Thermoelectric materials have gained wide attention to realize multilevel efficient energy management to alleviate the increasingly severe energy crisis. Oxide ceramics were well-explored as potential thermoelectric candidates because of their outstanding merits, including abundance, eco-friendliness, high-temperature stability, and chemical stability. AIn this work, we aim to provide a comprehensive summary of the diversified state-of-the-art oxide ceramics and establish the links between composition designing, preparation process, structural characteristics, and properties to summarize the underlying chemistry and physics mechanism of band engineering, doping, composited with the second phase, defects engineering, and entropy engineering is provided. Furthermore, advanced device design and applications such as thermoelectric modules, miniature generators, sensors, and coolers were summarizreviewed. Ultimately, the challenges and future perspective of oxides ceramics for the device design and thermoelectric applications in the development of energy harvesting technology have been prospected.
- thermoelectrics
- oxides ceramics
- ZT
- electrical conductivity
- phonon scattering
1. Introduction
1. Introduction
Thermoelectric materials (TEs) have been used as a potential energy harvesting technology because they can convert heat into electricity and have no requirements for waste heat temperature [1][2][3][1,2,3]. Thermoelectric devices generally consist of n-type and p-type TEs wired electrically in series (or partly parallel) and thermally in parallel. Furthermore, there are also thermoelectric devices with single n-type and p-type TEs. They have the advantages of no moving parts, no noise, small size, etc., and have significant application merits in the military, aerospace, and high-tech energy fields [4][5][4,5].
It has been more than 200 years since the thermoelectric effect was discovered, and people have been constantly exploring and developing its industrial applications. In the early 1920s, Altenkirch, a German physicist, developed the fundamentals of thermoelectric power generation and refrigeration and summarized the performance evaluation parameters of TEs [6]: electrical conductivity (σ), Seebeck coefficient (S), and thermal conductivity (κ). Dimensionless thermoelectric merit (ZT = S2σT/κ, S is Seebeck coefficient, σ is electrical conductivity, T is absolute temperature, κ is thermal conductivity) is usually used as an indicator to measure the thermoelectric performance [7]. TEs with large ZT values must meet the requirements of a high Seebeck coefficient to ensure the generation of the obvious thermoelectric effect—high electrical conductivity leading to small Joule heat, large output power, as well as low thermal conductivity, are required to generate a substantial temperature difference. The above three thermoelectric parameters have a strong coupled relationship because they are dependent on the carrier concentration in a conflicting manner that restricts and influences each other, making how to optimize thermoelectric performance a huge challenge. Therefore, the coordinated regulation of S, σ, and κ to improve ZT has become the key point to realize the industrial application of thermoelectric materials.
2. Thermoelectric Fundamentals
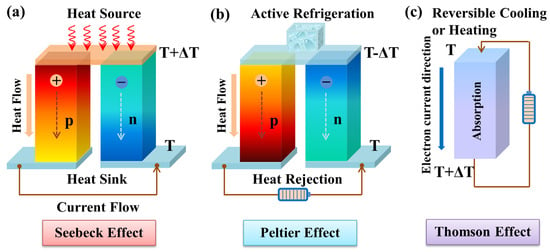
Figure 12. Schematic diagram of thermoelectric effects. (a) Seebeck effect for power generation, (b) Peltier effect for refrigeration, (c) Thomson effect for reversible cooling or heating.
