Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Alfred Zheng and Version 1 by Kazuki Santa.
A variety of phytocompounds contained in medical plants have been used as medication, including Kampo (traditional Japanese) medicine. Phytochemicals are one category of the chemical compounds mainly known as antioxidants. Phytochemicals generally consist of various classes of compounds, including terpenoids, carotenoids, flavonoids (polyphenols), etc. Here, people describe the effects of phytochemicals, including the polyphenols abundantly contained in onions, citrus fruits, tea, soybeans, turmeric, cacao, and grapes—familiar vegetables and fruits in Japan—combined with vitamin D, an attractive synergic formula for a healthy lifestyle and the prevention of diseases.
- phytochemicals
- vitamin D
1. Introduction
A variety of phytocompounds have been used as medicines in traditional medicine. Since ancient times, many drugs have been created from plant-derived ingredients, similarly to how antibiotics were developed from the research of bacteria. Aspirin is a typical plant-derived drug, isolated from willow trees [1], and currently, other plant-derived medicines such as artemisinin, an antimalarial drug derived from wormwood leaves, have been widely used [2]. Kampo (traditional Japanese) medicine is a good example of the use of such plant-derived ingredients; it was introduced from ancient mainland China before the spread of Western medicine in Japan in the late 18th century [3], and recently, a variety of plant-derived ingredients have been used for the promotion of a healthy lifestyle [4]. Furthermore, the COVID-19 pandemic has rekindled interest in the activities of natural substances for managing clinical outcomes. There are many kinds of plant-derived ingredients; however, recently, attention has focused on a group of plant-derived chemicals termed phytochemicals. Phytochemicals generally consist of various classes of compounds, including terpenoids, carotenoids, flavonoids (polyphenols), etc. [5]. Here, people describe the effects of phytochemicals, including the polyphenols abundantly contained in onions, citrus fruits, tea, soybeans, turmeric, cacao, and grapes—familiar vegetables and fruits in Japan—combined with vitamin D, an attractive synergic formula for a healthy lifestyle and the prevention of diseases.
2. Phytochemicals and Vitamin D
The term phytochemicals originates from the Greek, signifying “plant chemicals”. Phytochemicals are produced by plants to protect themselves from UV and other predatory presences. These chemicals range from toxins to drugs used for traditional medical purposes. Here, people explain phytochemicals well known in Japan, mainly those with anti-inflammatory properties contained in fruits and vegetables that are called phytonutrients, and attractive to use for the prevention of diseases and promotion of a healthy condition [15][6]. Nowadays, in addition to the three major nutrients (sugars, lipids, and proteins), Japanese nutritionists tend to regard minerals and vitamins the fourth and fifth major nutrients, with dietary fibres being the sixth. In addition, some nutritionists tend to regard phytochemicals as the seventh nutrient, and the recognition of their importance has been increasing [16][7]. Among phytochemicals, polyphenols are a generic name of phytocompounds containing phenolic hydroxyl groups within their chemical formulas. They comprise over 5000 pigments, and are bitter-tasting compounds produced by photosynthesis and present in most plants [17][8]. Typical polyphenols are catechins, anthocyanins, rutin, and isoflavones, which are classified as flavonoids, and others are curcumin and the polyphenols contained in cacao beans. Flavonoids are a generic term for plant secondary metabolites containing a flavan basic skeleton [18][9]. Generally, terpenoids, carotenoids, polyphenols (including flavonoids), and sulphuric compounds tend to be classified as phytochemicals.2.1. Onions
Quercetin is a representative flavonoid phytochemical found in onions (Allium cepa) and mainly contained in onion peel rather than the bulb [19,51][10][11]. The use of onion skin powder in food is increasing as of late as a pathway for the intake of phytochemicals for their health benefits. Quercetin is the strongest known anti-inflammatory flavonoid. In addition, quercetin is contained in the ingredients of many herbal medicines that feature in Kampo, such as Japanese hawthorn (Crataegus cuneata) [52][12]. Furthermore, onions are rich in rutin, which is a glycoside form of quercetin [20][13]. Allicin is another well-known phytochemical, contained in onions, garlic (Allium sativum), and leeks (Allium fistulosum), which is one of the allyl sulphides that characterises the spiciness and smell of onion. Figure 1 shows the chemical structure of quercetin. Onions have been used as a medical plant since ancient times, and their health benefits are still attractive [53][14].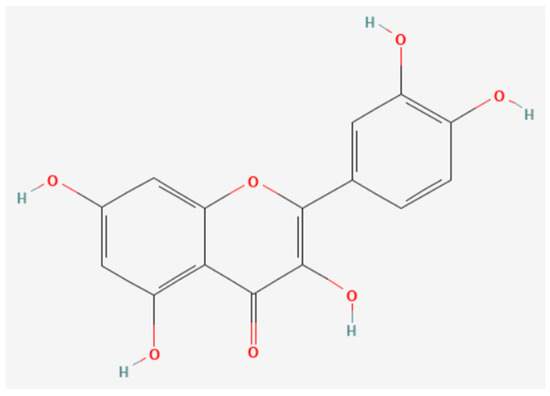
Figure 1.
