Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jun Wei and Version 2 by Rita Xu.
Li metal has emerged as a promising anode material for high energy density batteries, due to its low electrochemical potential and high specific capacity of 3860 mAh·g−1. These characteristics make it an attractive choice for electric vehicles and power grids.
- Li batteries
- Li metal anode
- dendrite issue
1. Introduction
The commercialization and renewal of energy storage devices has greatly promoted the developments of portable electronics and electric vehicles, which has hugely revolutionized our daily life [1][2][3][1,2,3]. Among various energy storage devices, Li ion batteries (LIBs) take a large share in the market owing to their high energy density, long cycle life and environmental friendliness [4][5][4,5]. However, the energy densities of current commercial LIBs are far below the expected value. The constant pursuit of the high-energy devices proposed demands high-capacity electrode materials. Graphite is one of the most commonly used anode materials in the market owing to its stable physical and electrochemical properties, low price and mature operation processes, but the specific capacity of a commercially used graphite anode is approaching its theoretical limit (372 mAh·g−1) [6][7][6,7]. To achieve a great enhancement in energy density, high-capacity anodes must be developed [8][9][8,9].
Li metal holds a high theoretical specific capacity of 3860 mAh·g−1 and the lowest redox potential (−3.04 V vs. the standard hydrogen potential electrode) [10][11][10,11], which has been regarded as the most promising anode candidates for Li batteries. Theoretically, in case of LIBs, if a graphite anode can be successfully replaced with a Li metal anode, the energy density can be significantly improved from ~250 Wh·kg−1 to ~440 Wh·kg−1 [12][13][12,13]. Furthermore, metallic Li is also an indispensable anode in the Li-S and Li-O2 energy storage systems, which are expected to exceed 500 Wh·kg−1 in energy densities [14][15][14,15]. However, the practical application of Li metal batteries is greatly hindered by the drastic dendrite issue and large volume change in the Li anode, which seriously affects the lifespan and safety of Li metal batteries [16]. Unlike the ions’ (de) intercalation energy storage mechanisms in commercial graphite anodes, Li metal anodes store energy thorough a Li plating/stripping process [17][18][17,18]. Typically, during the charging process, Li ions gain electrons from the Li anode, are reduced into metallic Li, and simultaneously deposit onto the Li anode. During the discharging process, metallic Li loses electrons and is oxidized into Li ions, which then transfer into electrolytes and ultimately intercalate into the cathode materials. However, during practical cycling, this plating/striping process is not quite reversible and faces great challenges. The ultrahigh reactivity of Li metal makes it prone to reacting with various liquid electrolytes, leading to the formation of a chaotic and heterogeneous solid electrolyte interphase (SEI) layer. This nonuniform SEI film induces the inhomogeneous diffusion of ions and irregular deposition of Li. Owing to the “tip effect” in which charge density tends to accumulate around sharp tips with high curvature, the subsequent Li tends to deposit on the protrusions; after long cycling, Li dendrites are formed [19]. Additionally, the uneven dissolution of Li also results in continuous electrolyte consumption and “dead Li”. These issues accelerate battery failure and pose potential safety hazards for Li metal batteries [20][21][22][20,21,22].
