Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by xiyang zhang.
Iron dysregulation is a common characteristic in many subtypes of acute lung injury (ALI). On the one hand, iron is needed to produce reactive oxygen species (ROS) as part of the immune response to an infection; on the other hand, iron can accelerate the occurrence of ferroptosis and extend host cell damage. Iron chelation represents a novel therapeutic strategy for alleviating lung injury and improving the survival of patients with ALI.
- acute lung injury
- iron overload
- ROS
- ferroptosis
1. Importance of Iron in Human Health
Iron is an essential trace mineral for normal biological function in almost all organisms. In order to maintain baseline homeostasis and resupply the minor unregulated loss of iron, 25 mg/day of iron is required for a human adult. About 65% of the total iron in humans is stored in RBCs in the form of hemoglobin, 20% is allocated in macrophages and hepatocytes, 10% is distributed in muscle fibers as myoglobin, and 5% is in bone marrow [1].
Under physiological conditions, there are two main sources of iron absorption in the body, namely endogenous iron absorption and exogenous iron absorption (Figure 1). Endogenous absorption is mainly from the recycling of iron from senescent erythrocytes. In the process of clearance of aging or damaged erythrocytes, macrophages phagocytose the erythrocytes and decompose heme, a component of hemoglobin with iron bonded, using heme oxygenase 1 (HO-1) to release iron, which can be recycled back into circulation by the iron exporter ferroportin (FPN), a transmembrane protein on the cell surface (Figure 1). Exogenous iron absorption mainly refers to dietary iron. Generally, about 1~2 mg of iron, in the form of either heme iron (Fe2+) or non-heme iron (Fe3+), is absorbed daily by duodenal enterocytes through different mechanisms (Figure 1). Fe2+ is directly absorbed in the apical surface of enterocytes via iron transport protein, divalent metal transporter 1 (DMT1), whereas Fe3+ is not bioavailable and needs to be converted to Fe2+ by duodenal cytochrome B (DcytB), a converting enzyme, before being absorbed by the apical membrane of duodenal enterocytes via DMT1[2]. Absorbed iron in duodenal enterocytes can be transported through FPN into the circulation to play its physiological role[3].
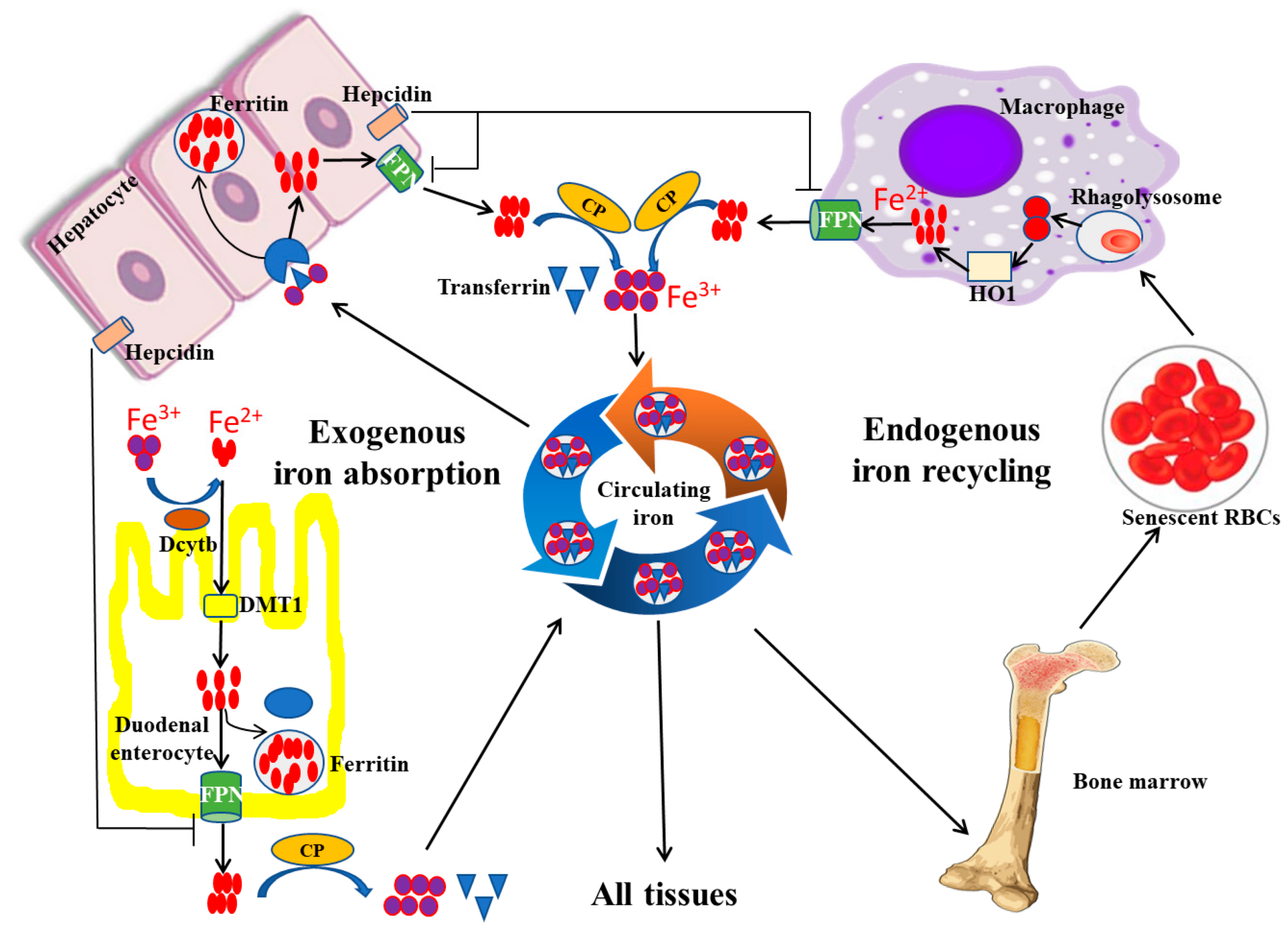