Chemical structure of quercetin, a typical phytochemical. (Downloaded from PubChem CID: 5280343.).
2.2. Citrus Fruits
Citrus fruits and oranges contain large amounts of phytochemicals. These are mainly contained in the skin and especially in the mesocarp instead of the pulp. Japanese mandarin (Unsyu-mikan) (Citrus unshiu) is the most popular citrus fruit in Japan, and its citrus peel, termed “chinpi”, is used to a high degree in Kampo medicine. To make chinpi, orange peel needs to be dried for over 1 year; its relevant phytochemicals include hesperidin, naringin, rutin, etc. [54][15]. Below are the well-known orange phytochemicals. They are not only used for pharmacological purposes in the manner of traditional medicines, but also are ingested in food through the use of healthy ingredients. Hesperidin is mainly contained in Japanese mandarins and Hassaku oranges (Citrus hassaku) and reduces cholesterol and blood pressure, prevents bone density loss, and shows protection against sepsis. Hesperidin is poorly soluble in water and, owing to its low absorption ratio in the body, hesperidin glycoside, a glucose-conjugated form, is frequently used [21,22][16][17]. β-cryptoxanthin is a xanthophyll carotenoid contained in a variety of citrus fruits such as the Japanese mandarin. β-cryptoxanthin is a provitamin A converted into vitamin A and is known to possess an antioxidant effect effective against liver disorders, arteriosclerosis, diabetes, and osteoporosis; in promoting bone metabolism; and in the prevention of cancer [55][18]. Naringin is a bitter component contained in oranges, grapefruits (Citrus paradisi), daidai (Citrus aurantium), Natsu-mikan (Citrus natsudaidai), etc., and is contained in some herbal medicines made from them. Naringin is effective in its anti-oxidative properties, obesity prevention, treatment of hypertension, and for lowering fasting blood sugar levels [23][19]. Nobiletin is contained in ponkan (Citrus poonensis) and shiikwaasa (Citrus depressa). It has anti-inflammatory effects, promotes skin metabolism, suppresses blood sugar levels by way of adiponectin production, reduces the size of enlarged adipocytes, burns fat, prevents chronic inflammation by suppressing histamine release, and prevents dementia resulting from Alzheimer’s disease [24,25][20][21]. Rutin is quercetin glycoside, a flavonoid glycoside found in Ruta graveolens. It is also found in some citrus fruits, including Japanese mandarins, grapefruits, lemons (Citrus limon), and limes (Citrus aurantiifolia), as well as buckwheat (Fagopyrum esculentum), and is used as a vascular protection medication in many countries [26][22]. Recently, Sudachitin, which is present in Sudachi (Citrus Sudachi), has been shown to regulate circadian rhythm and suppress the accumulation of fat in the liver [27][23].2.3. Tea
Tea (Camellia sinensis) contains catechins; raw tea leaves contain epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG). When heated, these change to catechin (C) and gallocatechin (GC), catechin gallate (CG), and gallocatechin gallate (GCG). Catechins have various physiological activities, controlling blood pressure, cholesterol, and sugar levels, and possessing anti-oxidative and anti-ageing effects [56][24]. An enhanced anti-obesity effect from the combined administration of citrus polyphenols and tea catechins has been reported [28][25]. The effects of catechins for viral inactivation are well known, and recent research has shown the effect of EGCG in the inactivation of SARS-CoV-2 virus [29,30][26][27]. Catechins from tea leaves have lipolytic properties and reduce the accumulation of visceral fat [31,32][28][29]. In addition, theaflavin, a catechin polymer, is produced during the fermentation process from green tea to black tea and has anti-inflammation, anti-oxidation, anti-cancer, and anti-obesity properties [33][30]. In addition, epidemiological studies have shown that catechins prevent dementia [34][31]. As it described above, catechins contained in tea leaves have various healthy properties.2.4. Soybeans
