Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Dipak Maity.
The introduction of cancer therapeutics and nanotechnology has resulted in a paradigm shift from conventional therapy to precision medicine. Nanotechnology, an interdisciplinary field with a focus on biomedical applications, holds immense promise in bringing about novel approaches for cancer detection, diagnosis, and therapy.
- nanoparticles
- quantum dots
- radioisotopes
- liposomes
- micelles
- nanobubbles
- theranostics
- therapeutics
1. Principle of Cancer Theranostics
Increased imaging capabilities and diagnostics have made it possible to identify cancers earlier and more thoroughly [30][1]. Recently, these methods have expanded into theranostics, in which the contrast agents that are typically used for imaging have been combined with therapeutics in order to diagnose and treat cancers simultaneously in a patient-specific manner [31][2].
Nanoparticles (NPs) play a wide range of roles in cancer diagnosis and have a number of advantages over other traditional methods of delivering chemotherapeutic drugs. Delivering drugs to the target tissue, or cells, or organs is more precise as well as effective with NPs, and the risk of side effects is reduced. With less drug removal from the system, NPs deliver drugs to tumor areas in passive and active modes [32][3]. The biodistribution of chemotherapy drugs in the body as well as the effectiveness of nanocarriers are significantly influenced by the size and surface properties of nanoparticles [33][4].
Phospholipids/surfactants are the primary constituents of self-assembled vesicular nanoparticles (NPs), which show promising potential for nanomedicine as novel biomedical nanocarriers. The two most common vesicle subcategories in recent decades, liposomes and noisome, have been utilized extensively in the treatment of cancer [34,35][5][6]. NPs can be administered into the body by several pathways having its own benefits.
2. Application of Cancer Theranostics
2.1. Quantum Dots
In anticancer studies, QDs are predominantly utilized for imaging purposes in conjunction with various nanoparticles to observe drug targeting and release. While QDs have been found to enhance drug activity in certain cases, there is limited research exploring their synergy with anticancer phytochemicals. It is important to note that QDs can exhibit cytotoxic effects, although coating the core can mitigate these toxicities. Nevertheless, the presence of heavy metals in QDs remains a health concern. On the other hand, carbon dots (CQDs) derived from natural sources offer a promising alternative as quasi-spherical particles with reduced health risks. These carbon dots possess optical properties and, if prepared from a natural product exhibiting anticancer activity, can independently elicit improved anticancer effects. Additionally, such carbon dots have demonstrated specificity towards cancer cells. To overcome the challenges associated with CQDs in drug delivery systems, comprehensive in vivo studies are needed, and the specific targeting can be achieved by attaching receptor ligands to the CQDs. Small semiconducting particles or nanocrystals known as QDs have extremely small diameters. They were initially found in 1980. The unbelievably high surface-to-volume ratios of these dots contribute to their unusual electronic properties, which are intermediate between those of discrete molecules and bulk semiconductors [45,46][7][8]. Fluorescence, where the nanocrystals can produce distinct colors depending on the size of the particles, is the most noticeable effect of this. The quantum dot is a type of nanoparticle with dimensions between tens of nanometers and a few hundredths of a nanometer, which are smaller than those of a typical nanoparticle. The tumor dot can be used in multiple fields, including solar cells, light-producing diodes, lasers, and biomedical applications because the quantum mechanical behavior associated with it exhibits various optical and electronic properties [47][9].