Figure 1. Iron absorption, recycling, and storage under physiological conditions. Exogenous iron absorption and endogenous iron recycling are two sources of iron in the human body. During the process of iron absorption and recycling, DcytB and DMT1 are responsible for the uptake of Fe2+ from the lumen, and macrophages are in charge of the cyclic utilization of iron from senescent erythrocytes. In addition, excess iron is usually stored in the hepatocyte in the form of ferritin, and FPN can export Fe2+ into the blood. FPN, ferroportin; HO-1, heme oxygenase 1; CP, ceruloplasmin; DcytB, duodenal cytochrome B; DMT1, divalent metal transporter 1 (modified according to Abbasi et al., 2021[4]).
To maintain iron homeostasis, hepcidin, a peptide hormone secreted by the liver, plays an important role by regulating the expression of FPN on the cell surface. Hepcidin can reduce FPN expression by binding FPN and inducing its internalization and degradation in lysosomes. In physical conditions, the absorbed iron can be stored and iron can be transported into the bloodstream via FPN. When iron levels exceed the body’s requirements, the increased secretion of hepcidin inhibits FPN expression, resulting in reduced iron transportation from cells into the blood circulation[5].
Transferrin (Tf), an iron transport protein, is responsible for the transportation and distribution of almost all iron within circulating blood (Figure 1). Specifically, most of the iron in the bloodstream exists in the form of Fe3+. About 25~30 mg/day of iron can be delivered by Tf from plasma to developing erythroid cells in the bone marrow for heme biosynthesis. Lower amounts of iron, up to 5 mg/day, are supplied to other tissues via Tf in order to meet various physiological needs. The pathway for iron transportation from Tf into cells is not fully defined, but the current evidence suggests that it involves endocytosis of the transferrin receptor (TfR) complex; the binding force between Tf and Fe3+ is then reduced, so as to allow for the release of iron from transferrin[6]. As a result, the free iron can then move into the cytoplasm and be used by the cell.
Under normal conditions, iron above immediate cell needs is safely stored within ferritin. Ferritin, as a hollow globular protein, is usually composed of 24 H and L subunits, so it can store up to 4500 ferrous ions. Furthermore, because hepatocytes are the major site for ferritin synthesis, the liver is the primary iron storage organ. In addition, some recycled iron is reincorporated into the bone marrow. When the iron demand increases, the stored iron in both the liver and bone marrow is mobilized to increase the iron content in circulation.