2. Origin of Li Dendrites
Commercial Li metal foil can be served as an anode directly, with no additional current collectors, which makes it an ideal candidate in terms of practical operation. However, the host-less Li metal faces serious challenges [23][24][27,28]. During a typical charging process, masses of solvated Li ions transfer through the electrolyte to the near-surface of the anode, where they are desolvated and reduced into Li atoms that are adsorbed onto the surface of anode. The final morphology of the electroplated Li anode is determined by the diffusion paths of these reduced Li atoms and their incorporation with metal lattice [25][26][29,30]. Conversely, during the discharging process, opposite electrochemical behaviors occur. The electroactive Li atoms are expected to conduct plating/striping in a two-dimensional layer-by-layer manner, resulting in a dense Li layer that provides a highly reversible anode for Li metal batteries. However, in practical occasions, the Li plating/striping processes are often drastically uncontrollable [27][28][29][31,32,33]. Prior to normal charging/discharging processes, an insulated SEI layer is formed on the interface of the Li metal anode and the electrolyte [30][31][32][34,35,36]. However, due to the uneven electric field and unavoidable convection of cations, it is difficult to ensure homogeneous Li metal nucleation sites. Moreover, the following Li atoms are thermodynamically driven to deposit towards one dimension, resulting in the formation of dendrite whiskers rather than a two-dimensional dense layer. This is because protrusion with high curvature often gains higher current densities, causing Li atoms to deposit on the top of these protrusions and form Li dendrites [33][37]. Furthermore, the cation gradient in electrolyte caused by fast Li deposition will lead to local space charge near the electrode and result in branched Li deposition [34][38]. As a result, dendritic Li is a common occurrence in Li metal batteries, especially under high current densities [35][36][39,40]. When the Li deposition process proceeds rapidly, the consumption rate of Li ions is much faster than the ions’ diffusion rate, and the concentration gradients are enlarged and highly inhomogeneous Li deposition occurs. Due to the high reactivity of Li metal, the dendritic Li reacts with organic electrolytes and forms an insulated SEI layer, exacerbating the uneven distribution of electric field and inducing more Li dendrites, as shown in Figure 1 [37][38][41,42]. To clearly show the nucleation and growth of Li dendrites, Akihiro’s group developed in-situ transmission electron microscopy (TEM) to observe this process directly, and they also provide quantitative analysis for the growth of a Li dendrite [39][43].
Figure 1. Schematic of Li dendrite growth.
3. Construction of 3D Li Metal Hosts
The fabrication of conductive 3D hosts for the accommodation of host-less Li metal has been proven to be an effective strategy to address both dendrite and volume change issues [40][41][42][44,45,46]. Conductive 3D porous hosts are beneficial to induce uniform Li deposition because the increased surface area helps to decrease the current density and offer more ion diffusion pathways [43][44][47,48]. Besides, the inner space of the 3D skeleton can provide enough space for Li deposition, making it possible to achieve high Li plating capacity. Conductive 3D hosts can be classified using the major materials of skeletons, such as conductive carbon-based and metallic-based hosts, both of which show great promise in suppressing the dendrite and volume change issues. Typically, carbon-based hosts are particularly advantageous due to their intrinsic lightweight, high conductivity, mechanical stability and the diversity of structures [43][45][47,49]. For instance, Hu’s group fabricated a 3D carbon nanotube sponge (CNTS) using a typical chemical vapor deposition method. The diameter of these as-prepared CNTs rages from 30 to 50 nm, and these self-assembled CNTs construct a 3D porous interconnected framework. Afterwards, a thin Al2O3 layer was coated on the surface of CNTs with the help of an ALD system. The obtained 3D porous ALD-CNTs hold high specific surface area and good electrical conductivity, and have also been reported as promising hosts for uniform Li metal deposition [46][50]. Because 3D CNTS could provide abundant active sites for Li deposition and promote uniform charge distribution, Li dendrite growth was effectively suppressed. Compared with the Li@Cu foil counterpart, the Li@ALD-CNTS electrode delivers 2.3 times higher Coulombic efficiency after longer cycles. Xu et al. [47][51] reported a graphene/MgF2 framework for Li accommodation, and they prepared the freestanding