3. Fabrication of Thermoelectric Oxide Ceramics
3.1. Lattice Structures of Thermoelectric Oxide Ceramics
3.1.1. n-Type Thermoelectric Oxides
3.1.2. p-Type Thermoelectric Oxides
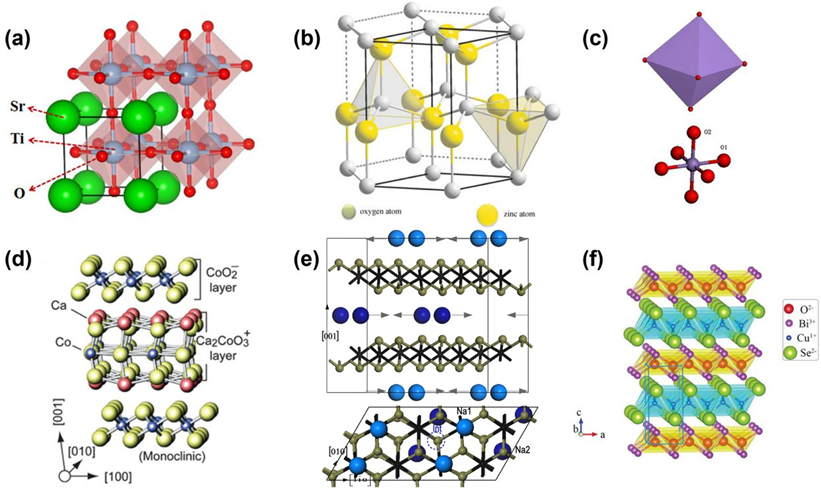
Figure 23. Schematic of the crystal structure of thermoelectric oxides. (a) SrTiO3, (b) ZnO [15][43], (c) CaMnO3 [16][29], (d) Ca3Co4O9 [17][9], (e) NaxCoO2 [18][16], (f) BiCuSeO [19][21].
3.2. Preparation Method
3.2.1. Bulk Crystal Growth
3.2.2. Solid-State Method
3.2.3. Spark Plasma Sintering
3.2.4. Mechanical Alloying
Mechanical alloying is a method to obtain solid alloy or solid solution by high-speed ball milling or grinding. Mechanical alloying has gained widespread attention for its advantages of a short cycle, low energy consumption, and simple process. Liu et al. studied the synthesis of La-doped SrTiO3 nanopowders using mechanical alloying in an air atmosphere. In comparison to the solid-phase reaction method, the mechanical alloying technique can eliminate the calcination stage and is more cost-effective and eco-friendly [39][76].3.2.5. Liquid-Phase Synthesis
Colloidal Synthesis
Solid-phase reaction method has the disadvantages of low purity and high energy consumption. To overcome the shortcoming of the high-temperature solid-phase method, the hydrothermal synthesis and sol-gel method became aroused researchers’ attention. The materials synthesized by the sol-gel method generally use inorganic salt or organic alkoxide as the precursor and, through a chemical reaction such as hydrolysis polymerization, nucleation, and growth transform from liquid precursors to sol and then to a network structure known as a ‘gel’, and then after dried and heating treatment to obtain the target material. Hydrothermal Synthesis The hydrothermal method is a solution reaction-based approach and can synthesize nanomaterials, which have been successfully applied for the preparation of TEs [40][78]. Usually, hydrothermal synthesis may occur over a wide temperature range from ambient temperature to extremely high temperatures and requires high-pressure conditions to generate high vapor pressures to trigger the chemical reaction or to control the morphology. Solvothermal Synthesis The solvothermal synthesis is analogous to the hydrothermal method and is based on heating the precursors and a solvent in a closed system at a temperature above the boiling point of the solvent used [27][60]. The synthetic procedure usually requires high temperature and high pressure to create the supercritical circumstances to develop the peculiar behavior of the solvent, exerting different influences on the precursors resulting in the desired product. Park et al. synthesized Ag-SrTiO3 nanocomposites by one-pot solvothermal method [41][81] using strontium nitrate (Sr(NO3)2), silver (I) nitrate (AgNO3), and titanium tetraisopropoxide [(CH3)2CHO]4Ti (TTIP) as starting ingredients. Furthermore, the loading quantity of Ag could be easily manipulated by adjusting the concentration of the AgNO3 precursor. In addition, the thermoelectric properties of layered TEs can be effectively controlled by bottom-up wet chemical synthesis of two-dimensional nanosheets/nanoplates [42][43][44][82,83,84].4. Development and Strategies to Improve the Thermoelectric Performance of Oxide Ceramics
Several of the selection rules are somewhat paradoxical as a result of the inherent trade-off effect between σ and S. Many optimized strategies may have a complicated correlated impact on S, σ, and κ. As an illustration, the increase of doping concentration will improve σ while decreasing S. Then, Ioffe’s finding in doped semiconductors was the first effort to empirically determine the carrier concentration “sweet spot” of excellent thermoelectrics is n = 1018–1020 cm−3 [45][86]. Consequently, an optimal power factor (PF = S2σ) versus doping concentration exists at a relatively high doping level. Furthermore, a further decrease of κ is necessary to produce a high ZT.