Serving as a prosthetic group for a variety of proteins, iron plays an important role in various vital physiological processes of cells. For example, iron is required for cell viability and proliferation. Iron functions as a catalyst for enzymes and plays a key role in DNA synthesis and repair, and cellular energy metabolism. Moreover, iron is also thought to play a role in the immune system. However, iron overload can attenuate the phagocytosis of macrophages, and affect the function of T lymphocytes, so as to disrupt the immune response[7][8].
By acquiring iron from hosts, invading microbes can also utilize iron to promote their own growth, disturb the homeostatic balance of the body, and bring potential adverse consequences[9][10][11]. Therefore, it is a physiological challenge to maintain the balance of the body’s requirement for iron, particularly in states of infection and inflammation.
2. Iron Regulation
In order to maintain an appropriate iron balance, organisms have evolved complex systemic homeostatic and cellular iron transport mechanisms. For example, hepcidin, produced primarily by hepatocytes, is the key regulator of systemic iron homeostasis. When the iron circulating concentration is increased in plasma, the body responds by elevating hepcidin release, which can downregulate FPN mRNA and protein levels, reducing the efficiency of delivering iron from storage sites to plasma, and thus lowering the plasma iron level[5][12]. Similarly, when the iron concentration is decreased, the body responds by reducing hepcidin release, which can upregulate the expression levels of FPN, partly resulting in a recovery of iron reabsorption and an increase in iron concentration[8].
In addition to the hepcidin/FPN pathway, the iron regulatory protein/iron-responsive elements (IRP/IRE) system also plays a key role in maintaining iron homeostasis. IREs are mainly located in the untranslated regions of mRNAs encoding proteins related to the absorption, storage, utilization, and export of iron. Interestingly, the 5′-end IREs mainly regulate gene expression related to iron storage and transport, such as ferritin and FPN. However, the 3′-end IREs are closely related to the expression of iron absorption genes, such as TfR1 and DMT1. Moreover, IRP1 and IRP2, via binding to IREs of the 5′ and 3′ regions, can control the mRNA translation or stability. Generally, the translation of the mRNAs, including ferritin and FPN, will be inhibited under the condition of IRPs combined with 5′ IREs, whereas the stability of mRNAs, including TfR1 and DMT1, will be reinforced when IRP binds to 3′ IREs. For example, under iron overload conditions, IRP1 is converted to c-aconitase by SCF (SKP1-CUL1-F-box) E3 ligase complex and IRP2 is degraded by proteasomes, resulting in decreased IRP binding to IREs. This dissociation of IRP–IRE interaction will increase the translation of FPN and ferritin mRNA and disturb TfR1 and DMT1 mRNA, resulting in an increase in iron storage and export and a decline in iron absorption[13][14][15][16].
Recent studies in mouse models have begun characterizing the roles of zinc transporter SLC39A8 (ZIP8) and other soluble proteins in regulating body iron homeostasis. ZIP8 is a zinc transporter and is encoded by the SLC39A8 gene, and has also been proven to have the ability to import iron into the cytosolic space[17]. In in vitro and animal studies, ZIP8 expression can be greatly increased after inflammatory stimulation with LPS, suggesting that increased ZIP8 via iron transporting has a function in host defense[18][19] (Figure 2). Lipocalin-2 is a soluble protein and is secreted by epithelial cells and neutrophils, and can also promote cellular and tissue iron uptake through the siderophore–lipocalin-2 complex. It is reported that circulating concentrations of lipocalin-2 are positively associated with adiposity, hypertriglyceridemia, hyperglycemia, insulin resistance, and circulating levels of C-reactive protein. Moreover, lipocalin-2 can bind to iron with a much higher affinity than the endogenous carrier protein transferrin, thus potentially aiding pathogen survival and growth during infection[11][20][21].
