The idea of supporting the Sustainable Development Goals (SDGs) has inspired researchers around the world to explore more environmentally friendly energy generation and production methods, especially those related to solar and hydrogen energy. Among the various available sustainable energy technologies, photo(electro)catalytic hydrogen production has been competitively explored, benefiting from its versatile platform to utilize solar energy for green hydrogen production. Nevertheless, the bottleneck of this photo(electro)catalytic system lies within its high voltage required for water electrolysis (>1.23 V), which affects the economic prospects of this sustainable technology. In this regard, coupling the photo(electro)catalytic system with a solar-powered photovoltaic (PV) system (PV-PEC) to unleash the fascinating properties and readiness of this system has heightened attention among the scientific community.
- photovoltaic
- photo(electro)catalytic
- hydrogen
- solar
- renewable
1. Introduction
2. Basic Principle of the PV-PEC Integrated System
2.1. Basic Principle of the PV System
The concept of the photovoltaics (PV) system lies within the conversion of direct sunlight energy into electricity in the presence of the PV cell. The term “photovoltaic” stems from the word “photo” meaning light and “voltaic” meaning electricity [4,36][4][29]. When the cell is illuminated by light, the PV cell absorbs the photon generated from the sunlight which causes the electrons to be excited and move from the atoms of the cell, resulting in the creation of holes in the system. Typically, a single PV cell is only capable of producing around 0.5 V, whereas the current generation depends on several factors such as weather, the intensity of the sunlight, and the surface of the cell [37,38,39][30][31][32]. Thus, to enhance the capability of the PV cell, it is common to see that the PV cell is usually designed in a big module or panel in which the individual cell is either connected in a series arrangement or parallel arrangement. It is worth noting that different arrangements of the cell result in different enhancements.2.2. Basic Principle of the PEC System
The photoelectrochemical (PEC) system employs photoelectrodes, comprising semiconductor photocatalysts deposited on a substrate, usually fluorine tin oxide (FTO) or indium tin oxide (ITO). The PEC system requires a source of energy to initiate the water splitting process. The photoelectrode is typically composed of a photoactive semiconductor that separates photogenerated charge carriers through the space-charge field [19,24][19][24]. The minority of these carriers then migrate to the interface between the semiconductor and liquid for the reaction to take place.3. Types of Solar PV Technology
The development of solar PV technology has witnessed a surge of interest among the community and society as one of the most promising renewable energies. The use of solar PV technology to produce electricity has attracted multifarious attention across the globe due to its versatile offer which includes less reliance on fossil fuels, less emission of greenhouse gases, and better energy independence. As solar PV technology progressively becomes affordable, the yearning to cut the price for solar PV technology has seen significant efforts.3.1. First Generation of PV Technology