Soybeans (Glycine max) contain isoflavones such as genistein and daidzein that work as oestrogen-like substances, that is, as oestrogen receptor agonists [35][32]. The oestrogenic effect of soy isoflavones comes from equol. The conversion of equol from isoflavones requires the metabolism of gut microbes, but the population of people who have gut microbes that can convert isoflavone to equol is limited by region and age [36,37][33][34].2.5. Turmeric
Turmeric (Curcuma longa) is known as a spice used in Indian cuisine and is quite familiar to Japanese people, as it is the essential ingredient of Japanese curry rice. The turmeric rhizome contains a large amount of the polyphenol curcumin. Spices such as curcumin are known to upregulate immunity, and its health-promoting abilities have proven attractive around the world [57][35]. Turmeric has anti-inflammatory, anti-oxidative, and anti-cancer properties, and much research has shown that curcumin has protective effects for a variety of disorders [58][36]. In addition, curcumin is well known for its effect in enhancing liver function [38][37].2.6. Cacao
Cacao (Theobroma cacao) is a raw material for chocolates and cocoa drinks consumed all over the world. Cacao phytochemicals mainly consist of polyphenols that have been confirmed as anti-inflammatory and anti-oxidative and ameliorate the effects of metabolic-syndrome-related disorders, similarly to other polyphenols contained in plants [59][38]. Phytochemicals in cacao beans are called cacao polyphenols and consist of the family of flavan-3-ols (flavonoids), comprising epicatechin, catechin, and their polymers, the procyanidins [60,61][39][40]. A recent study investigated the mechanism of cocoa flavanols in ameliorating metabolic-syndrome-related disorders [39][41]. Another study has shown the preventive effects of cacao polyphenol against blood sugar spikes via the enhancement of GLP-1 and insulin production [40][42].2.7. Grapes
Grapes (Vitis spp.) are one of the food crops with the longest histories. They were cultivated for wine production rather than for food in ancient times. The polyphenol resveratrol is a well-known grape phytochemical, which made headlines in the form of the French paradox. In this phenomenon, phytochemicals contained in red wine have been reportedly associated with there being fewer heart disease patients in France compared with America, making polyphenols widely known to the public [41][43]. Grape phytochemicals are mainly contained in the skins or seeds and are classified into terpenoids, carotenoids, and flavonoids. The terpenoids include oleanolic acid, which exists on the surface of the fruit; the carotenoids include β-carotene; and the flavonoids are subdivided into flavon-3-ols including quercetin, flavan-3-ols including catechins, and anthocyanins including malvidin [62][44]. Tannins and procyanidin, which are polymers of catechins, are rich in grapes as well. Quercetin in grapes is contained in the skin and seeds, but mostly in the skin. Quercetin glucoside, a water-soluble glycoside, is decomposed into quercetin by bacteria in the large intestine and is absorbed in the gut. After absorption, quercetin conjugates again with glucuronic acid to become quercetin glucuronide and circulates into the bloodstream. Quercetin has anti-inflammatory properties, but it requires an activation process. When blood vessels induce inflammation, macrophages gathered there secrete β-glucuronidase. This enzyme cuts glucuronic acid from quercetin glucuronide to make the active form of quercetin. This activated quercetin suppresses the production of TNF-α and ameliorates inflammation [42][45]. Anthocyanins are water-soluble pigments, of red, blue, and purple colour, widely found in the flowers and fruits of plants [63][46]. Anthocyanins, abundant in grape skin and also found in grape seeds, mainly consist of malvidin, cyanidin, and delphinidin. They have anti-inflammatory and anti-oxidative effects and reduce the incidence of cardiovascular diseases and type-2 diabetes, as well as the mortality rate [43][47]. Procyanidins are oligomers and polymers of epicatechins or catechins which are abundantly present in cacao beans as