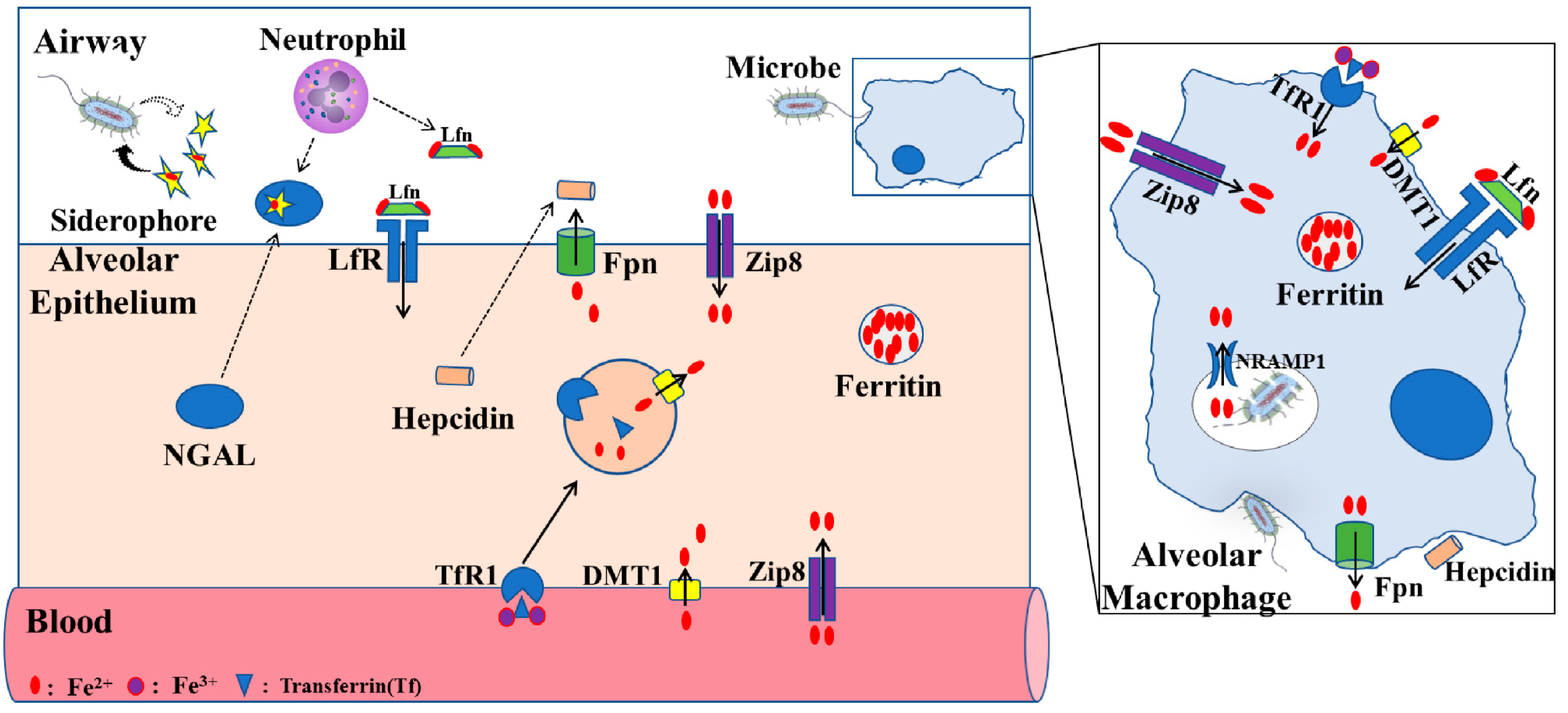
Figure 2. Proposed iron regulation in lung. The level of iron in alveolar epithelial cells is mainly regulated by TfR1, DMT1, ZIP8, LfR, and FPN receptors. Alveolar macrophages, through phagocytosis of bacteria, play an important role in lung defense response. Fpn, ferroportin; ZIP8, zinc transporter SLC39A8; Lfn, lactoferrin; LfR, lactoferrin receptor; DMT1, divalent metal transporter 1; TfR1, transferrin receptor 1; NRAMP1, natural resistance–associated macrophage protein 1NGAL, neutrophil gelatinase–associated lipocalin (modified according to Zhang et al., 2019[22]).