MgF2 − rGO film by mixing homogeneous MgF2 + rGO solution before vacuum filtration and drying processes. After that, molten Li was absorbed into the dried composite film, forming MgxLiy/LiF-Li-rGO composite anode. During cycling in both symmetric full batteries, the 3D porous rGO framework can maintain a robust structure for stable Li plating/striping, and the well-distributed lithiophilic MgF2 sites ensure uniform Li deposition. The asymmetric cells assembled with MgxLiy/LiF-Li-rGO composite anode delivers a good cycling stability of 450 h at 1 mA·cm−2 with a capacity of 1 mAh·cm−2. The reasonable structure design of the 3D host is crucial, but the feasibility, simplicity and economy of manufacturing processes are also non-negligible factors. Lu’s group [48][52] deployed a one-step method to construct a 3D porous composite anode (Figure 2a). They chose copper meshes with various pore sizes and cut them into a circle shape, and then Li metal was embedded onto the copper mesh with the aid of external mechanical pressure. After this simple and cost-effective operation, the 3D Cu/Li component electrodes were successfully fabricated. Benefiting from the porous structure, this Cu/Li composite anode exhibited favored charge transfer kinetics and a smooth Li deposition process, effectively preventing dendrite growth and volume expansion. After 100 cycles, the composite anode still shows a high coulombic efficiency of 93.8%, which is much higher than that of the common anode with only 30.9% at 70 cycles. Zhang’s group [49][53] obtained a 3D CuO and SnO2 co-coated Cu host (3D CSCC) via the oxidation of commercial bronze foil by diluted aqueous ammonia solution. After holding in the ammonia solution for 36 h at 5–8 °C, the flat foil transformed into a spongy 3D structure (Figure 2b), providing an enlarged surface area and lower current density, as well as good accommodation for Li metal to release the volume change. Moreover, the modified lithophilic CuO and SnO2 layer helped to lower the Li deposition overpotential and ensure homogeneous Li plating. As a result, this 3D CSCC demonstrated an excellent stability of 3000 h at 1.0 mA·cm−2. To obtain high energy density, double-sided cathodes and anodes are commonly applied in commercial batteries. Pointing at the double-sided Li anodes for commercial Li batteries, Ma’s group [50][54] fabricated a bidirectional porous Cu film with well-connected and tunable pores as a Li host, the through-pore structure was advantageous to the suppression of Li dendrite and the reduction of weight owing to the absence of a dense Cu layer. Benefiting from this well-designed, light-weight host, Li dendrite was released and allowed for full utilization of the anode, resulting in significant improvements in both energy density (~187.5%) and cycling life. In addition to the above monodispersed pore frameworks, the rational design and fabrication of 3D hosts with gradient pore size can lead to better performance. Lee’s group [51][55] explored the influence of pore size distribution on Li deposition behavior. They fabricated gradient-sized pores along the pore depth direction by staking and sintering a commercial Cu particle. When used as a Li metal host, superconformal Li deposition was observed. Compared to the conformal Li deposition behavior in the monodisperse pore host counterpart, this superconformal deposition demonstrated superior cycling stability of 760 cycles at 2 mA·cm−2 (Figure 2c). This work sheds lights on the gradient structure design and its potential in stabilizing the Li metal anode. Zheng’s group [52][56] synthesized a Cu-decorated carbon nanofiber (Cu-CNF) matrix through an electrospinning process in which the superior lithiophilic nanocopper seeds can induce preferable Li nucleation, while the 3D host enables controlled Li growth (Figure 2d). As a result, homogeneous Li deposition was realized, and the symmetric cell assembled with Cu-CNF-scaffolded Li delivered a long cycle stability of 500 h at 5 mA·cm−2. When coupled with different cathodes including NCM811 and LFP, the half-batteries show satisfactory electrochemical performance. In contrast, a bare CNF skeleton was also prepared as a counterpart, but due to the serious mismatch of metallic Li and carbon, the nucleation and deposition of Li were uncontrollable, which also demonstrates the importance of lithiophilic character for the 3D skeleton.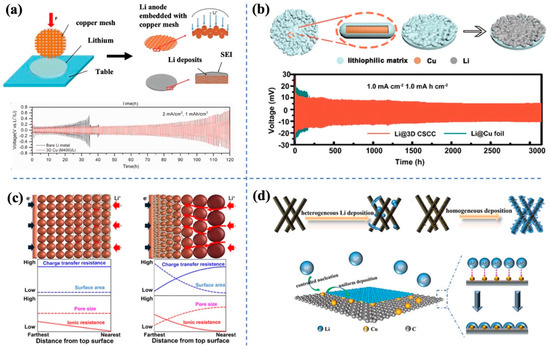
Figure 2. (a) Schematic of 3D Cu/Li component electrodes and the cycling performance of symmetric cells. (b) Schematic of 3D CSCC and the cycling performance of symmetric cells. (c) monodisperse pore size framework (left) and gradient pore size framework (right). (d) Schematic of Cu-CNF and Li deposition behavior.