The first generation of solar PV cells that debuted into the market is made up of silicon-based cells. At present, more than 80% of the global installation of solar PV technology comes from this first generation of silicon-based PV technologies [16,17][16][17]. This is attributed to its mature efficiency that is suitable for market needs. Typically, the first generation of PV technology, which is also known as wafer-based technology, is composed of a thick crystalline layer of silicon either in the form of mono- or polycrystalline silicon. The advantages of this wafer-based PV technology are that the source of the material is abundantly available in the Earth’s crust and the non-toxic properties of the silicon allow delays in the contamination process and durability loss. In the case of monocrystalline silicon PV technology, the entire set of the PV cells is made up of a single crystal of silicon that was extracted from the sand. Nevertheless, the process of purifying the silicon from the unwanted impurities required a multi-step process of extraction, purification, and heating at high temperatures before the Czochralski process [41,42][33][34]. As per the brief description of the manufacturing process, the complicated manufacturing process of this monocrystalline silicon has greatly impacted the production cost of solar PV technology despite its advantages of high efficiency around 26–27% [2,8][2][8].3.2. Second Generation of PV Technology
The second generation of PV technology is known as thin film solar PV technology, which as the name says, is composed of either single or multiple thin layers of PV elements. These single or multiple layers are usually printed on a glass or a metal substrate depending on the desired thickness ranging from several nanometers to tens of micrometers. As such, the aforesaid second generation of PV technology has a good advantage in terms of a thinner PV cell in comparison to the first generation of solar PV technology. There are several types of the second generation of solar PV technologies that have been developed which include amorphous silicon PV, Copper indium gallium selenide PV, Gallium arsenide PV, Cadmium telluride PV, and Copper zinc tin sulfide PV systems [43,44,45][35][36][37]. On account of the second generation of PV technology, amorphous silicon PV systems can be considered one of the most matured thin-film PV technologies in this generation. In the amorphous silicon PV system, the structure is composed of either a p-i-n or n-i-p type junction in which each p-layer and n-layer are responsible for creating an internal electric field (i-layer). The amorphous silicon PV system is also known to have a high absorption capacity which ranges from 1.1 to 1.7 eV. This absorption capacity range is higher than the first generation of solar PV technology which only accounted for up to 1.1 eV [42,46][34][38]. It is worth noting that the amorphous silicon is composed of a low structural homogeneity which impacts the electron and hole movement and separation, consequently affecting the absorption capacity. In addition, the unsaturated silicon atoms in the amorphous silicon PV system are observed to have structural changes at different light intensity exposures. Thus, overcoming this limitation in amorphous silicon PV systems has been of great interest to the scientific community. Among the recent approaches to mitigate this issue is forming a heterojunction of amorphous silicon with crystalline silicon, commonly referred to as HJT cells. On the other hand, gallium arsenide (GaAs) and cadmium telluride (CdTe) PV systems are other examples of the second generation of PV technologies. Typically, the GaAs PV system is used in space applications owing to its promising properties for having strong resistance to the radiation source in space. Meanwhile, the CdTe PV system has been considered as one of the promising PV absorbing materials concerning its bandgap energy of 1.4 eV. The promising bandgap energy of the CdTe PV system allows the system to be capable of absorbing a wide range of light intensities, which later has urged the scientific community to escalate the maturity of this type of PV technology.3.3. Third Generation of PV Technology