4.1. Band Engineering
Since S, σ, and κ of TEs are determined by the transport and interaction of carriers and phonons, adjusting one parameter often may sacrifice other physical parameters, making achieving collaborative optimization of thermoelectric performance a huge challenge. In bulk TEs, the band gap, the degeneracy of the conduction band, the extreme value of the valence band, and the effective mass in the band structure are fundamental parameters that determine the thermoelectric performance. According to the Mott formula [46][36], the parameter of S is in direct proportion to the slope of the energy band near the Fermi level. A greater slope means a greater S. Furthermore, the S is a function of the variation of σ near the Fermi surface, and increasing the complexity of the crystal structure can increase the complexity and the degeneracy of the band structure, leading to more extreme values in the conduction band and valence band, thus increasing S. In a word, factors of the band gap, energy band shape, effective mass of carriers, mobility, etc., determined by band structure are closely related and produce a trade-off effect on thermoelectric performa4.2. Doping
Element doping is one of the widely used basic strategies to regulate carrier concentration for enhancing the thermoelectric performance of TEs. By properly doping elements with different valence states into different cationic lattice sites, a well-regulated n can be obtained, which leads to an optimized S2σ. Doping will cause a change in cell parameters, induce point defects, and bring lattice distortion because of the position of atoms and the length of valence bonds changed, resulting in variations of S, κ, and σ. Furthermore, the length and strength of the valence bond is an important parameter that determines thermal conductivity. In n-type SrTiO3-based TEs, trivalent states of La [32][34][39][40][46][47][48][49][23,25,36,65,67,76,78,91], Ce [49][91], Nd [49][91], Sm [49][50][91,92], Gd [49][91], Dy [49][91], Pr [51][93], and Y [52][74] can be used to dope in Sr2+ site. The valence states of ≥5+, such as Ta [53][94], Nb [54][24], and W [55][95], can be used to dope in the Ti4+ site. K. Singsoog et al. [56][96] investigated the influence of La doping amount on Sr1-xLaxTiO3 (x = 0, 0.06, 0.13, 0.25) through theoretical calculation; the results show that the parameters (S, σ, κ, PF, and ZT) increase first and then decrease. When the doping concentration of La = 0.13, it has the best thermoelectric performance of S900K = −450 μ V/K, PF1200K = 2.55 mW/m/K2. In the n-type CaMnO3 system, three types of elements doped at the Ca position are rare earth elements (La, Y, Ce, Sm, Pu, Nd, Lu, etc.) [57][58][99,100], main group elements such as Bi [59][101] and alkaline earth elements (Ba, Sr, Mg, etc.) [60][102]. Additionally, the doped elements in the Mn site mainly include Ta, V, Nb, Ru, etc. [61][103]. The doping cases such as Ca1-xBixMnO3 (0 ≤ x ≤ 0.10) [59][101], CaMn1-xNbxO3 (x = 0.02, 0.05, 0.08) [62][104], Dy and Yb co-doping on Ca1-2xDyxYbxMnO3 (0 ≤ x ≤ 0.10) [63][105], and Ca0.92-xPr0.08SrxMnO3 (x = 0.01, 0.02, 0.03, 0.04) [64][106] demonstrated that Bi, Nb, Dy, Yb, Pr doping at CaMnO3 obtained the ZT values range of 0.18–0.27 which were much improved compared with the pure phase of CaMnO3 (ZT = 0.04). To improve the thermoelectric properties of ZnO, commonly used doping elements are C, Si, Fe, In, Ga, Al, Ni, and Sb [21][65][66][67][34,107,108,109]. Among them, Al-doped ZnO is the one widely studied and has the best TE performance at present. In the case of Zn0.96Al0.02Ga0.02O [65][107], ZT = 0.65 at 1247 K was obtained, which is one of the high levels of ZT values in n-type oxide thermoelectric materials. Additionally, this outstanding achievement can be attributed to the Al and Ga co-doping can significantly reduce the thermal conductivity from 40 W/m/K of Zn0.98Al0.02O to 5 W/m/K of Zn0.93Al0.02Ga0.05O. In the p-type Ca3Co4O9-based TEs, much research focused on the partial substitution of cations on the Ca-site such as Na [68][110], Sr [69][111], Ba [70][112], La [71][12], Ag [71][12], Fe [72][113], Pb [73][114], Ag [74][115], Ga [75][116], Tb [76][117], and Yb [77][118], while Ni [78][119], Mn [79][120], Cu [79][120], and Ir [80][121] were introduced into the Co-site to optimize the thermoelectric properties in the Ca3Co4O9 system. Specifically, as R3+ has a higher ox4.3. Entropy Engineering
Due to its superior characteristics, such as low thermal conductivity [26][28][81][82][83][59,61,134,135,136], high-entropy ceramics (HECs) containing five or more cations have lately gained considerable interest in the TE field. High-entropy engineering has been confirmed to increase the S and reduce the κl in TE oxide ceramics, making it a promising strategy for optimizing thermoelectric performance by broadening the composition design. High-throughput screening has been used to construct multi-component TEs with high entropy, as reported by Zhang et al. [84][137] and Liu et al. [85][138]. By decreasing the κl and/or increasing S, high-entropy engineering can become an efficient technique to considerably improve thermoelectric performance (Figure 3).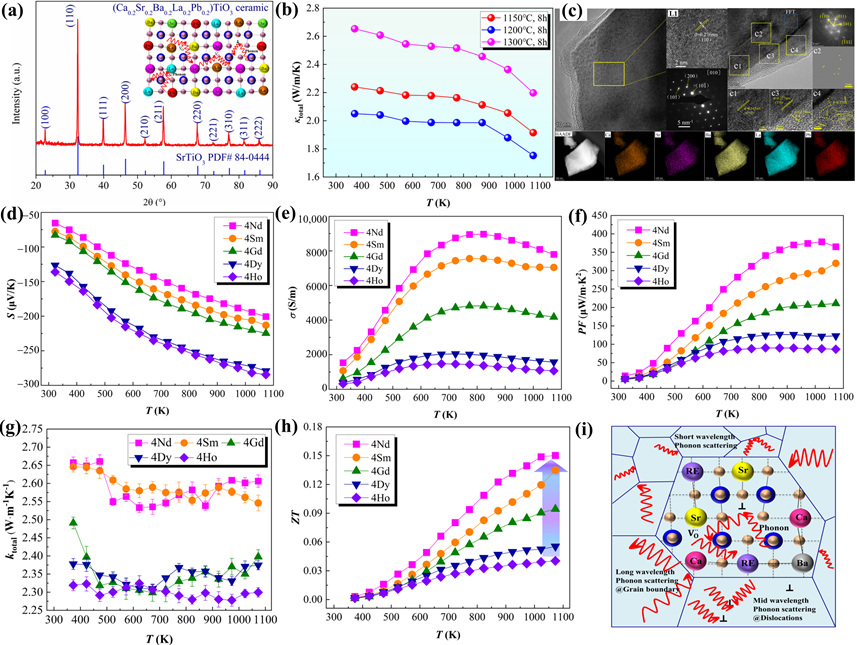
Figure 37. (a) XRD patterns, (b) κ, (c) TEM results of the high-entropy (Ca0.2Sr0.2Ba0.2La0.2Pb0.2)TiO3 ceramics [86][59]. (d–h) S, σ, PF, κtotal, and ZT values of the high-entropy (Sr0.2Ca0.255Ba0.25RE0.25)TiO3 ceramics [87][134]. (i) Schematic diagram of the possible phonon scattering in reducing lattice thermal conductivity for high-entropy ceramic [88][135].