well. Recently, procyanidins have gained much attention for their anti-ageing activities attained through the suppression of senescent cell accumulation, which causes the senescence-associated secretory phenotype (SASP) [44][48]. In addition, procyanidins are dose-dependently associated with the gut–brain axis in the central nervous system via neurotransmitter receptors [45][49]. Furthermore, oleanolic acid is a component of the white powder, called bloom, found on the surface of grapes. Japanese hawthorn contains a large amount of oleanolic acid as well. This compound activates intestinal peristalsis as an agonist of the bile acid receptor TGR5 [46][50]. In addition, oleanolic acid suppresses cholestasis and releases liver cholesterol into the intestine as bile acids, ultimately excreting them from the body [64][51]. Grape phytochemicals have already been used for medical purposes and in functional foods such as grape seed extract (GSE) [47,48][52][53].2.8. Health Promotion Effect of Vitamin D and Interactions with Phytochemicals
Vitamins and minerals are classified as two of the five major nutrients in Japanese nutrition science. Vitamins are essential nutrients for the existence of every organism, and most of them are organic compounds that our body cannot synthesise. However, vitamin D is different from every other vitamin as to one point: it is synthesised from cholesterol in skin cells under UV exposure [80][54]. Vitamin D works as a hormone associated with bone formation and breakdown and its deficiency causes rickets and osteomalacia [81][55]. In addition, the importance of vitamin D has been further indicated by the fact that its deficiency correlates with a variety of disorders, including hypertension, tuberculosis, cancer, periodontal disease, multiple sclerosis, and autoimmune disorders [82,83][56][57]. The intake of vitamin D and phytochemicals was recommended during the COVID-19 crisis to upregulate immunity. Since the combined intake of vitamin D with phytochemicals promotes a healthy lifestyle, vitamin D seems to have an adjuvant effect on phytochemicals [84][58]. On the other hand, it has been reported that 98% of the Japanese population has an insufficient amount of serum vitamin D (under 30 ng/mL) [85][59]. The following descriptions are the interactions between vitamin D and phytochemicals: Vitamin D is susceptible to oxygen and high temperature, but quercetin suppresses the decomposition of vitamin D [86][60]. In addition, both vitamin D and phytochemicals possess anti-inflammatory effects and upregulating properties towards immune responses [87][61]. They are both important for maintaining healthy gut microbiota [88][62]. There has been intriguing genetic research showing the benefit of vitamin D and phytochemicals in the remission of inflammatory bowel disease [89][63]. In a dietary intervention study, they were found to downregulate the level of TNF-α, an important factor in chronic inflammation [90][64].References
- Dragoș, D.; Petran, M.; Gradinaru, T.C.; Gilca, M. Phytochemicals and Inflammation: Is Bitter Better? Plants 2022, 11, 2991.
- Peplow, M. Synthetic biology’s first malaria drug meets market resistance. Nature 2016, 530, 389–390.
- Yamamoto, M. KAMPOmics: A framework for multidisciplinary and comprehensive research on Japanese traditional medicine. Gene 2022, 831, 146555.
- Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461.
- Santa, K.; Kumazawa, Y.; Nagaoka, I. The Potential Use of Grape Phytochemicals for Preventing the Development of Intestine-Related and Subsequent Inflammatory Diseases. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 794–802.
- Shen, J.; Shan, J.; Zhong, L.; Liang, B.; Zhang, D.; Li, M.; Tang, H. Dietary Phytochemicals that Can Extend Longevity by Regulation of Metabolism. Plant Foods Hum. Nutr. 2022, 77, 12–19.
- Arai, S.; Morinaga, Y.; Yoshikawa, T.; Ichiishi, E.; Kiso, Y.; Yamazaki, M.; Morotomi, M.; Shimizu, M.; Kuwata, T.; Kaminogawa, S. Recent trends in functional food science and the industry in Japan. Biosci. Biotechnol. Biochem. 2002, 66, 2017–2029.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621.