3. Iron in Pathology and Diseases
Iron deficiency results in the impairment of multiple cellular functions and, in particular, erythropoiesis and red blood cell heme contents are reduced[4][23]. Research has demonstrated that iron deficiency is associated with up to 60% of patients with cardiovascular disease, including coronary artery disease, heart failure, and pulmonary hypertension. In elderly patients with reduced ejection fraction, both anemia and iron deficiency may confer independent risk due to a poor prognosis[24]. Iron deficiency is usually caused by an underlying inflammatory disease, such as inflammatory bowel disease (IBD) or chronic kidney disease (CKD)[25]. In IBD, iron deficiency is markedly associated with decreased intestinal iron absorption, malnutrition, chronic inflammation, and blood loss. In patients diagnosed with CKD, anemia is induced by disturbed renal erythropoietin (EPO) production, which is responsible for erythropoiesis. Iron supplementation or recombinant EPO have been used to improve anemia in these patients[26]. In addition, iron deficiency is closely related to tumor progression. At the cellular level, the expression of iron metabolism–related proteins is altered in cancer. For example, TfR1 and DMT1, which increase iron uptake, are highly overexpressed in many tumor types, increasing intracellular iron levels[27][28]. Also, it is reported that FPN, which is in charge of iron release, is downregulated in prostate and breast cancer[29][30]. Therefore, iron deficiency, especially iron-deficiency anemia, has become one of the most important contributors to the global burden of disease, which mainly affects children, as well as people in low-income countries[31].
On the other hand, iron overload also has adverse consequences for the host, including oxidative stress, vascular dysfunction, ferroptosis, and peroxidation of lipid membranes [32][33][34]. Generally, under pathological conditions, iron overload mainly refers to non-transferrin-bound iron. Because humans lack a proper physiological pathway to clear out iron, it is important for iron to be maintained in homeostasis via a complex feedback mechanism between iron uptake, utilization, and storage. However, under pathological conditions, this balance will be disturbed. Initially, the excess iron can be safely combined with transferrin or stored in ferritin. However, transferrin will be saturated quickly by an accumulation of iron, raising the level of non-transferrin-bound-iron, which is rapidly taken up by various organs. As a result, the body will have a pathological situation of iron overload, resulting in organ damage. For example, a clinical study showed that due to high glucose toxicity in diabetic patients, the release of serum iron was inhibited, which resulted in iron overload. What is more, by facilitating lipid peroxidation and catalyzing excess hydroxyl free radicals, the increase of serum iron can promote the occurrence of acute kidney injury (AKI); therefore, iron overload is considered an independent risk factor for AKI in critically ill patients with diabetes[35]. It is also reported that iron is necessary for the continuous and rapid proliferation of cancer cells, resulting in accelerated cancer cell growth and increased morbidity and mortality in cancer patients[8]. Moreover, excess iron can promote the generation of free radicals and reactive oxygen species, which damage healthy cells and induce inflammation.
Furthermore, increased iron availability is also associated with the increased virulence of multiple microbial pathogens[36][37]. Toni et al. described the link between iron overload and an increased susceptibility to various invasive fungal infections, and suggested that reducing the level of iron may be a better way to prevent infections in patients with hematological malignancies[38]. Although different bacteria and viruses have different ways of acquiring and utilizing iron, they all use iron with the same goal of promoting their own growth, increasing virulence, and ultimately increasing pathogenicity. Therefore, the use of iron chelators to limit iron availability and control microbial survival may be a useful way to hinder the development of infections[39].
References
- Manuel Muñoz; Isabel Villar; José Antonio García-Erce; An update on iron physiology. World J. Gastroenterol. 2009, 15, 4617-4626.
- Bryan Mackenzie; Michael D. Garrick; Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am. J. Physiol. Liver Physiol. 2005, 289, G981-G986.
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 2005, 1, 945-972.
- Usama Abbasi; Srinivas Abbina; Arshdeep Gill; Lily E. Takuechi; Jayachandran N. Kizhakkedathu; Role of Iron in the Molecular Pathogenesis of Diseases and Therapeutic Opportunities. ACS Chem. Biol. 2021, 16, 945-972.
- Mitchell D. Knutson; Iron transport proteins: Gateways of cellular and systemic iron homeostasis. J. Biol. Chem. 2017, 292, 12735-12743.
- Hiroshi Kawabata; Transferrin and transferrin receptors update. Free. Radic. Biol. Med. 2019, 133, 46-54.
- Yan Yang; Yu Wang; Lin Guo; Wen Gao; Ting-Li Tang; Miao Yan; Interaction between macrophages and ferroptosis. Cell Death Dis. 2022, 13, 1-10.
