Gout is the monosodium urate (MSU) crystal deposition disease. The experts from the Gout, Hyperuricemia, and Crystal-Associated Disease Network (G-CAN) defined the disease as being present when MSU crystal deposition is accompanied by clinical manifestations such as flares, persistent arthritis, and/or tophi. Gout is intimately associated with cardiovascular disease—especially in cases of an atherosclerosis origin, but also with others such as heart failure, atrial fibrillation, or aortic valve stenosis. Besides the common presence of vascular comorbidities in gout sufferers, the disease is—in itself—an independent cardiovascular risk factor, with disease events and mortality attributable to having this condition. Crystal-derived persistent inflammation is likely behind the association.
- gout
- cardiovascular disease
- cardiovascular risk
- tophi
- inflammation
- hyperuricemia
1. Introduction
| Paper | Population | Type of Cardiovascular Disease | Gout Population, Compared to Controls a |
|---|---|---|---|
| Choi, 2007 [29] | Health Professionals Follow-up Cohort | Cardiovascular mortality | RR 1.35 (1.19–1.55) |
| Kuo, 2010 [30] | Chang Gung Memorial Hospital, Taiwan | Cardiovascular mortality | HR 1.97 (1.08–3.59) |
| Kuo, 2011 [31] | National Death Registry of Taiwan | Cardiovascular mortality | SMR 1.58 (1.39–1.78) in men |
| SMR 1.81 (1.46–2.23) in women | |||
| Stack, 2013 [32] | NHANES-III | Cardiovascular mortality | HR 1.46 (1.07–2.00) |
| Dehlin, 2022 [15] | Western Sweden | Cardiovascular mortality | HR 1.17 (1.12–1.23) |
| Abbott, 1988 [33] | Framingham Study | Coronary heart disease | RR 1.6 (1.1–2.5) |
| Krishnan, 2006 [34] | Multiple Risk Factor Intervention Trial | Coronary heart disease | OR 1.26 (1.14–1.40) |
| Seminog, 2013 [35] | UK National Linked Dataset of Admissions and Deaths | Coronary heart disease | RR 1.82 (1.78–1.85) in England data |
| Clarson, 2015 [36] | UK Clinical Practice Research Datalink | Coronary heart disease | HR 1.08 (1.01–1.15) in men |
| HR 1.25 (1.12–1.39) in women | |||
| Huang, 2021 [38] | Taiwan National Health Insurance database | Coronary heart disease | HR 1.36 (1.04–2.76) |
| Singh, 2018 [37] | US Medicare dataset | Coronary heart disease (older adults) | HR 1.79 (1.68–1.90) |
| De Vera, 2010 [39] | Former Medical Students Cohort | Coronary heart disease (women) | RR 1.39 (1.20–1.61) in females |
| RR 1.11 (0.99–1.23) in males | |||
| Kuo, 2013 [40] | Taiwan National Health Insurance Database | Coronary heart disease (Young patients with no CVDRF) | HR 1.59 (1.12–2.24) in age 20–44 |
| HR 1.24 (1.08–1.41) in age 45–69 | |||
| HR 1.11 (0.94–1.32) in age ≥ 70 | |||
| Seminog, 2013 [35] | UK National Linked Dataset Admissions and Deaths | Stroke | RR 1.71 (1.68–1.75) in England data |
| Haddadin, 2021 [41] | US National Inpatient Sample | Stroke | OR 1.10 (1.01–1.11) in an AF population |
| Clarson, 2015 [36] | UK Clinical Practice Research Datalink | Peripheral artery disease | HR 1.18 (1.01–1.38) in men, HR 1.89 (1.50–2.38) in women |
| Schlesinger, 2015 [42] | Rutgers-Robert Wood Johnson Rheumatology Department | Erectile dysfunction | OR 2.94 (1.41–6.06) |
| Chen, 2015 [43] | Taiwan National Health Insurance Database | Erectile dysfunction | HR 1.40 (1.11–1.77) in those without comorbidities |
| HR 2.04 (1.63–2.57) in those with comorbidities | |||
| Abdul Sultan, 2017 [44] | UK Clinical Practice Research Datalink | Erectile dysfunction | HR 1.31 (1.24–1.40) |
2. Risk Assessment Tools for Cardiovascular Diseases
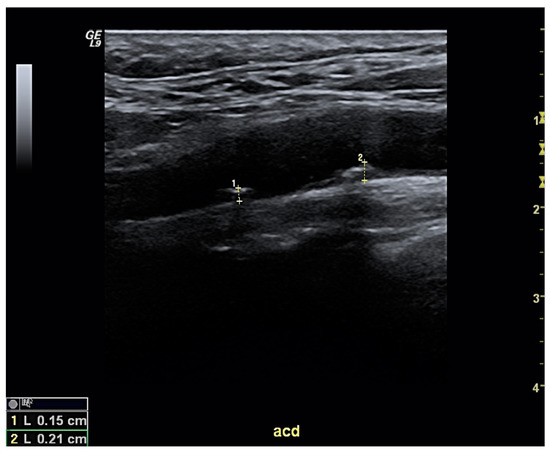
As cardiovascular risk levels vary across individuals, predicting the specific risk for any given patient with gout is vital to tailoring his/her clinical management—both from a cardiovascular perspective and in terms of gout. This aspect is especially relevant for people without established CVD. To this end, some risk assessment tools have been developed to simultaneously evaluate individual cardiovascular risk factors and assess the risk of developing CVD, including fatal events. Some tools largely used in primary care include the Framingham Heart Study (FHS) [51][79] and the Systematic Coronary Risk Evaluation (SCORE) [52][80], which has been recently updated as SCORE2 [53][81]. However, no validation studies have been performed in patients with gout. Data from other inflammatory diseases such as rheumatoid arthritis have pointed to risk underestimation [54][82], which has led to the development of specific risk calculators; for example, the ERS-RA [55][83]. FHS and SCORE were only moderately accurate in detecting the presence of subclinical carotid atherosclerosis patients with gout, with areas under the curve of 0.707 and 0.705, respectively [13]. Both scales lacked sufficient sensitivity (22.5% and 49.0%) despite good specificity results (89.3% and 80.4%), suggesting the possibility that high-risk cardiovascular subjects can go undetected. Gamala and colleagues later reported that a substantial risk reclassification of gout patients can be achieved if they are classified as having inflammatory arthritis as per the Dutch-adapted SCORE [56][84]; however, longitudinal data is needed to validate this prediction. The screening for subclinical atherosclerosis using ultrasound is simple, accessible, and reliable—especially for carotid arteries (Figure 1), which carry prognostic consequences. The European cardiovascular guidelines recommend that the identification of subclinical atherosclerosis should prompt the physician to classify the patient as being in the highest cardiovascular risk category [57][85], since this factor independently predicts both coronary and cerebrovascular events [58][59][86,87]. On the other hand, the guidelines caution against making risk estimations based on intima-media thickness measurements. The reported prevalence of carotid atheroma plaques in patients with gout ranges from 29.1% to 59.2% [13][49][60][61][62][63][13,49,88,89,90,91]—percentages that vary depending on several factors (such as patient demographics and/or disease duration). In any case, the percentage is likely higher than in people without gout. Our group demonstrated that a cardiovascular screening strategy incorporating risk assessment tools and carotid ultrasound is capable of reclassifying the risk of more than half of new gout patients seen in rheumatology clinics, with two-thirds of them ultimately meeting the very-high risk level threshold [13].