4.4. Defect Engineering
In ionic compounds, the heat transport mechanism is dominated by κl. Additionally, κl can be lowered due to phonon scattering. Based on Debye–Callaway’s model and the relaxation-time approximation, the κ is a function of phonon scattering rates (1/τ) or the relaxation time (τ) [89][142]. τ has contributions from grain boundary scattering (τB), point defect scattering (τPD), phonon-phonon Umklapp scattering (τU), and electron-phonon scattering (τep) as represented by Equation (8) [89][90][142,143]. Here, vm is the mean acoustic velocity, L is the average grain size, ΘD is the Debye temperature, ω is the phonon frequency, and A, B, and C are parameters corresponding to the point defects, Umklapp process, and electron-phonon scattering, respectively. Phonons are more likely to be strongly scattered by lattice defects of considerable size. Therefore, during the defect regulation, attention should be paid to building a full-scale hierarchical structure containing defects of various forms and scales to achieve “total phonon scattering” in a broad frequency range. Specifically, the atomic scale point defects, such as vacancy, replacement atoms, etc., can realize effective phonon scattering by the high-frequency short wavelength [81][83] (Figure 4 and Figure 5)[134,136].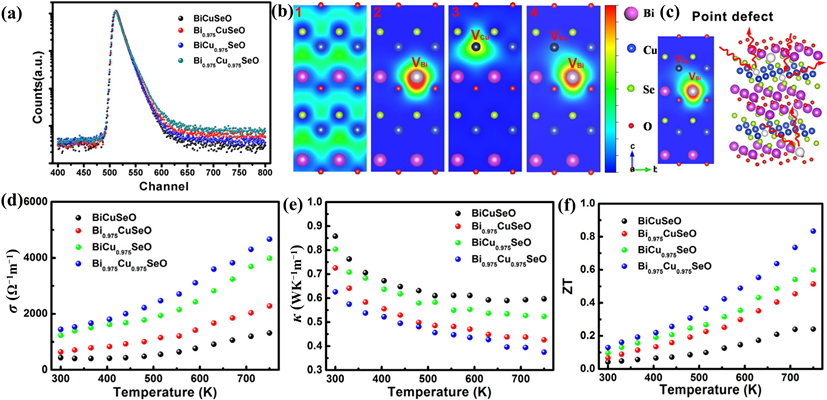
Figure 48. (a) Positron lifetime spectrum. (b) Schematic representation of trapped positrons for Bi1–xCu1–ySeO samples in (100) plane. (c) Schematic representation of phonon scattering with Bi/Cu vacancies. (d–f) σ, κ, and ZT values of the Bi1–xCu1–ySeO samples [91][148].
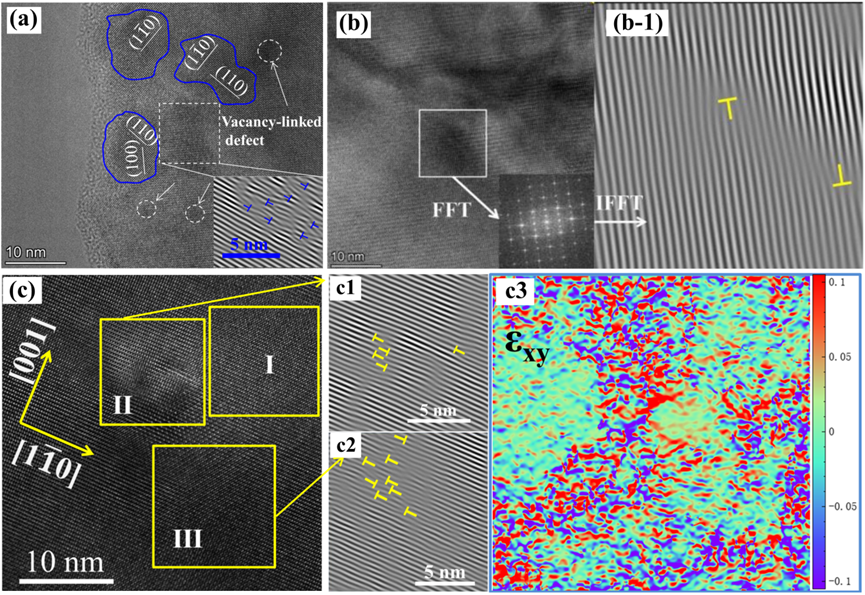
4.5. Grain Boundary and Nanostructure Engineering
Grain boundaries (GBs) form ubiquitous microstructures in polycrystalline oxide ceramics, which play a significant role in tuning their ZT. Different grain size has a varied impact on σ and κ. To ensure that the effect of GBs or/and the nanostructure on decreasing κ is larger than its deterioration in σ, the grain sizes should be appropriately tuned. The energy filtering mechanism was proposed, meaning that randomly distributed potential barriers can filter away low-energy carriers to cause a decrease in the actual carrier density to improve S. In the nanostructured polycrystalline ceramic TEs, low energy carrier filtering effects