Vitamin D seems to be involved in infections, autoimmune diseases, cardiometabolic diseases, and cancer development. The relationship between vitamin D and insulin resistance has been a topic of growing interest. Low 25-hydroxyvitamin D (25(OH)D) levels appear to be associated with most of the insulin resistance disorders described to date. In fact, vitamin D deficiency may be one of the factors accelerating the development of insulin resistance. Vitamin D deficiency is a common problem in the population and may be associated with the pathogenesis of diseases related to insulin resistance, such as obesity, diabetes, metabolic syndrome (MS) and polycystic ovary syndrome (PCOS). An important question is the identification of 25(OH)D levels capable of generating an effect on insulin resistance, glucose metabolism and to decrease the risk of developing insulin resistance related disorders.
1. Background
Currently, vitamin D insufficiency affects more than half the population of all ages
[1]. The role of vitamin D in bone health is well known. In addition, vitamin D may also play a role in extra-skeletal functions. Vitamin D is a fat-soluble prohormone steroid that has endocrine, autocrine, and paracrine functions
[2]. Vitamin D acts as a chemical messenger
[3][4][3,4] and is involved in the regulation of transcription in approximately 3% of the human genome
[5]. Most tissues and organs have receptors for vitamin D, and it appears to be involved in many biological functions. In fact, some studies have shown that low 25(OH)D levels are related to other pathological conditions such as autoimmune diseases, hypertension, cardiovascular disease (CVD)
[4][6][4,6], and cancer
[7]. Insulin resistance has also been linked to vitamin D deficiency. Furthermore, insulin resistance has been linked to multiple disorders such as obesity, type 2 diabetes (T2D), and its complications, MS, and PCOS. In this context, all these diseases could potentially be linked with vitamin D deficiency.
Obesity is generally described as a condition of excessive fat accumulation, with abdominal fat being the main risk factor for insulin resistance
[8]. Obesity has become a major public health problem worldwide, being the main cause to the development of diseases such as T2D and CVD
[9]. The state of insulin-resistant obesity is often associated with a low circulating concentration of 25(OH)D
[10]. However, the possible mechanisms underlying this hypovitaminosis D remain to be elucidated. Furthermore, the role of vitamin D supplementation in obesity is also being studied.
The incidence of T2D is increasingly common and alarming worldwide, in part, to higher obesity rates
[11]. The World Health Organization (WHO) reported that most of the diabetes cases (90%) constitute T2D with 15 million affected people worldwide. Moreover, this number could double by 2025
[12]. The systemic inflammation, pancreatic β-cells disfunction, and defect in insulin signaling pathway are engaged in insulin resistance and T2D development
[11]. A reduction in the level of some metabolic parameters associated with vitamin D supplementation has been reported by several clinical studies
[13][14][13,14]. However, the level of 25(OH)D required to obtain an improvement in glycemic homeostasis and to decrease the risk of developing T2D is not fully established.
MS is characterized by a combination of some risk factors such as central (intraabdominal) obesity, hypertension, increased triglyceride (TG) serum levels, decreased high-density lipoprotein cholesterol (HDL-C) levels, hyperglycemia, and insulin resistance
[15]. The dysfunction and distribution of adipose tissue has also been considered as an important factor, and the abdominal location of excess adipose tissue has been most closely associated with insulin resistance
[16]. The prevalence of MS has increased in recent years, which has been attributed besides to the aging of the population, to the increase in obesity rates related to lifestyle changes, such as low physical activity and poor healthy eating habits
[17][18][17,18]. It has been proposed that low serum 25(OH)D levels are associated with a higher risk of MS and with the different components that define MS. However, this relationship is not fully established. There is also insufficient scientific evidence, as the results of the different studies are discordant, on the effect of vitamin D supplementation in MS.
PCOS affects up to 25% of women throughout the reproductive years, making it the most common endocrine disorder
[19]. PCOS is a heterogeneous disorder, related to metabolic abnormalities such as insulin resistance, systemic inflammation, dyslipidemia, and endothelial dysfunction. The Rotterdam workshop consensus
[20] established diagnostic criteria for PCOS based on the combination of at least two of the following three clinical features: hyperandrogenism (clinical and/or biochemical), with acne, androgenic alopecia, and hirsutism; chronic oligo-anovulation; and polycystic ovaries on ultrasound
[21]. Although insulin resistance was not included in the Rotterdam criteria, it is a recurrent sign in PCOS. In fact, the key role of compensatory hyperinsulinemia in the onset and progression of PCOS is supported by scientific evidence
[22]. Approximately 30–40% of normal-weight PCOS patients and up to 80% of PCOS women with upper body obesity (increased waist-hip circumference) have hyperinsulinemia secondary to insulin resistance
[23][24][23,24]. There are publications suggesting a molecular implication of 25(OH)D deficiency in insulin resistance, dyslipidemia, inflammation, and decreased fertility, i.e., clinical and metabolic phenomena frequently found in PCOS. However, the molecular mechanism relating 25(OH)D levels and the development of PCOS, as well as vitamin D supplementation and PCOS improvement are currently unknown.
The main circulating form of vitamin D used to determine vitamin D status is calcidiol or 25(OH)D
[25]. The definition of adequate 25(OH)D concentrations remains controversial, so there is no clear cutoff point for defining optimal levels of vitamin D. Several studies have proposed that 25(OH)D levels above 30 ng/mL would ensure adequate bone health
[26]. However, there has been no evidence regarding which levels of 25(OH)D would be optimal to obtain benefits with respect to glucose and energy metabolism and other health targets
[27][28][27,28].