2. 2. Liposomes
By increasing drug efficacy and enhancing cell type-specific delivery, liposomes have amazing benefits that could strengthen immune responses and cancer immunotherapy [66][10]. Liposomes are lipid-based nanoparticles that have a high potential for enhancing cancer immunotherapies because they can incorporate and/or associate a variety of cancer drug molecules [67][11]. The preparation of liposomes typically involves four fundamental stages. First, lipids are dried down from an organic solvent. Next, the dried lipids are dispersed in an aqueous medium. Subsequently, the resulting liposome formulation undergoes a purification process. Finally, the final product is analyzed to ensure its quality and characteristics. These four stages are commonly employed in various methods of liposome preparation. One commonly employed technique is the ether injection (solvent vaporization) method. In this approach, a solution of lipids dissolved in diethyl ether or an ether–methanol mixture is slowly injected into an aqueous solution containing the material to be encapsulated. This process typically takes place at temperatures ranging from 55 °C to 65 °C or under reduced pressure. As the ether is subsequently removed under vacuum, liposomes are formed. However, this method has some drawbacks, including the heterogeneity of the resulting population of liposomes (ranging from 70 to 200 nm) and the exposure of the compounds to be encapsulated to organic solvents at elevated temperatures. Liposomes can be used for a variety of immunotherapeutic cancer treatments, such as vaccination or checkpoint blockade, making them very flexible. Low-molecular-weight anticancer drugs, immunomodulators, and antigens are frequently delivered using these mechanisms [68,69][12][13]. Anticancer agents have been formulated as liposomal formulations to increase antitumor efficacy by rising deposition of tumor drug, reducing toxicity of drug by avoiding critical normal tissues, and extend drug circulating lifetime [70][14]. The clinical application of micro- and nanoparticulate formulations and their development, along with the combination of the novel agents with conventional medications and standard-of-care therapies, still presents challenges in spite of clinical approval of chemotherapeutics which are multiple liposome-based [71][15]. Control over drug biodistribution, encapsulated drug release rates, and target cell uptake are all factors that need to be optimized. Several liposome-based goods have been authorized or put on the market. To improve liposomes’ therapeutic indices for applications in oncology, antineoplastic agent classes have been added. Anthracyclines, analogues of platinum, camptothecins, vinca alkaloid, and antimetabolite are a few of them. Many liposomal anticancer medications are either clinically approved or in late-stage clinical development [72,73,74,75][16][17][18][19].2.3. Extracellular Vesicles and Exosomes
Extracellular vesicles (EVs) are small biological particles that consist of exosomes, microvesicles, and various other types of nanoscopic vesicles. They are naturally produced by many different cell types and are believed to play important roles in intercellular communication. Recently, researchers have started exploring the potential of using EVs as vehicles for drug delivery. Initial findings suggest that EVs may offer several advantages over synthetic nanoparticle delivery systems for specific applications. They have emerged as a promising cell-free approach for the treatment of various diseases, including cancer. With their inherent ability to facilitate cell-to-cell communication, along with their remarkable physiochemical stability and biocompatibility, EVs have gained recognition as exceptional carriers for delivering therapeutic agents such as nucleic acids, proteins, drugs, and nanomaterials. Ongoing