The third generation of PV technology can be classified as a frontier and progressive solution developed by the scientific community to further heighten the efficiency and versatility of solar PV systems in comparison with the second generation of PV technologies that has been studied since the late 1970s [11,17][11][17]. Among the current third generation PV technologies that received significant attention and have been progressively optimized are organic PV, dye-sensitized solar cells (DSSCs), quantum dots PV, and perovskite PV systems. The emergence of this latest generation of PV technology aimed to alleviate the inherent limitations possessed by the previous generation of PV technology such as the high manufacturing cost, sluggish solar conversion efficiency, and limited applicability in certain environments. Due to the aforementioned virtues of this third generation of PV technology, the scientific community has engaged in utilizing novel materials that can be used for capturing a broader range of solar radiation such as organic dyes, quantum dots, and perovskite-based materials in comparison to the old generation of PV technology that focused on silicon-based materials. In this approach, the selection of materials plays an important role in improving the light absorption capacity and thus improving the overall efficiency of the solar PV system and ultimately reducing the cost per watt of the new solar PV technology.4. PV-PEC Systems
The work of Landman and co-workers reported the new conceptual idea of cell separation in the PEC system for decoupling the PEC system into individual oxygen and hydrogen cells as illustrated in Figure 9 [51][39]. Figure 9 illustrates the redox reaction that occurred in a separate compartment, where Figure 9a is dedicated for the O2 evolution reaction and Figure 9b is designed for the H2 evolution reaction. In this decoupled PEC system, the authors used the first generation of silicon PV modules for assisting the photoelectrocatalytic hydrogen production in a separate cell system. The Si PV module has a capacity of 164.5 mA and 5.4 V of a short-circuit current and open-circuit voltage, respectively. It is worth noting that the charge carrier separation in both individual cells was obtained by integrating a battery-grade nickel hydroxide into both cells’ compartments. This indicated that nickel hydroxide plays an important role in mediating the ion exchange in both individual hydrogen and oxygen compartments if a decoupled PEC was designed. Apart from that, the STH efficiency of this decoupled PV-PEC system is 0.68%, taking into account that the Faradaic efficiency is 100% and short-circuit current is 55.2 mA. Nevertheless, the idea of decoupling the PEC system into individual H2 and O2 evolution reactions has its own drawback, because a decrease of 5% of the overall STH efficiency was observed.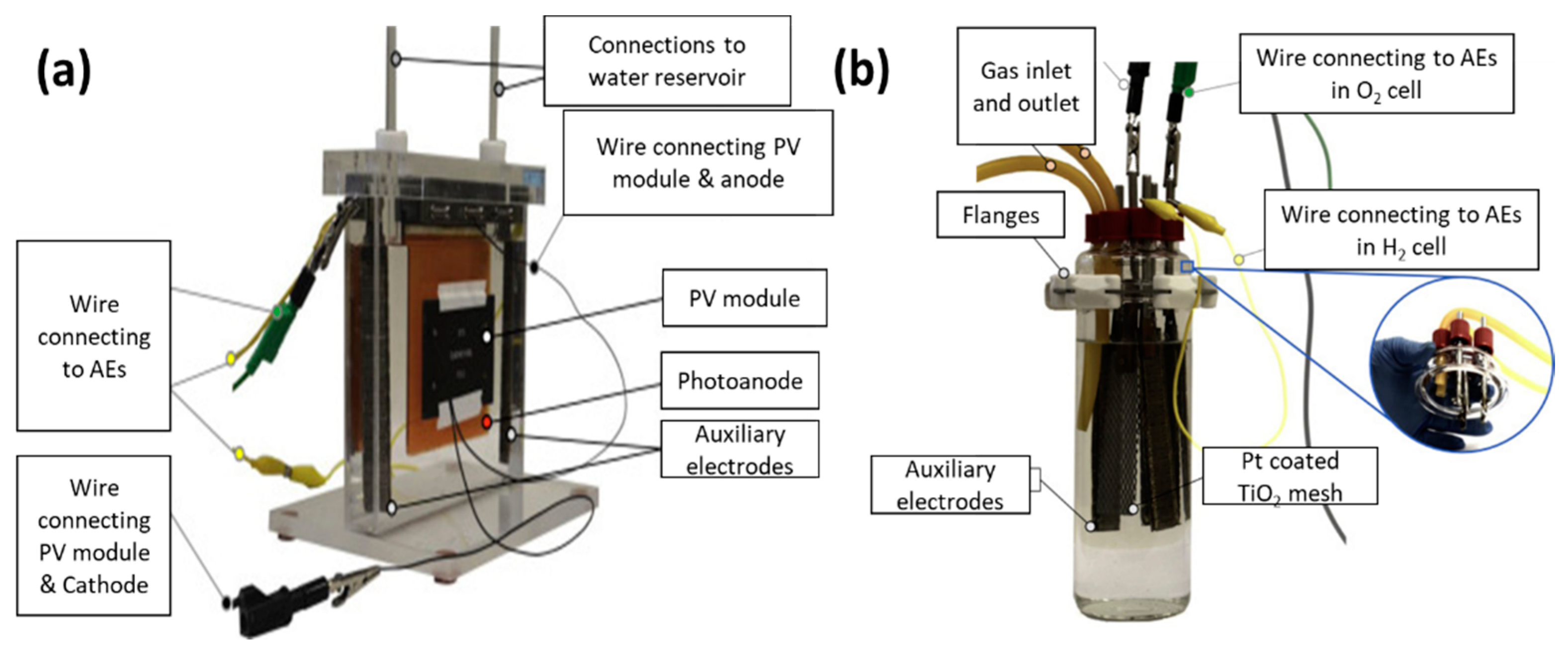
5. Different Types of Semiconductor Materials Used in PEC System
6. Challenges in the PV-PEC System
On account of the huge potential of the PV-PEC system as the next alternative for sustainable energy technologies, there are several challenges that need to be taken into consideration prior to debuting this PV-PEC system into a commercial and large-scale application. Firstly, as discussed in Section 4, the STH efficiency reported by certain published studies is relatively low. This requires further improvement of the reported efficiency in order to ensure that such PV-PEC systems become more competitive for practical usage. The low efficiency monitored can be due to the resistance losses in the series arrangement of the PV-PEC system, which critically needs to be mitigated to further heighten the STH efficiency of this