combined with the enhanced phonon scattering can effectively suppress κl [32][40][95][96][97][26,65,78,150,151], confirmed by both computational and experimental results. Optimized thermoelectric performance by grain boundary and nanostructure are mainly reflected in lowering κ [40][98][78,152], or the formation of a conductive path at grain boundary to promote the carrier mobility; these two factors play a synergistic effect on the electron and phonon transport process contribute to a trade-off ZT values of 0.3–0.4. In the cases of the nanostructured Sr0.91La0.09TiO3 sample, ZT = 0.37 was achieved at 973 K [99][153], a greatly improved performance ZT = 0.35 in Pr-doped of SrTiO3−δ because of the Pr-rich grain boundaries contributed to improving carrier mobility [99][100][153,154], and the improving ZT = 0.38 obtained in Sr0.8La0.067Ti0.8Nb0.2O3-δ system containing Cu or Fe inclusions [101][155].4.6. Textured Engineering
Texture engineering offers a strategy for gaining better thermoelectric performance in bulk ceramics by creating crystallographic anisotropy. Especially in layered crystal structure TEs, the electron and phonon transport properties are highly anisotropic. Multi-scale parallel interfaces, including zigzag interfaces and “core-shell” interfaces inside the orientation of the stripe-like grains, the paralleled grain boundaries, and lattice stacking faults were observed in the “brick-wall” microstructure can contribute to suppressing the thermal conductivity [71][102][12,156]. The physical properties of this special crystallographic orientation in textured ceramics can approach 60–80% of the properties of single crystals of the same composition. Creating the textured structure helps to the decoupling of electrical and thermal properties (Figure 6).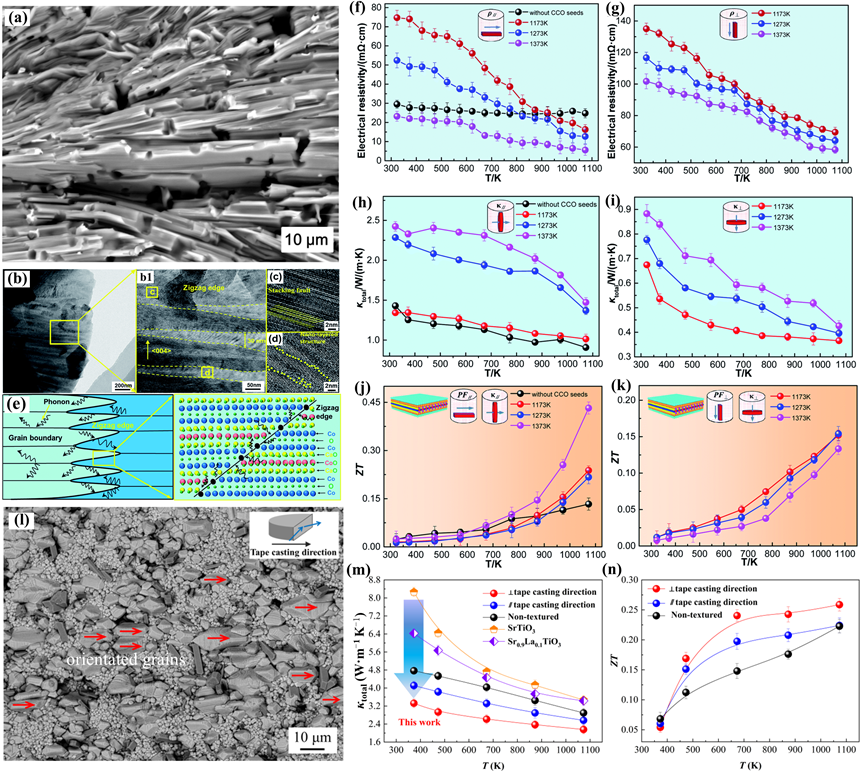
Figure 611. (a) BSE image, (b–d) TEM images, and (e) the sketch diagram of phonon scattering at the grain boundary and zigzag edge of the (Ca0.87Ag0.1La0.03)3Co4O9 textured ceramics, and temperature dependence of the thermoelectric properties of the samples in parallel and perpendicular to the tape-casting direction. (f,g) Electrical resistivity, (h,i) thermal conductivity, (j,k) ZT values [103][12]. (l) BSE image, (m) Total thermal conductivity, and (n) ZT values of the Sr0.9La0.1TiO3-based textured ceramic [104][156].