research indicates that EVs can be modified, engineered, or purposefully designed to enhance their effectiveness, specificity, and safety in cancer therapy [81][20]. Furthermore, the presence of heterogeneity and complex components within EVs poses challenges to their therapeutic efficacy and raises safety concerns when used as carriers for therapeutic cargos. Additionally, the lack of efficient methods for isolating and scaling up EV production, as well as accurately monitoring the dosage of therapeutic cargos within EVs, are potential issues in clinical applications. Consequently, engineered EVs have emerged as a promising alternative approach for EV-based therapy addressing these concerns [82][21]. Increasing evidence suggests that engineering EVs enhances their loading efficiency, targeting capability, and therapeutic effectiveness [83][22]. Currently, two primary strategies are employed to load desired cargos into EVs. One approach involves incorporating the cargo into producer cells, which then undergoes the natural biogenesis process to obtain cargo-loaded EVs. The other approach involves harvesting EVs from various sources (such as cultured cells, human blood, and milk) and introducing cargos into EVs using traditional and advanced biotechniques [84][23]. There is also a growing interest in modifying EV membranes to target specific tissues and combining EVs with other nanomaterials to achieve improved or synergistic therapeutic effects [85][24]. Extensive exploration has also been conducted to fabricate bio-inspired or biomimetic EVs with higher production yields and loading efficiencies [86][25]. Numerous studies have established a strong association between exosomes and the development and progression of cancers [87,88][26][27]. Within the TME, exosomes facilitate the transfer of bioactive molecules among tumor cells, immune cells, and stromal cells, enabling cancer cells to evade immune surveillance and induce immune tolerance. Conversely, exosomes originating from immune cells exhibit the ability to inhibit tumor growth, proliferation, and metastasis. Consequently, exosomes play a dual role as a double-edged sword in cancer immunity. The primary objective of cancer immunotherapy is to stimulate the immune-suppressed host to recognize and eliminate cancer cells. By modifying exosome-secreting cells, exosomes can be utilized as delivery systems for immunotherapy drugs, antigens, or genes [89][28]. Both immature and mature dendritic cells are capable of producing exosomes, and exosomes derived from dendritic cells (DEXs) have demonstrated the capacity to overcome tumor-induced immunosuppression through direct and indirect mechanisms.2.4. Polymeric Nanoparticles
Polymeric nanoparticles (NPs) have a significant role in the precise delivery of cancer drugs. Polymeric nanocarriers, also referred to as polymeric nanomedicine, can attach or enclose anticancer drugs. The use of polymeric nanomedicine has demonstrated promising results by offering controlled drug release with decreased toxicity and improved tumor retention. However, there is still a scarcity of drug-loaded delivery systems that meet the requirements of clinical applications. Therefore, additional endeavors are necessary to address this gap [98][29]. They can be synthesized in two main ways: (I) The dialysis technique is a straightforward and efficient method used to prepare polymeric nanoparticles with a narrow size distribution. To summarize the process, both the drug and polymers are dissolved in water-miscible organic solvents of appropriate molecular weight cut-off and placed inside a dialysis tube or membrane. The organic phase gradually diffuses out through the dialysis tube or membrane into the aqueous phase, reducing the interfacial tension between the two. As a result, the solvent displacement within the membrane leads to the