system. Secondly, the stability of the catalyst used in both PV and PEC cells is often unstable and prone to have a shortened lifetime over time. This leads to a high maintenance cost of this system if the cells need to be replaced frequently. Moreover, the stability of the catalyst in converting the light energy during cloudy weather remains a huge challenge in this field. Thus, designing a stable catalyst that can withstand harsh conditions of the environment while retaining its efficiency is highly desirable. Next, although the price for the first generation of solar cells has declined over the years, there is still room for improvement in the market price particularly for the latest generation of the solar PV and PEC systems. This is due to the potential of achieving greater efficiency in comparison to those that are available in the market via the latest generation of solar PV and PEC systems. It is recommended that, apart from excavating the full-fledged potential of the system, the scientific community also needs to embark on finding a way to minimize the cost of the PV-PEC system. Finally, the scale of study for this PV-PEV system is still limited to a small scale, whereas a pilot scale of this system is yet to be proven. Thus, it is timely for the scientific community to start exploring the pilot scale of this PV-PEC system, which can further escalate the readiness of this technology.References
- Wang, Z.; Huang, X.; Wang, X. Recent Progresses in the Design of BiVO4-Based Photocatalysts for Efficient Solar Water Splitting. Catal. Today 2019, 335, 31–38.
- Allouhi, A.; Rehman, S.; Buker, M.S.; Said, Z. Up-to-Date Literature Review on Solar PV Systems: Technology Progress, Market Status and R&D. J. Clean. Prod. 2022, 362, 132339.
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421.
- Mekhilef, S.; Safari, A.; Mustaffa, W.E.S.; Saidur, R.; Omar, R.; Younis, M.A.A. Solar Energy in Malaysia: Current State and Prospects. Renew. Sustain. Energy Rev. 2012, 16, 386–396.
- Younis, S.A.; Kwon, E.E.; Qasim, M.; Kim, K.H.; Kim, T.; Kukkar, D.; Dou, X.; Ali, I. Metal-Organic Framework as a Photocatalyst: Progress in Modulation Strategies and Environmental/Energy Applications. Prog. Energy Combust. Sci. 2020, 81, 100870.
- He, Y.; Hamann, T.; Wang, D. Thin Film Photoelectrodes for Solar Water Splitting. Chem. Soc. Rev. 2019, 48, 2182–2215.
- Licht, S.; Wang, B.; Mukerji, S.; Soga, T.; Umeno, M.; Tributsch, H. Over 18% Solar Energy Conversion to Generation of Hydrogen Fuel; Theory and Experiment for Efficient Solar Water Splitting. Int. J. Hydrogen Energy 2001, 26, 653–659.
- Samsudin, M.F.R.; Frebilot, C.; Kaddoury, Y.; Sufian, S.; Ong, W.J. Bifunctional Z-Scheme Ag/AgVO3/g-C3N4 photocatalysts for expired ciprofloxacin degradation and hydrogen production from natural rainwater without using scavengers. J. Environ. Manag. 2020, 270, 110803.
- Rekioua, D.; Matagne, E. Optimization of Photovoltaic Power Systems: Modelization, Simulation and Control; Springer: Berlin/Heidelberg, Germany, 2012; Volume 102.
- Rekioua, D.; Rekioua, T.; Soufi, Y. Control of a Grid Connected Photovoltaic System. In Proceedings of the 2015 International Conference on Renewable Energy Research and Applications (ICRERA) 2015, Palermo, Italy, 22–25 November 2015; pp. 1382–1387.
- Yan, J.; Saunders, B.R. Third-Generation Solar Cells: A Review and Comparison of Polymer:Fullerene, Hybrid Polymer and Perovskite Solar Cells. RSC Adv. 2014, 4, 43286–43314.
- Tao, M.; Azzolini, J.A.; Stechel, E.B.; Ayers, K.E.; Valdez, T.I. Review—Engineering Challenges in Green Hydrogen Production Systems. J. Electrochem. Soc. 2022, 169, 054503.
- Fischer, M. Review of Hydrogen Production with Photovoltaic Electrolysis Systems. Int. J. Hydrogen Energy 1986, 11, 495–501.