4.7. Composites
For the ionic compound of oxide ceramics, their resistivity after doping modification, nanostructuring, or entropy engineering is still relatively high. To further reduce the resistivity of oxide ceramics, some compounds with excellent electrical conductivity, such as metal particles, graphene, and its derivatives, are added to the matrix to improve the thermoelectric properties. In addition, it has been confirmed that the 2D graphene can be introduced into SrTiO3-based ceramics for grain refinement and promoting more oxygen vacancy formation to enhance carrier mobility [105][162]. Therefore, SrTiO3-based ceramics containing graphite or its derivatives achieve single-crystal-like electron mobility [105][106][107][108][162,163,164,165] because the delocalization of Anderson localized electrons aided by graphite leads to a manifold improvement in weighted mobility and further the enhanced electron transport properties.5. Device Applications
5.1. Power Generation
In recent years, thermoelectric modules have attracted wide attention due to their great potential for power generation and electronic refrigeration. At present, oxide thermoelectric devices can be used in a wide temperature range, especially in high temperatures, but there are few types of research focused on oxide thermoelectric devices, and the efficiency and power of thermoelectric conversion still need to be further improved. Most of these modules are made of Ca3Co4O9 as p-type materials, and the most common thermoelectric n-type oxides are CaMnO3, SrTiO3, ZnO, etc. [109][45]. TE modules usually adopt the silver bar and silver paste [110][170], Cu, Ni, etc., as electrode contacts.5.2. Sensor Devices
One of the primary applications of TEs is the temperature sensors, namely thermocouples, in which the temperature measurement of thin film thermocouples belongs to the contact in situ temperature measurement technology. For example, indium tin oxide (ITO) -In2O3 film thermocouple [111][173] have a limit measuring temperature reaching 1300 °C and the Seebeck coefficient reaches 160 μV/°C, the MoSi2-Al2O3 and TaSi2-Al2O3 thin film thermocouple [112][174] prepared by screen printing technology presented the thermoelectric output of 16 mV at the high-temperature stage.5.3. Flexibility and Wearable Devices
Flexible thermoelectric materials with excellent plastic deformation, light, inconspicuous, and other characteristics have a widespread application in portable electronic devices and wearable devices. Due to the ductility of inorganic semiconductors and ceramic insulators rarely observed, the application of inorganic oxides in flexible TEs field is rarely reported.5.4. Other Application
Other applications of thermoelectric modules include aerospace, automotive waste heat recovery systems, refrigeration, etc. At present, the application of alloy thermoelectric has been well-researched, while oxide needs to be further studied. Recently, organic-inorganic composites and hybrid materials have promoted a flexible TE devices’ design and exploration, which can be used in wearable electronic devices, and sensors to apply to the “Internet of Things” (IoT) field [113][177] with the spring-up and development of novel two-dimensional (2D) materials growth, superlattice growth, and inorganic-organic composites. Furthermore, using of high-performance nanostructured TEs combined with spectrally selective solar absorbers to develop solar thermoelectric generators (STEGs) obtained a peak efficiency of 4.6% [114][178], opening up the door to the comprehensive application of TEs for energy conversion.