formation of a homogeneous nanoparticle suspension, followed by progressive polymer aggregation due to reduced solubility. To obtain a fine powder of nanoparticles, the resulting nanosuspension is freeze-dried using 5% mannitol as a cryoprotectant. (II) Polymers play a crucial role in the development of oral delivery systems. The utilization of natural polymers, such as chitosan and alginates, instead of toxic chemical polymers can enhance the drug’s permeation effect, enzyme inhibitory ability, and mucoadhesive properties. In brief, the drug and polymer are dissolved in a weak acidic medium or water, depending on their solubility. The resulting solution is then added drop by drop, with constant stirring, to a solution containing counter ions and a stabilizer. This process leads to the formation of spherical particles through the complexation of oppositely charged species, resulting in gelation and precipitation. To reduce the particle size to the nanometric range, the resulting solution is sonicated. The nanosuspension obtained is subsequently freeze-dried, using 5% mannitol as a cryoprotectant, to yield a fine powder of nanoparticles [99][30]. Currently, drug-loaded NPs have emerged as a promising approach for effective cancer therapy, offering improved drug delivery and therapeutic outcomes across different cancer types [100][31]. These polymeric nanocarriers, known as polymeric nanomedicines, exhibit diverse architectures such as polymer-drug conjugates, micelles, nanospheres, nanogels, vesicles, and dendrimers [101,102][32][33]. They have garnered significant attention for their ability to simultaneously deliver multiple therapeutic agents and target tumor cells [16][34]. A wide range of therapeutics, including cytotoxic agents, small interfering RNA (siRNA), chemosensitizers, and antiangiogenic agents, can be transported by these nanoparticle platforms [103,104][35][36].2.5. Radioisotopes
A radioisotope is an atom that is unique in that it has the ability to emit radiation. As a result of internal changes in unstable or radioactive atoms, radiations are waves or particles that are sent out in all directions [112][37]. For the large-scale development, high-current medical systems producing enhanced yields of radioisotopes, experimental thick target yields and functions of excitation—i.e., cross sections of isotope production that depend on energy—are crucial [113,114][38][39]. In modern medicine, accelerator-produced radioisotopes are frequently used for imaging, cancer therapy, and therapy and diagnostic combination (theranostics) [115][40]. Several radioisotope-based treatments are in advanced stages of clinical trials, which may pave the way for a strong demand for specific accelerator systems devoted to radioisotope production [116][41].2.6. Micelles
Polymeric micelles have emerged as a popular option for delivering chemotherapeutic agents that are difficult to dissolve for cancer patients in pre-clinical studies [120][42]. These micelles are formed by the self-assembly of amphiphilic polymers and can be customized with different polymeric combinations to achieve optimal loading, stability, systemic circulation, and delivery to the target cancer tissues as demonstrated below in Figure 1. Moreover, the surface of the nanocarriers can be modified with additional ligands to facilitate active cancer cell targeting, internalization, and organelle-specific drug targeting. Polymeric micelles offer several advantages over other types of nanoparticles. They are formed by amphiphilic polymers that self-assemble in an aqueous environment, and different polymeric building blocks can be used to achieve the desired hydrophobic/lipophilic balance, size, drug loading capacity, micellization ability, and stability in the systemic circulation [121,122,123][43][44][45].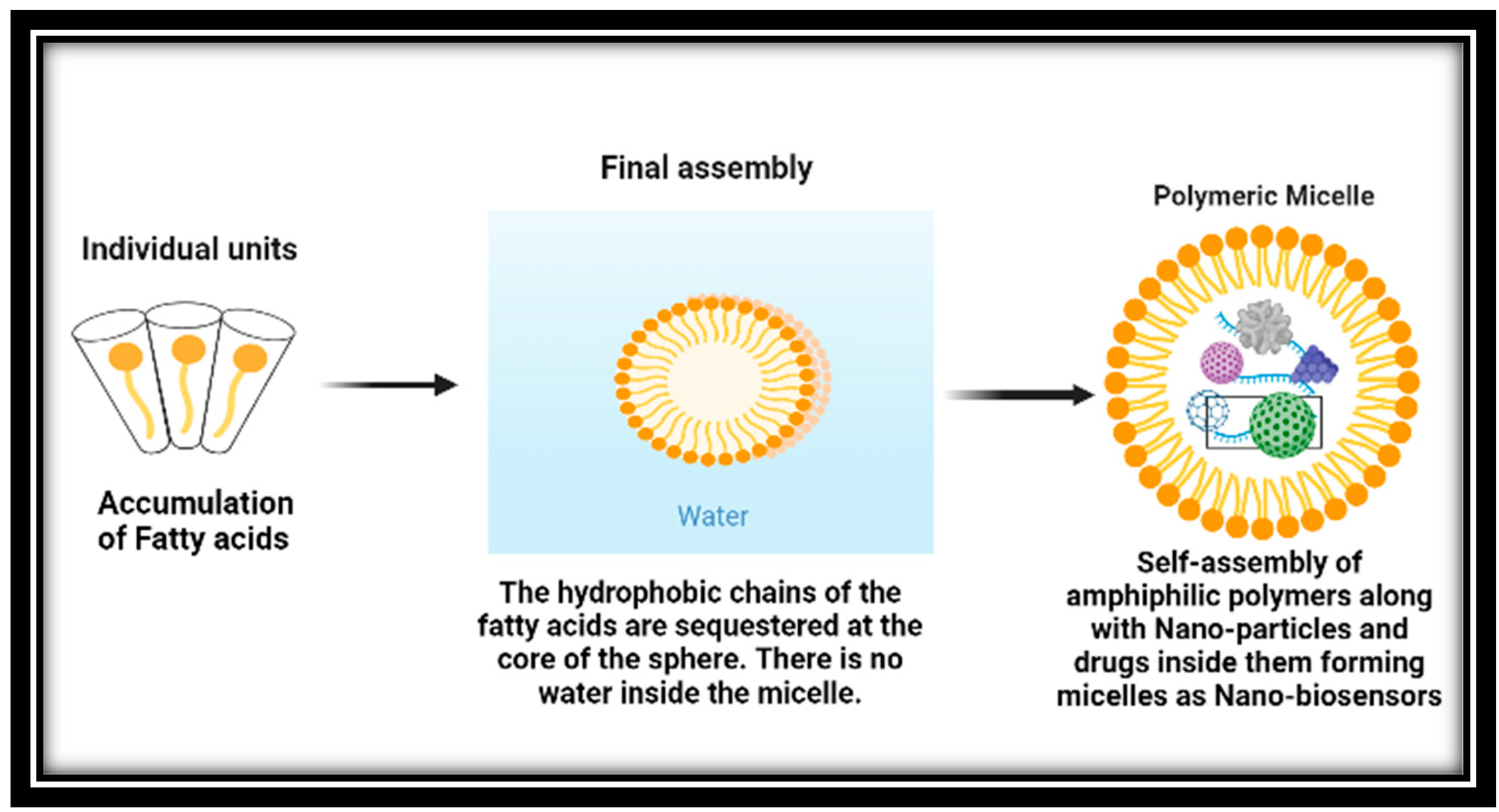
Figure 1.