- Wang, Z.; Gu, Y.; Wang, L. Revisiting Solar Hydrogen Production through Photovoltaic-Electrocatalytic and Photoelectrochemical Water Splitting. Front. Energy 2021, 15, 596–599.
- Khan, M.A.; Al-Shankiti, I.; Ziani, A.; Idriss, H. Demonstration of Green Hydrogen Production Using Solar Energy at 28% Efficiency and Evaluation of Its Economic Viability. Sustain. Energy Fuels 2021, 5, 1085–1094.
- Özçelep, Y.; Bekdaş, G.; Apak, S. Meeting the Electricity Demand for the Heating of Greenhouses with Hydrogen: Solar Photovoltaic-Hydrogen-Heat Pump System Application in Turkey. Int. J. Hydrogen Energy 2023, 48, 2510–2517.
- Pastuszak, J.; Węgierek, P. Photovoltaic Cell Generations and Current Research Directions for Their Development. Materials 2022, 15, 5542.
- Samsudin, M.F.R.; Ullah, H.; Bashiri, R.; Mohamed, N.M.; Sufian, S.; Ng, Y.H. Experimental and DFT Insights on Microflower g-C3N4/BiVO4 Photocatalyst for Enhanced Photoelectrochemical Hydrogen Generation from Lake Water. ACS Sustain. Chem. Eng. 2020, 8, 9393–9403.
- Samsudin, M.F.R.; Bacho, N.; Sufian, S. Recent Development of Graphitic Carbon Nitride-Based Photocatalyst for Environmental Pollution Remediation. In Nanocatalysts; IntechOpen: London, UK, 2018; Volume 1, pp. 1–15.
- Ahmad, I.; Zou, Y.; Yan, J.; Liu, Y.; Shukrullah, S.; Naz, M.Y.; Hussain, H.; Khan, W.Q.; Khalid, N.R. Semiconductor Photocatalysts: A Critical Review Highlighting the Various Strategies to Boost the Photocatalytic Performances for Diverse Applications. Adv. Colloid Interface Sci. 2023, 311, 102830.
- Zhao, F.; Li, X.; Zuo, M.; Liang, Y.; Qin, P. Preparation of Photocatalysts Decorated by Carbon Quantum Dots (CQDs) and Their Applications: A Review. J. Environ. Chem. Eng. 2023, 11, 109487.
- Samsudin, M.F.R.; Sufian, S.; Hameed, B.H. Epigrammatic Progress and Perspective on the Photocatalytic Properties of BiVO4-Based Photocatalyst in Photocatalytic Water Treatment Technology: A Review. J. Mol. Liq. 2018, 268, 438–459.
- Samsudin, M.F.R.; Bashiri, R.; Mohamed, N.M.; Ng, Y.H.; Sufian, S. Tailoring the Morphological Structure of BiVO4 Photocatalyst for Enhanced Photoelectrochemical Solar Hydrogen Production from Natural Lake Water. Appl. Surf. Sci. 2020, 504, 144417.
- Saafie, N.; Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Current Scenario of MXene-Based Nanomaterials for Wastewater Remediation: A Review. Chemistry 2022, 4, 1576–1608.
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renew. Sustain. Energy Rev. 2007, 11, 401–425.
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A Review on BiVO4 photocatalyst: Activity Enhancement Methods for Solar Photocatalytic Applications. Appl. Catal. A Gen. 2018, 555, 47–74.
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for Photoelectrochemical Water Splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817.
- Peerakiatkhajohn, P.; Yun, J.-H.; Wang, S.; Wang, L. Review of Recent Progress in Unassisted Photoelectrochemical Water Splitting: From Material Modification to Configuration Design. J. Photon. Energy 2016, 7, 012006.
- El Hammoumi, A.; Chtita, S.; Motahhir, S.; El Ghzizal, A. Solar PV Energy: From Material to Use, and the Most Commonly Used Techniques to Maximize the Power Output of PV Systems: A Focus on Solar Trackers and Floating Solar Panels. Energy Rep. 2022, 8, 11992–12010.
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting-Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660.
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, 1904717.