2. Vitamin D and Insulin Resistance Physiology
The term of vitamin D includes ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). The main vitamin D metabolites according to their hydroxylation patterns, are calcidiol (25(OH)D) and calcitriol (1,25-dihydroxyvitamin D3 or 1,25(OH)
2D). Vitamin D in humans comes mainly from the skin and, to a lesser extent, from the diet including foods rich in vitamins D2 and D3 or supplements. Serum vitamin D is bound to vitamin D binding protein (DBP), through which it is transported to the liver where is converted to 25(OH)D by 25-hydroxylase. 25(OH)D leads to 1,25(OH)
2D, the most biologically active form of vitamin D, in the kidneys through the action of the enzyme 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). The presence of CYP27B1, along with the vitamin D receptor (VDR) in several tissues, suggests that vitamin D could have a key function beyond bone metabolism
[29][30][29,30].
VDR belongs to the nuclear receptor family participating in DNA transcription
[31][32][31,32]. Although the VDR acts primarily as a nuclear transcription factor, non-genomic actions of vitamin D have been postulated that involve rapid binding of 1,25(OH)
2D to cytosolic and membrane VDR that activates several second messenger systems
[33]. A vitamin D response element region was identified in the promoter of the insulin receptor gene, so that vitamin D may be involved in the transcriptional control of insulin
[34].
Retinoid X receptors (RXRs) are ligand-inducible transcription factors belonging to the superfamily of nuclear receptors which, in the presence of their ligand, can form homodimers or heterodimers with other receptors, including VDR regulating important genes involved in energy homeostasis
[35][36][37][35,36,37]. Vitamin A metabolite 9-cis retinoic acid acts as a ligand of RXR and it has been found to be a pancreas-specific autacoid expressed by β-cells capable of exert an effect on the control of glucose levels. Some studies have found increased 9-cis retinoic acid levels associated with mouse models of obesity
[38][39][38,39]. Based on this, the interaction between VDR, RXR and their ligands could play a relevant role in the pathophysiology of insulin resistance.
The level of glutathione has also been shown to play an important role in the regulation of vitamin D levels. Animal and human studies have found that glutathione is essential for the conversion of provided vitamin D into active vitamin D metabolites: 25(OH)D and 1,25(OH)2D and that it positively regulates the bioavailability of 25(OH)D
[40][41][40,41]. In addition, vitamin D has also been shown to increase glutathione thus contributing to the reduction of the oxidative stress
[42]. Based on these findings, insulin resistance could be related to the glutathione deficiency that exists in many diseases, such as obesity and diabetes
[43].
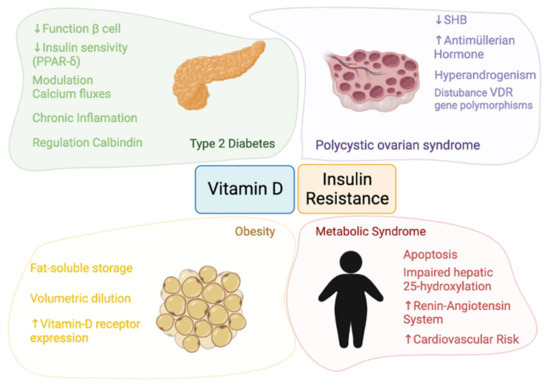
The pathophysiology linking vitamin D and insulin resistance and the four themes involved in this
re
ntryview—obesity, T2D, MS, and PCOS—are detailed in
Figure 1.
Figure 1.
Pathophysiology of the relationship between vitamin D and insulin resistance.
In summary, vitamin D may play an important role in the regulation and of pancreatic β-cells function in T2D patients
[13][14][13,14] since calcitriol (1,25(OH)
2D) acts as a chemical messenger by interacting with calcium flux-regulating receptors on the β-cells
[44][45][44,45]. Moreover, vitamin D is able to reduce hyperactivity of the renin-angiotensin system and to improve the function of β-cells
[46]. On the other hand, vitamin D could influence the insulin secretion regulated by the opening and closing of calcium channels and 1,25(OH)
2D may also improve insulin sensitivity by stimulating the expression of insulin receptors and activating peroxisome proliferator-activated receptor delta (PPAR-δ)
[46]. Finally, the effects of chronic inflammation may be reduced by vitamin D, because vitamin D was shown to deactivate inflammatory cytokines associated with insulin resistance and to promote calbindin expression, leading to protection from apoptosis
[47][48][47,48].
In the case of obesity, VDR mRNA expression has been identified in visceral and subcutaneous adipose tissue as well as in primary adipocytes. This, coupled with the fact that adipose tissue constitutes a reservoir of vitamin D, suggests that vitamin D plays a key fat-associated role
[8][10][8,10].
Regarding to MS, vitamin D deficiency may reduce the ability of β-cells to convert proinsulin to insulin
[49]. Furthermore, the two most widely accepted hypotheses linking vitamin D and MS are the possible sequestration of vitamin D and its volumetric dilution.
In PCOS, the resulting hyperinsulinemia inhibits hepatic synthesis of sex hormone binding globulin (SHBG), leading to increased circulating free androgens
[50][51][50,51]. A positive correlation has been found between serum 25(OH)D and SHBG levels. Moreover, an association between vitamin D deficiency with VDR gene polymorphisms has been reported in PCOS. In a review, the authors found that vitamin D was a predictor of insulin resistance in both PCOS and control women
[52]. The authors point out that only one study demonstrates no effect of vitamin D3 supplementation on insulin resistance
[52][53][52,53].