Compared to other drug delivery methods, micelles have an advantage due to their small size, which enables them to extravasate more effectively through leaky vasculature. The hydrophilic polymeric coating on their surface also enables them to evade detection by the reticuloendothelial system during circulation [124][46]. Furthermore, micelles can be directed towards the tumor site by chemically conjugating tumor-homing ligands onto their surface [125][47].
Micelles are nanoparticles that self-assemble when amphiphilic block copolymers are dissolved in specific solvents at concentrations above the critical micelle concentration (CMC). In recent years, nanosized delivery systems like micelles have gained attention due to their improved in vivo stability, ability to protect entrapped drugs, release kinetics, ease of cellular penetration, and increased therapeutic efficacy [126][48].
The figure shows the formation of polymeric micelles used in cancer therapy.
2.7. Nanobubbles
Nanobubbles, which are gas-filled cavities in aqueous solutions, have distinctive features attributed to their low internal pressure and surface tension resulting from the charged gas/liquid interface [132][49]. To induce cell death in the tumor lymph node by delivering particles, DOX medications are loaded into magnetic Poly (lactic-co-glycolic acid) PLGA microbubbles that contain perfluorocarbon gas. The results indicate that this approach can be utilized to improve lymphatic targeting and mitigate tumor metastasis [133][50].2.8. Diagnosis Using Cancer Theranostics
Nanoparticle-based therapy has been suggested as a potential solution for overcoming multiple drug resistance in various types of cancer, including breast cancer, ovarian cancer, and prostate cancer [140][51]. The intersection of nanotechnology and medicine has opened up a new phase in cancer treatment, and further exploration of this field is needed. This revisewarch explores current challenges, future research directions, and the underlying principles of using the nanocarrier system in cancer therapy [141,142][52][53]. Nanotheranostics is seen as a promising strategy for combating cancer by slowing down cancer progression during the initial diagnostic procedure, reducing the overall cancer burden and making the ensuing anticancer therapy easier [143][54]. Developing a molecular therapy system that can circulate undetected in the bloodstream, identify the target, and efficiently deliver drugs or silence genes is a significant challenge. Nanotechnology is essential for creating new types of nanotherapeutics that can provide efficient treatments with minimal side effects and high specificity [144,145][55][56].References
- Kastner, M.A. Mesoscopic physics and artificial atoms. AIP Conf. Proc. 1993, 275, 573–586.
- Collier, C.P.; Vossmeyer, T.; Heath, J.R. Nanoparticles Superlattices. Anu. Rev. Phys. Phys. Chem. 1998, 49, 371.
- Tiwari, K.P.; Sahu, M.; Kumar, G.; Ashourian, M. Pivotal Role of Quantum Dots in the Advancement of Healthcare Research. Comput. Intell. Neurosci. 2021, 2021, 2096208.
- Malavika, J.P.; Shobana, C.; Sundarraj, S.; Ganeshbabu, M.; Kumar, P.; Selvan, R.K. Green synthesis of multifunctional carbon quantum dots: An approach in cancer theranostics. Biomater. Adv. 2022, 136, 212756.
- Dhas, N.; Pastagia, M.; Sharma, A.; Khera, A.; Kudarha, R.; Kulkarni, S.; Soman, S.; Mutalik, S.; Barnwal, R.P.; Singh, G.; et al. Organic quantum dots: An ultrasmall nanoplatform for cancer theranostics. J. Control. Release 2022, 348, 798–824.
- Shen, C.L.; Liu, H.R.; Lou, Q.; Wang, F.; Liu, K.K.; Dong, L.; Shan, C.X. Recent progress of carbon dots in targeted bioimaging and cancer therapy. Theranostics 2022, 12, 2860.
- Krishna, R.; Webb, M.S.; Onge, G.S.; Mayer, L.D. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J. Pharmacol. Exp. Ther. 2001, 298, 1206–1212.
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-encapsulated vincristine, vinblastine and vinorelbine: A comparative study of drug loading and retention. J. Control. Release 2005, 104, 103–111.
- Young, R.C.; Ozols, R.F.; Myers, C.E. The anthracycline antineoplastic drugs. N. Engl. J. Med. 1981, 305, 139–153.