- Sufia Islam; Nazia Hoque; Nishat Nasrin; Mehnaz Hossain; Farhana Rizwan; Kushal Biswas; Muhammad Asaduzzaman; Sabera Rahman; David W. Hoskin; Saki Sultana; et al.Christian Lehmann Iron Overload and Breast Cancer: Iron Chelation as a Potential Therapeutic Approach. Life 2022, 12, 963.
- Nermi L. Parrow; Robert E. Fleming; Michael F. Minnick; Sequestration and Scavenging of Iron in Infection. Infect. Immun. 2013, 81, 3503-3514.
- Kathryn R. Michels; Zhimin Zhang; Alexandra M. Bettina; R. Elaine Cagnina; Debora Stefanova; Marie D. Burdick; Sophie Vaulont; Elizabeta Nemeth; Tomas Ganz; Borna Mehrad; et al. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. J. Clin. Investig. 2017, 2, e92002.
- Jordi Mayneris-Perxachs; José María Moreno-Navarrete; José Manuel Fernández-Real; The role of iron in host–microbiota crosstalk and its effects on systemic glucose metabolism. Nat. Rev. Endocrinol. 2022, 18, 683-698.
- Gaetano Bergamaschi; Antonio Di Sabatino; Alessandra Pasini; Cristina Ubezio; Filippo Costanzo; Davide Grataroli; Michela Masotti; Costanza Alvisi; Gino R. Corazza; Intestinal expression of genes implicated in iron absorption and their regulation by hepcidin. Clin. Nutr. 2016, 36, 1427-1433.
- Joana Neves; Thomas Haider; Max Gassmann; Martina U. Muckenthaler; Iron Homeostasis in the Lungs—A Balance between Health and Disease. Pharm. 2019, 12, 5.
- Cole P. Anderson; Macy Shen; Richard S. Eisenstein; Elizabeth A. Leibold; Mammalian iron metabolism and its control by iron regulatory proteins. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2012, 1823, 1468-1483.
- Nicole Wilkinson; Kostas Pantopoulos; The IRP/IRE system in vivo: insights from mouse models. Front. Pharmacol. 2014, 5, 176.
- Long-Xia Li; Fang-Fang Guo; Hong Liu; Tao Zeng; Iron overload in alcoholic liver disease: underlying mechanisms, detrimental effects, and potential therapeutic targets. Cell. Mol. Life Sci. 2022, 79, 1-13.
- Chia-Yu Wang; Supak Jenkitkasemwong; Stephanie Duarte; Brian K. Sparkman; Ali Shawki; Bryan Mackenzie; Mitchell D. Knutson; ZIP8 Is an Iron and Zinc Transporter Whose Cell-surface Expression Is Up-regulated by Cellular Iron Loading. J. Biol. Chem. 2012, 287, 34032-34043.
- Ming-Jie Liu; Shengying Bao; Marina Gálvez-Peralta; Charlie J. Pyle; Andrew C. Rudawsky; Ryan E. Pavlovicz; David W. Killilea; Chenglong Li; Daniel W. Nebert; Mark D. Wewers; et al.Daren L. Knoell ZIP8 Regulates Host Defense through Zinc-Mediated Inhibition of NF-κB. Cell Rep. 2013, 3, 386-400.
- Charlie J. Pyle; Saife Akhter; ShengYing Bao; Claire E. Dodd; Larry S. Schlesinger; Daren L. Knoell; Zinc Modulates Endotoxin-Induced Human Macrophage Inflammation through ZIP8 Induction and C/EBPβ Inhibition. PLOS ONE 2017, 12, e0169531.
- Laxminarayana R. Devireddy; Claude Gazin; Xiaochun Zhu; Michael R. Green; A Cell-Surface Receptor for Lipocalin 24p3 Selectively Mediates Apoptosis and Iron Uptake. Cell 2005, 123, 1293-1305.
- Colin Ratledge; Lynn G Dover; Iron Metabolism in Pathogenic Bacteria. Annu. Rev. Microbiol. 2000, 54, 881-941.
- Vida Zhang; Elizabeta Nemeth; Airie Kim; Iron in Lung Pathology. Pharm. 2019, 12, 30.
- Chanté L. Richardson; Lorrie L. Delehanty; Grant C. Bullock; Claudia M. Rival; Kenneth S. Tung; Donald L. Kimpel; Sara Gardenghi; Stefano Rivella; Adam N. Goldfarb; Isocitrate ameliorates anemia by suppressing the erythroid iron restriction response. J. Clin. Investig. 2013, 123, 3614-3623.