- Chan, C.K.; Tüysüz, H.; Braun, A.; Ranjan, C.; La Mantia, F.; Miller, B.K.; Zhang, L.; Crozier, P.A.; Haber, J.A.; Gregoire, J.M.; et al. Advanced and in Situ Analytical Methods for Solar Fuel Materials; Springer: Berlin/Heidelberg, Germany, 2016; Volume 371, ISBN 9783319230986.
- Saga, T. Advances in Crystalline Silicon Solar Cell Technology for Industrial Mass Production. NPG Asia Mater. 2010, 2, 96–102.
- Kenu, E. Sarah A Review of Solar Photovoltaic Technologies. Int. J. Eng. Res. 2020, V9, 741–749.
- Huang, C.; Wang, L. Simulation Study on the Degradation Process of Photovoltaic Modules. Energy Convers. Manag. 2018, 165, 236–243.
- Radue, C.; van Dyk, E.E. A Comparison of Degradation in Three Amorphous Silicon PV Module Technologies. Sol. Energy Mater. Sol. Cells 2010, 94, 617–622.
- Mateo, C.; Hernández-Fenollosa, M.A.; Montero; Seguí-Chilet, S. Ageing and Seasonal Effects on Amorphous Silicon Photovoltaic Modules in a Mediterranean Climate. Renew. Energy 2022, 186, 74–88.
- Mikolasek, M.; Kemeny, M.; Chymo, F.; Ondrejka, P.; Huran, J. Amorphous Silicon PEC-PV Hybrid Structure for Photo-Electrochemical Water Splitting. J. Electr. Eng. 2019, 70, 107–111.
- Landman, A.; Halabi, R.; Dias, P.; Dotan, H.; Mehlmann, A.; Shter, G.E.; Halabi, M.; Naseraldeen, O.; Mendes, A.; Grader, G.S.; et al. Decoupled Photoelectrochemical Water Splitting System for Centralized Hydrogen Production. Joule 2020, 4, 448–471.
- Welter, K.; Hamzelui, N.; Smirnov, V.; Becker, J.P.; Jaegermann, W.; Finger, F. Catalysts from Earth Abundant Materials in a Scalable, Stand-Alone Photovoltaic-Electrochemical Module for Solar Water Splitting. J. Mater. Chem. A 2018, 6, 15968–15976.
- Bashiri, R.; Mohamed, N.M.; Fai Kait, C.; Sufian, S.; Khatani, M. Enhanced Hydrogen Production over Incorporated Cu and Ni into Titania Photocatalyst in Glycerol-Based Photoelectrochemical Cell: Effect of Total Metal Loading and Calcination Temperature. Int. J. Hydrogen Energy 2017, 42, 9553–9566.
- Li, J.; Huang, J.; Zeng, G.; Zhang, C.; Yu, H.; Wan, Q.; Yi, K.; Zhang, W.; Pang, H.; Liu, S.; et al. Efficient Photosynthesis of H2O2 via Two-Electron Oxygen Reduction Reaction by Defective g-C3N4 with Terminal Cyano Groups and Nitrogen Vacancies. Chem. Eng. J. 2023, 463, 142512.
- Wang, J.; Pan, R.; Hao, Q.; Gao, Y.; Ye, J.; Wu, Y.; van Ree, T. Constructing Defect-Mediated CdS/g-C3N4 by an In-Situ Interlocking Strategy for Cocatalyst-Free Photocatalytic H2 Production. Appl. Surf. Sci. 2022, 599, 153875.
- Wang, J.; Sun, Y.; Lai, J.; Pan, R.; Fan, Y.; Wu, X.; Ou, M.; Zhu, Y.; Fu, L.; Shi, F.; et al. Two-Dimensional Graphitic Carbon Nitride/N-Doped Carbon with a Direct Z-Scheme Heterojunction for Photocatalytic Generation of Hydrogen. Nanoscale Adv. 2021, 3, 6580–6586.
- Wang, Y.; Li, Y.; Zhao, J.; Wang, J.; Li, Z. g-C3N4/B Doped g-C3N4 Quantum Dots Heterojunction Photocatalysts for Hydrogen Evolution under Visible Light. Int. J. Hydrogen Energy 2018, 44, 618–628.
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on g-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955.