- Wande, D.P.; Trevaskis, N.; Farooq, M.A.; Jabeen, A.; Nayak, A.K. Theranostic nanostructures as nanomedicines: Benefits, costs, and future challenges. Des. Appl. Theranostic Nanomed. 2023, 1, 3–24.
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68.
- Yang, F.; Zhao, Z.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Nanotherapeutics for antimetastatic treatment. Trends Cancer 2020, 6, 645–659.
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics—Application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660.
- Sabir, F.; Asad, M.I.; Qindeel, M.; Afzal, I.; Dar, M.J.; Shah, K.U.; Zeb, A.; Khan, G.M.; Ahmed, N.; Din, F.U. Polymeric nanogels as versatile nanoplatforms for biomedical applications. J. Nanomater. 2019, 2019, 1526186.
- Shao, L.; Li, Q.; Zhao, C.; Lu, J.; Li, X.; Chen, L.; Deng, X.; Ge, G.; Wu, Y. Auto-fluorescent polymer nanotheranostics for self-monitoring of cancer therapy via triple-collaborative strategy. Biomaterials 2019, 194, 105–116.
- Panigrahi, B.K.; Nayak, A.K. Carbon nanotubes: An emerging drug delivery carrier in cancer therapeutics. Curr. Drug Deliv. 2020, 17, 558–576.
- Costa, P.M.; Wang, J.T.W.; Morfin, J.F.; Khanum, T.; To, W.; Sosabowski, J.; Tóth, E.; Al-Jamal, K.T. Functionalised carbon nanotubes enhance brain delivery of amyloid-targeting Pittsburgh compound B (PiB)-derived ligands. Nanotheranostics 2018, 2, 168.
- Wang, J.T.; Hodgins, N.O.; Maher, J.; Sosabowski, J.K.; Al-Jamal, K.T. Organ biodistribution of radiolabelled γδ T cells following liposomal alendronate administration in different mouse tumour models. Nanotheranostics 2020, 4, 71.
- Medalsy, I.; Dgany, O.; Sowwan, M.; Cohen, H.; Yukashevska, A.; Wolf, S.G.; Wolf, A.; Koster, A.; Almog, O.; Marton, I.; et al. SP1 protein-based nanostructures and arrays. Nano Lett. 2008, 8, 473–477.
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156.
- Yong, T.; Wang, D.; Li, X.; Yan, Y.; Hu, J.; Gan, L.; Yang, X. Extracellular vesicles for tumor targeting delivery based on five features principle. J. Control. Release 2020, 322, 555–565.
- Yang, B.; Chen, Y.; Shi, J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv. Mater. 2019, 31, 1802896.
- Tran, P.H.; Xiang, D.; Tran, T.T.; Yin, W.; Zhang, Y.; Kong, L.; Chen, K.; Sun, M.; Li, Y.; Hou, Y.; et al. Exosomes and nanoengineering: A match made for precision therapeutics. Adv. Mater. 2020, 32, 1904040.
- Lu, M.; Huang, Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925.
- LeBleu, V.S.; Kalluri, R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer 2020, 6, 767–774.
- Kim, H.; Kim, D.W.; Cho, J.Y. Exploring the key communicator role of exosomes in cancer microenvironment through proteomics. Proteome Sci. 2019, 17, 5.
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines 2018, 6, 69.
- Wang, Y.; Xu, F.; Zhong, J.Y.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Yuan, L.Q. Exosomes as mediators of cell-to-cell communication in thyroid disease. Int. J. Endocrinol. 2020, 2020, 4378345.
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle technologies for cancer therapy. Drug Deliv. 2010, 55–86.
- Liu, Y.; Feng, L.; Liu, T.; Zhang, L.; Yao, Y.; Yu, D.; Wang, L.; Zhang, N. Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 2014, 6, 3231–3242.
- Liu, J.; Xu, M.; Yuan, Z. Immunoscore guided cold tumors to acquire “temperature” through integrating physicochemical and biological methods. BIO Integr. 2020, 1, 6–14.
- Hu, C.M.J.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334.
- Liao, L.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A convergent synthetic platform for single-nanoparticle combination cancer therapy: Ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899.
- Siddique, S.; Chow, J.C. Recent advances in functionalized nanoparticles in cancer theranostics. Nanomaterials 2022, 12, 2826.