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918.
- Nishimura, M.; Muro, T.; Kobori, M.; Nishihira, J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2019, 12, 91.
- Chadorshabi, S.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red onion skin active ingredients, extraction and biological properties for functional food applications. Food Chem. 2022, 386, 132737.
- Cui, Y.; Du, K.; Hou, S.; Yang, R.; Qi, L.; Li, J.; Chang, Y. A comprehensive strategy integrating metabolomics with multiple chemometric for discovery of function related active markers for assessment of foodstuffs: A case of hawthorn (Crataegus cuneata) fruits. Food Chem 2022, 383, 132464.
- Miyake, T.; Kuge, M.; Matsumoto, Y.; Shimada, M. α-glucosyl-rutin activates immediate early genes in human induced pluripotent stem cells. Stem Cell Res. 2021, 56, 102511.
- Kumari, N.; Kumar, M.; Radha; Lorenzo, J.M.; Sharma, D.; Puri, S.; Pundir, A.; Dhumal, S.; Bhuyan, D.J.; Jayanthy, G.; et al. Onion and garlic polysaccharides: A review on extraction, characterization, bioactivity, and modifications. Int. J. Biol. Macromol. 2022, 219, 1047–1061.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114.
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648.
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent For Obesity. Drug Des. Devel. Ther. 2019, 13, 3855–3866.
- Nishino, A.; Maoka, T.; Yasui, H. Preventive Effects of β-Cryptoxanthin, a Potent Antioxidant and Provitamin A Carotenoid, on Lifestyle-Related Diseases-A Central Focus on Its Effects on Non-Alcoholic Fatty Liver Disease (NAFLD). Antioxidants 2021, 11, 43.
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133.
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750.
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380.
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312.
- Mawatari, K.; Koike, N.; Nohara, K.; Wirianto, M.; Uebanso, T.; Shimohata, T.; Shikishima, Y.; Miura, H.; Nii, Y.; Burish, M.J.; et al. The Polymethoxyflavone Sudachitin Modulates the Circadian Clock and Improves Liver Physiology. Mol. Nutr. Food Res. 2023, 67, e2200270.
- Bansal, S.; Vyas, S.; Bhattacharya, S.; Sharma, M. Catechin prodrugs and analogs: A new array of chemical entities with improved pharmacological and pharmacokinetic properties. Nat. Prod. Rep. 2013, 30, 1438–1454.
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 19067.
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Significant Inactivation of SARS-CoV-2 in vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules 2021, 26, 3572.
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Rapid Inactivation In Vitro of SARS-CoV-2 in Saliva by Black Tea and Green Tea. Pathogens 2021, 10, 721.
- Chen, S.; Osaki, N.; Shimotoyodome, A. Green tea catechins enhance norepinephrine-induced lipolysis via a protein kinase A-dependent pathway in adipocytes. Biochem. Biophys. Res. Commun. 2015, 461, 1–7.
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. 2002, 26, 1459–1464.
- Kakutani, S.; Watanabe, H.; Murayama, N. Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review. Nutrients 2019, 11, 1165.
- Shan, Z.; Nisar, M.F.; Li, M.; Zhang, C.; Wan, C.C. Theaflavin Chemistry and Its Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 6256618.
- Nakai, S.; Fujita, M.; Kamei, Y. Health Promotion Effects of Soy Isoflavones. J. Nutr. Sci. Vitaminol. (Tokyo) 2020, 66, 502–507.
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231.
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules 2021, 26, 5863.
- Soni, V.K.; Mehta, A.; Ratre, Y.K.; Tiwari, A.K.; Amit, A.; Singh, R.P.; Sonkar, S.C.; Chaturvedi, N.; Shukla, D.; Vishvakarma, N.K. Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur. J. Pharmacol. 2020, 886, 173551.
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895.
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 133.
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. (Paris) 2019, 175, 724–741.
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295.
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061.
