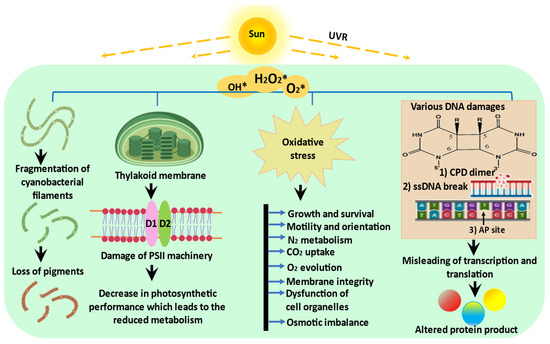
2. Impact of UVR on Cyanobacteria
The surface of the Earth receives very small amounts of solar UVR (UV-C, 0%; UV-B, <1%; UV-A, <7%), but this part of the solar spectrum is extremely energetically active
[1]. In cyanobacteria, there are several direct targets for harmful UV-B radiation, such as proteins and DNA, which have absorption maxima in this region, whereas UV-A irradiation has indirect effects through energy transfer from UV-A-stimulated chromophores to the DNA target
[1]. After being exposed to UV-B radiation for 9 h, the levels of photosynthetic pigments, total chlorophyll, total carotenoids, and c-phycocyanin were reduced in
Arthrospira platensis [11]. According to Vega et al.
[12], numerous cyanobacteria and microalgae are affected by UV-B exposure in terms of their development, survival, pigmentation, orientation, growth, general metabolism, photosynthesis, nitrogen fixation, and nitrogen uptake (
Figure 1).
Figure 1. Schematic representation of possible effects of UVR on cyanobacteria: UVR exposure triggers the production of ROS which negatively affects the morphology, physiology, and biochemistry of cyanobacteria (modified from Rastogi et al.
[2]).
2.1. Photosynthesis
UVR inhibits a number of photosynthetic processes in cyanobacteria, including the uptake of
14CO
2, O
2 evolution, and ribulose-1,5 bisphosphate carboxylase (RuBISCO) activity. RuBISCO, a holoenzyme, consists of two subunits: the 55 kDa larger subunit (LSU) and the 14 kDa smaller subunit (SSU)
[13]. When exposed to UV-B radiation, RuBISCO becomes susceptible to a variety of modifications, including photo-degradation, polypeptide chain fragmentation, denaturation, active site modification, and the enhanced solubility of membrane proteins
[14]. The availability of ATP and NADPH
2 may also be reduced, which could prevent the fixation of carbon dioxide. Additionally, UV-B radiation negatively impacts tyrosine electron donors, quinine electron acceptors, and D1 and D2 proteins of photosystem II (PSII)
[15]. Damage to the water-oxidizing Mn cluster in the PSII reaction centre (RC) leads to electron transport chain (ETC) inactivation
[16]. The cyanobacterium
Anabaena variabilis PCC 7937, when exposed to UVR, showed a reduction in the overall photosynthetic yield because of a reduction in the relative electron transport rate (rETR)
[17]. Under UV-B radiation,
Spirulina platensis showed a distorted thylakoid membrane with decreased Chl
a content
[18]. After exposing a
Phormidium strain to UV radiation for only a few minutes, Häder
[19] observed a reduction in O
2 evolution. Additionally, UV-B radiation causes oxidative damage which leads to the lipid peroxidation of polyunsaturated fatty acids (PUFAs), which in turn weaken the strength of cells and thylakoid membranes and harm photosynthetic components
[20].
2.2. Growth, Cell Differentiation, and Motility
UV-B irradiation severely affects cyanobacteria’s biochemical and physiological life processes, such as morphology, survival, cell differentiation, growth, development, pigmentation, orientation, and motility
[21]. Döhler et al.
[22], Häder et al.
[23], and Newton et al.