- Lammers, T.; Ferrari, M. The success of nanomedicine. Nano Today 2020, 31, 100853.
- Li, X.; He, G.; Jin, H.; Tao, J.; Li, X.; Zhai, C.; Luo, Y.; Liu, X. Dual-therapeutics-loaded mesoporous silica nanoparticles applied for breast tumor therapy. ACS Appl. Mater. Interfaces 2019, 11, 46497–46503.
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 2021, 26, 6389.
- Kargozar, S.; Mollazadeh, S.; Kermani, F.; Webster, T.J.; Nazarnezhad, S.; Hamzehlou, S.; Baino, F. Hydroxyapatite nanoparticles for improved cancer theranostics. J. Funct. Biomater. 2022, 13, 100.
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-black-phosphorus-reinforced 3D-printed scaffolds: A stepwise countermeasure for osteosarcoma. Adv. Mater. 2018, 30, 1705611.
- Wang, Z.; Zhao, J.; Tang, W.; Hu, L.; Chen, X.; Su, Y.; Zou, C.; Wang, J.; Lu, W.W.; Zhen, W.; et al. Multifunctional nanoengineered hydrogels consisting of black phosphorus nanosheets upregulate bone formation. Small 2019, 15, 1901560.
- Geng, S.; Pan, T.; Zhou, W.; Cui, H.; Wu, L.; Li, Z.; Chu, P.K.; Yu, X.F. Bioactive phospho-therapy with black phosphorus for in vivo tumor suppression. Theranostics 2020, 10, 4720.
- Li, Y.; Feng, P.; Wang, C.; Miao, W.; Huang, H. Black phosphorus nanophototherapeutics with enhanced stability and safety for breast cancer treatment. Chem. Eng. J. 2020, 400, 125851.
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black phosphorus nanosheets immobilizing Ce6 for imaging-guided photothermal/photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440.
- Stern, S.T.; Hall, J.B.; Lee, L.Y.; Wood, L.J.; Paciotti, G.F.; Tamarkin, L.; Long, S.E.; McNeil, S.E. Translational considerations for cancer nanomedicine. J. Control. Release 2010, 146, 164–174.
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147.
- Jain, S.; Hirst, D.G.; O’Sullivan, J. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113.
- Staves, B. Pilot Study of AurolaseTM Therapy in Refractory and/or Recurrent Tumors of the Head and Neck,. 2010. ClinicalTrials.gov, Identifier: NCT00848042. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00848042 (accessed on 17 May 2023).
- El-Sayed, I.H. Nanotechnology in head and neck cancer: The race is on. Curr. Oncol. Rep. 2010, 12, 121–128.
- Moodley, T.; Singh, M. Current stimuli-responsive mesoporous silica nanoparticles for cancer therapy. Pharmaceutics 2021, 13, 71.
- Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent progress in lipid nanoparticles for cancer theranostics: Opportunity and challenges. Pharmaceutics 2021, 13, 840.
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Hussain, H.; Ullah, S.; Assiri, M.A. Current and future perspectives of multifunctional magnetic nanoparticles based controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102946.
- Stanicki, D.; Vangijzegem, T.; Ternad, I.; Laurent, S. An update on the applications and characteristics of magnetic iron oxide nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2022, 19, 321–335.
- Endo-Takahashi, Y.; Negishi, Y. Gene and oligonucleotide delivery via micro-and nanobubbles by ultrasound exposure. Drug Metab. Pharmacokinet. 2022, 44, 100445.
- Bismuth, M.; Katz, S.; Mano, T.; Aronovich, R.; Hershkovitz, D.; Exner, A.A.; Ilovitsh, T. Low frequency nanobubble-enhanced ultrasound mechanotherapy for noninvasive cancer surgery. Nanoscale 2022, 14, 13614–13627.
- Ghafary, S.M.; Rahimjazi, E.; Hamzehil, H.; Mousavi, S.M.M.; Nikkhah, M.; Hosseinkhani, S. Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of quaorubicin/curcumin and graphene quantum dots. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102544.
- Alavi, M.; Webster, T.J.; Li, L. Theranostic safe quantum dots for anticancer and bioimaging applications. Micro Nano Bio Asp. 2022, 1, 1–11.
More