- Strat, K.M.; Rowley, T.J., 4th; Smithson, A.T.; Tessem, J.S.; Hulver, M.W.; Liu, D.; Davy, B.M.; Davy, K.P.; Neilson, A.P. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J. Nutr. Biochem. 2016, 35, 1–21.
- Kawakami, Y.; Watanabe, Y.; Mazuka, M.; Yagi, N.; Sawazaki, A.; Koganei, M.; Natsume, M.; Kuriki, K.; Morimoto, T.; Asai, T.; et al. Effect of cacao polyphenol-rich chocolate on postprandial glycemia, insulin, and incretin secretion in healthy participants. Nutrition 2021, 85, 111128.
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250.
- Santa, K.; Kumazawa, Y.; Nagaoka, I. Prevention of Metabolic Syndrome by Phytochemicals and Vitamin, D. Int. J. Mol. Sci. 2023, 24, 2627.
- Kawai, Y.; Nishikawa, T.; Shiba, Y.; Saito, S.; Murota, K.; Shibata, N.; Kobayashi, M.; Kanayama, M.; Uchida, K.; Terao, J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem. 2008, 283, 9424–9434.
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500.
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236.
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726.
- Osakabe, N.; Fushimi, T.; Fujii, Y. Hormetic response to B-type procyanidin ingestion involves stress-related neuromodulation via the gut-brain axis: Preclinical and clinical observations. Front. Nutr. 2022, 9, 969823.
- Alemi, F.; Poole, D.P.; Chiu, J.; Schoonjans, K.; Cattaruzza, F.; Grider, J.R.; Bunnett, N.W.; Corvera, C.U. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 2013, 144, 145–154.
- Liu, J.; Liu, J.; Meng, C.; Huang, C.; Liu, F.; Xia, C. Oleanolic acid alleviates ANIT-induced cholestatic liver injury by activating Fxr and Nrf2 pathways to ameliorate disordered bile acids homeostasis. Phytomedicine 2022, 102, 154173.
- Schön, C.; Allegrini, P.; Engelhart-Jentzsch, K.; Riva, A.; Petrangolini, G. Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients 2021, 13, 654.
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844.
- Nanri, A.; Mizoue, T.; Goto, A.; Noda, M.; Sawada, N.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Vitamin D intake and all-cause and cause-specific mortality in Japanese men and women: The Japan Public Health Center-based prospective study. Eur. J. Epidemiol. 2023, 38, 291–300.
- Urena-Torres, P.; Souberbielle, J.C. Pharmacologic role of vitamin D natural products. Curr. Vasc. Pharmacol. 2014, 12, 278–285.
- Gallagher, J.C.; Rosen, C.J. Vitamin D: 100 years of discoveries, yet controversy continues. Lancet Diabetes Endocrinol. 2023, 11, 362–374.
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165.
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562.
- Miyamoto, H.; Kawakami, D.; Hanafusa, N.; Nakanishi, T.; Miyasaka, M.; Furutani, Y.; Ikeda, Y.; Ito, K.; Kato, T.; Yokoyama, K.; et al. Determination of a Serum 25-Hydroxyvitamin D Reference Ranges in Japanese Adults Using Fully Automated Liquid Chromatography-Tandem Mass Spectrometry. J. Nutr. 2023, 153, 1253–1264.
- Chang, S.N.; Lee, J.J.; Kim, H.J.; Kang, S.C. Quercetin enhances vitamin D2 stability and mitigate the degradation influenced by elevated temperature and pH value. Turk. J. Chem. 2021, 45, 1155–1161.
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122.
- Uranga, J.A.; López-Miranda, V.; Lombó, F.; Abalo, R. Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. Pharmacol. Rep. 2016, 68, 816–826.
- Ferguson, L.R. Nutritional Modulation of Gene Expression: Might This be of Benefit to Individuals with Crohn’s Disease? Front. Immunol. 2015, 6, 467.
- Quarta, S.; Massaro, M.; Carluccio, M.A.; Calabriso, N.; Bravo, L.; Sarria, B.; García-Conesa, M.T. An Exploratory Critical Review on TNF-α as a Potential Inflammatory Biomarker Responsive to Dietary Intervention with Bioactive Foods and Derived Products. Foods 2022, 11, 2524.
More