[24] reported the inhibitory effects of UV-B radiation on certain cyanobacteria, such as
Anabaena flos-aquae,
Synechococcus leopoliensis, and
Phormidium uncinatum. It has been suggested that UV-B radiation damages cellular components that absorb radiation between 280 and 320 nm, leading to cell death. The tolerance of different species to UV-B radiation varies, and even strains that are closely related exhibit differences in sensitivity. In Antarctica cyanobacterium,
Oscillatoria priestleyi growth was completely suppressed, whereas it was 62% in the case of
Phormidium murrayi following a similar dosage of UV exposure. UVR significantly reduces the proportion of motile filaments and reduces the linear velocity of cyanobacterial cells, which affects their capacity to protect themselves from harmful UVR
[2]. After 10–30 min of UVR exposure, a study has shown a significant reduction in the number of motile filaments of
Anabaena variabilis, Oscillatoria tenuis, and
Phormidium uncinatum. UV-B radiation hinders the ability of cyanobacteria to establish themselves in their photo environment, which ultimately leads to their premature death
[25]. The differentiation of vegetative cells into heterocysts or akinetes and the fragmentation of filaments have been reported in the cyanobacterium
Anabaena siamensis TISTR-8012 due to exposure to UV-B radiation
[2]. Under UV-B radiation, the cyanobacterium
Anabaena sp. PCC 7120 showed heterocyst differentiation of vegetative cells and a reduction of up to 49% in trichome length
[9]. The three main heterocyst polypeptides (26, 54, and 55 kDa) are believed to be depleted after UV-B treatment as a result of the breakdown of the multi-layered heterocyst wall, which is crucial for maintaining the active form of the enzyme nitrogenase
[26].
2.3. Nitrogen Metabolism
Nitrogenase is the key enzyme for nitrogen fixation, and UV-B radiation also significantly inhibits the process of nitrogen fixation, either directly or indirectly, due to the highly sensitive nature of the nitrogenase enzyme to UVR
[27]. The activity of nitrogenase in a
Nostoc species was lost after 45 min of exposure to UV-B, but nitrate reductase and the glutamine synthetase activity were found to be unaffected
[28]. Strong protection against nitrogenase inactivation was provided by ascorbic acid or reduced glutathione. By preserving the sulfhydryl groups or disulfide bonds, reducing agents are known to maintain the native structure of various proteins in their original condition. Nitrogenase is extremely sensitive to oxygen and is likely to exhibit changes in its sulfhydryl groups. In different species, different levels of UV-B protection mechanisms have been observed, which may account for the variation in the amount of time needed to completely kill and inactivate nitrogenase activity
[29]. Many researchers believe that the high susceptibility of nitrogenase to UV-B radiation is caused by either the presence of aromatic amino acids or the enzyme’s native structure
[25].
2.4. Biomolecules
UV radiation has been shown to have an effect on the structural and functional integrity of accessory photo-harvesting pigments, such as phycocyanin, phycoerythrin, and allophycocyanin, in the marine cyanobacterium
Lyngbya sp. A09DM
[30]. UV-B radiation severely affects low-molecular-weight proteins. In
Nostoc sp., αβ monomers of phycocyanin with a very low size (approximately 20 kDa) were the most affected. When
Nostoc carmium and
Anabaena sp. were exposed to UV-B radiation for 90 or 120 min, proteins with a size of 14.5–45 kDa were completely lost, but proteins with a size of about 55–66 kDa were unaffected, even after 120 min of UV-B irradiation. After 150 min of exposure to UV-B radiation, the proteins in
Nostoc commune and
Scytonema sp. completely disappeared
[31]. The total protein profile of
Nostoc spongiaeforme and
Phormidium corium was changed both qualitatively and quantitatively after UV-B radiation and high light treatment
[32]. The number and quantity of protein bands in various cyanobacteria were found to decrease linearly with the increased duration of UV exposure.
When cyanobacteria are directly exposed to UVR, their DNA is subjected to several kinds of damage and their cells also suffer from oxidative stress. One such type of damage that is specifically caused by UV-B radiation is covalent linkage between the bases, which results in lesions such as cyclobutane pyrimidine dimers (CPDs), pyrimidine photoproducts (6-4PPs), and their Dewar isomers. These CPDs and 6-4PPs DNA lesions produced by UV-B radiation can produce primary and secondary breaks in DNA, respectively
[33]. These breaks stall the DNA polymerase and prevent it from progressing, thus inhibiting transcription and translation machinery
[33], which eventually results in mutations or the death of the organism
[34]. Numerous filamentous and unicellular cyanobacterial species such as
Anabaena sp.,
Nostoc sp., and
Scytonema sp. produce thymine dimers (T< >T) when exposed to UV radiation
[35]. Thymine dimers appear more frequently under continuous UVR exposure. A cyanobacterium,
Arthrospira platensis, formed CPD under UV stress in a way that was dependent on the temperature and its biomass. UV-induced DNA damage is reported in
Anabaena variabilis PCC7937 and
Synechocystis PCC 6308. Mosca et al.
[36] observed an increased accumulation of DNA lesions in dried UV-irradiated biofilms compared to dried biofilms of the desert cyanobacterium
Chroococcidiopsis.