M. Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochemistry 2009, 70, 492–500.
- Jensen, M.; Mouritsen, O.G. Lipids do influence protein function-The hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta-Biomembr. 2004, 1666, 205–226.
- Calanca, P.P. Effects of Abiotic Stress in Crop Production. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2017; pp. 165–180.
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38.
- Dresselhaus, T.; Hückelhoven, R. Biotic and abiotic stress responses in crop plants. Agronomy 2018, 8, 267.
- Lisar, S.Y.S.; Motafakkerazad, R.; Hossain, M.M.; Rahm, I.M.M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; InTech: Rijeka, Croatia, 2012.
- Mcelrone, A.J.; Choat, B.; Gambetta, G.A.; Brodersen, C.R. Water Uptake and Transport in Vascular Plants. Nat. Educ. Knowl. 2013, 4, 6.
- Pawłowicz, I.; Masajada, K. Aquaporins as a link between water relations and photosynthetic pathway in abiotic stress tolerance in plants. Gene 2019, 687, 166–172.
- Pawłowicz, I.; Rapacz, M.; Perlikowski, D.; Gondek, K.; Kosmala, A. Abiotic stresses influence the transcript abundance of PIP and TIP aquaporins in Festuca species. J. Appl. Genet. 2017, 58, 421–435.
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51.
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9.
- Wallace, I.S.; Choi, W.G.; Roberts, D.M. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 1165–1175.
- López-Berenguer, C.; García-Viguera, C.; Carvajal, M. Are root hydraulic conductivity responses to salinity controlled by aquaporins in broccoli plants? Plant Soil 2006, 279, 13–23.
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254.
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587.
- Maurel, C. Plant aquaporins: Novel functions and regulation properties. FEBS Lett. 2007, 581, 2227–2236.
- Forrest, K.L.; Bhave, M. Major intrinsic proteins (MIPs) in plants: A complex gene family with major impacts on plant phenotype. Funct. Integr. Genom. 2007, 7, 263–289.
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; Van Den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805.
- Muries, B.; Mohamed, F.; Carvajal, M.; Martínez-Ballesta, M.C. Identification and differential induction of the expression of aquaporins by salinity in broccoli plants. Mol. Biosyst. 2011, 7, 1322–1335.
- Patankar, H.V.; Al-Harrasi, I.; Al-Yahyai, R.; Yaish, M.W. Functional characterization of date palm aquaporin gene pdpip1;2 confers drought and salinity tolerance to yeast and arabidopsis. Genes 2019, 10, 390.
- Xu, Y.; Hu, W.; Liu, J.; Song, S.; Hou, X.; Jia, C.; Li, J.; Miao, H.; Wang, Z.; Tie, W.; et al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. Biochem. 2020, 147, 66–76.
- Wang, X.; Gao, F.; Bing, J.; Sun, W.; Feng, X.; Ma, X.; Zhou, Y.; Zhang, G. Overexpression of the Jojoba aquaporin gene, ScPIP1, enhances drought and salt tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 153.
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and Root Water Uptake. In Plant Aquaporins; Springer: Cham, Switzerland, 2017; pp. 133–153.
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460.
- Martins, C.; Pedrosa, A.M.; Du, D.; Gonçalves, L.P.; Yu, Q.; Gmitter, F.G.; Costa, M.G.C. Genome-wide characterization and expression analysis of major intrinsic proteins during abiotic and biotic stresses in sweet orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0138786.
- Kammerloher, W.; Fischer, U.; Piechottka, G.P.; Schäffner, A.R. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 1994, 6, 187–199.
- Suga, S.; Komatsu, S.; Maeshima, M. Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol. 2002, 43, 1229–1237.
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New Insights into the Regulation of Aquaporins by the Arbuscular Mycorrhizal Symbiosis in Maize Plants under Drought Stress and Possible Implications for Plant Performance e-Xtra. Mol. Plant-Microbe Interact. 2014, 27, 349–363.
- Pou, A.; Jeanguenin, L.; Milhiet, T.; Batoko, H.; Chaumont, F.; Hachez, C. Salinity-mediated transcriptional and post-translational regulation of the Arabidopsis aquaporin PIP2;7. Plant Mol. Biol. 2016, 92, 731–744.
- Katsuhara, M.; Shibasaka, M. Barley root hydraulic conductivity and aquaporins expression in relation to salt tolerance. Soil Sci. Plant Nutr. 2007, 53, 466–470.
- Katsuhara, M.; Akiyama, Y.; Koshio, K.; Shibasaka, M.; Kasamo, K. Functional analysis of water channels in barley roots. Plant Cell Physiol. 2002, 43, 885–893.
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005, 59, 469–484.
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjövall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369.
- Jang, J.Y.; Kim, D.G.; Kim, Y.O.; Kim, J.S.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725.
- Martínez-Ballesta, M.; Moreno-Fernández, D.A.; Castejón, D.; Ochando, C.; Morandini, P.A.; Carvajal, M. The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 524.
- Sutka, M.; Li, G.; Boudet, J.; Boursiac, Y.; Doumas, P.; Maurel, C. Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 2011, 155, 1264–1276.
- Lee, S.H.; Zwiazek, J.J. Regulation of aquaporin-mediated water transport in arabidopsis roots exposed to NaCl. Plant Cell Physiol. 2015, 56, 750–758.
- Alexandersson, E.; Danielson, J.Å.H.; Råde, J.; Moparthi, V.K.; Fontes, M.; Kjellbom, P.; Johanson, U. Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J. 2010, 61, 650–660.
- Provart, N.J.; Alonso, J.; Assmann, S.M.; Bergmann, D.; Brady, S.M.; Brkljacic, J.; Browse, J.; Chapple, C.; Colot, V.; Cutler, S.; et al. 50 years of Arabidopsis research: Highlights and future directions. New Phytol. 2016, 209, 921–944.
- Staiger, D.; Zecca, L.; Wieczorek Kirk, D.A.; Apel, K.; Eckstein, L. The circadian clock regulated RNA-binding protein AtGRP7 autoregulates its expression by influencing alternative splicing of its own pre-mRNA. Plant J. 2003, 33, 361–371.