- Gianluigi Savarese; Stephan von Haehling; Javed Butler; John G F Cleland; Piotr Ponikowski; Stefan D Anker; Iron deficiency and cardiovascular disease. Eur. Hear. J. 2022, 44, 14-27.
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011-1023.
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet (Lond. Engl.) 2017, 389, 1238-1252.
- Iryna Kindrat; Volodymyr Tryndyak; Aline de Conti; Svitlana Shpyleva; Thilak K. Mudalige; Tetyana Kobets; Anna M. Erstenyuk; Frederick A. Beland; Igor P. Pogribny; MicroRNA-152-mediated dysregulation of hepatic transferrin receptor 1 in liver carcinogenesis. Oncotarget 2015, 7, 1276-1287.
- Xiang Xue; Sadeesh K. Ramakrishnan; Kevin Weisz; Daniel Triner; Liwei Xie; Durga Attili; Asha Pant; Balázs Győrffy; Mingkun Zhan; Christin Carter-Su; et al.Karin M. HardimanThomas D. WangMichael K. DameJames VaraniDean BrennerEric R. FearonYatrik M. Shah Iron Uptake via DMT1 Integrates Cell Cycle with JAK-STAT3 Signaling to Promote Colorectal Tumorigenesis. Cell Metab. 2016, 24, 447-461.
- Dong Xue; Cui-Xing Zhou; Yun-Bo Shi; Hao Lu; Xiao-Zhou He; Decreased expression of ferroportin in prostate cancer. Oncol. Lett. 2015, 10, 913-916.
- Pinnix, Z.K.; Miller, L.D.; Wang, W.; D’Agostino, R., Jr.; Kute, T.; Willingham, M.C.; Hatcher, H.; Tesfay, L.; Sui, G.; Di, X.; et al.et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2010, 2, 43ra56.
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet (Lond. Engl.) 2021, 397, 233-248.
- Wenchao Wang; Xingzhi Jing; Ting Du; Jiabin Ren; Xiaoyang Liu; Feifei Chen; Yuandong Shao; Shengyao Sun; Guihe Yang; Xingang Cui; et al. Iron overload promotes intervertebral disc degeneration via inducing oxidative stress and ferroptosis in endplate chondrocytes. Free. Radic. Biol. Med. 2022, 190, 234-246.
- Grant Ramm; Richard G Ruddell; Iron Homeostasis, Hepatocellular Injury, and Fibrogenesis in Hemochromatosis: The Role of Inflammation in a Noninflammatory Liver Disease. Semin. Liver Dis. 2010, 30, 271-287.
- Leonardo dos Santos; Sabrina Rodrigues Bertoli; Renata Andrade Ávila; Vinícius Bermond Marques; Iron overload, oxidative stress and vascular dysfunction: Evidences from clinical studies and animal models. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2022, 1866, 130172.
- Manqiu Mo; Yunqing Gao; Ling Deng; Yuzhen Liang; Ning Xia; Ling Pan; Association Between Iron Metabolism and Acute Kidney Injury in Critically Ill Patients With Diabetes. Front. Endocrinol. 2022, 13, 892811.
- Shoshana Cook-Libin; Ellen M. E. Sykes; Vanessa Kornelsen; Ayush Kumar; Iron Acquisition Mechanisms and Their Role in the Virulence of Acinetobacter baumannii. Infect. Immun. 2022, 90, e0022322.
- Annie Yap; Heribert Talasz; Herbert Lindner; Reinhard Würzner; Hubertus Haas; Ambient Availability of Amino Acids, Proteins, and Iron Impacts Copper Resistance of Aspergillus fumigatus. Front. Cell. Infect. Microbiol. 2022, 12, 847846.
- Toni Valković; Marija Stanić Damić; Role of Iron and Iron Overload in the Pathogenesis of Invasive Fungal Infections in Patients with Hematological Malignancies. J. Clin. Med. 2022, 11, 4457.
- Ravneet Chhabra; Aishwarya Saha; Ashkon Chamani; Nicole Schneider; Riya Shah; Meera Nanjundan; Iron Pathways and Iron Chelation Approaches in Viral, Microbial, and Fungal Infections. Pharm. 2020, 13, 275.
More
