ImmLunotherapy for non-small cell lung cg cancer is divided to Non-Small Cell Lung Cancer (NSCLC) is incorporated increasingly in first line treatments protocols. Multiple phase 3 studies have tested different medications targeting programmed death receptor 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), with or withoutcomprising about 85% of lung cancer cases, and small cell lung cancer (15% of lung cancer cases). Non-small cell lung cancer (NSCLC) has several subtypes: a. Adenocarcinoma, b. Squamous cell carcinoma, c. Large cell carcinoma, or d. mixed histology. Treatment of localized NSCLC is surgical resection, stereotactic ablative radiation therapy, or combination of chemotherapy. The inclusion criteria differ between the various clinical trials, and radiation (chemoradiation). Treatment of advanced / metastatic disease including the cut-off levels of PD-L1 expression on tumor cells, and the tumor histology (squamous or non-squamous)es targeted therapies, chemotherapy and immunotherapy.
- Non-Small Cell Lung Cancer
1. Introduction
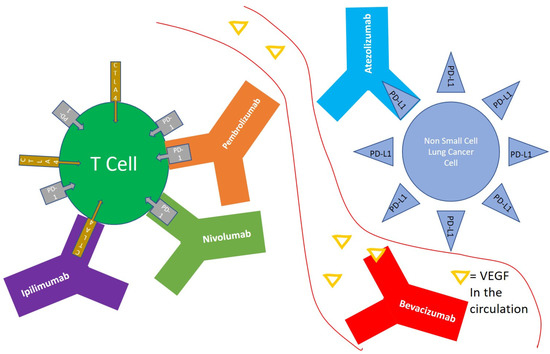
2. Monoclonal Antibodies Targeting Immune Checkpoints
|
Generic Name |
Brand Name |
Antibody Type |
Indications and Usage Other than NSCLC |
Target |
Half-Life (Days) |
|
Pembrolizumab |
Keytruda |
Humanized IgG4 kappa |
· Melanoma · Small Cell Lung Cancer · Head and Neck Squamous Cell Cancer · Classical Hodgkin Lymphoma · Primary Mediastinal Large B-Cell Lymphoma · Urothelial Carcinoma · MSI-H or dMMR Cancers · Gastric Cancer · Esophageal Cancer · Cervical Cancer · Hepatocellular Carcinoma · Merkel Cell Carcinoma · Renal Cell Carcinoma · Endometrial Carcinoma · Tumor Mutational Burden-High Cancer · Cutaneous Squamous Cell Carcinoma |
PD-1 |
22 |
|
Nivolumab |
Opdivo |
Fully human IgG4 kappa |
· Melanoma · Small Cell Lung Cancer · Head and Neck Squamous Cell Cancer · Classical Hodgkin Lymphoma · Urothelial Carcinoma · MSI-H or dMMR colorectal cancer · Hepatocellular Carcinoma · Renal Cell Carcinoma · Esophageal Squamous Cell Carcinoma |
PD-1 |
25 |
|
Atezolizumab |
Tecentriq |
Humanized non-glycosylated IgG1 kappa |
· Urothelial Carcinoma · Triple-Negative Breast Cancer · Small Cell Lung Cancer · Hepatocellular Carcinoma · Melanoma |
PD-L1 |
27 |
|
Ipilimumab |
Yervoy |
Fully human IgG1 kappa |
· Melanoma · Renal Cell Carcinoma · MSI-H or dMMR colorectal cancer · Hepatocellular Carcinoma |
CTLA-4 |
15 |
|
Durvalumab |
Imfinzi |
Fully human IgG1 kappa |
· Urothelial Carcinoma · Small Cell Lung Cancer |
PD-L1 |
18 |
2.1. Nivolumab
2.2. Pembrolizumab
2.3. Atezolizumab
2.4. Ipilimumab
2.5. Durvalumab
3. Chemotherapeutic Agents Used for Treatment of NSCLC Together with Immunotherapy
3.1. Platinum Based Chemotherapeutic Agents
3.1.1. Cisplatin
3.1.2. Carboplatin
3.2. Taxanes
3.2.1. Paclitaxel
3.2.2. Docetaxel
3.2.3. Nanoparticle Albumin-Bound Paclitaxel
3.3. Gemcitabine
3.4. Pemetrexed
4. Phase 3 Randomized Controlled Trials that Includes Immunotherapy for NSCLC
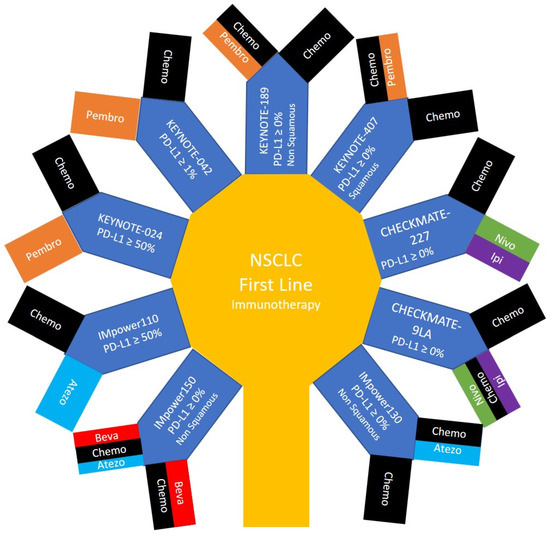
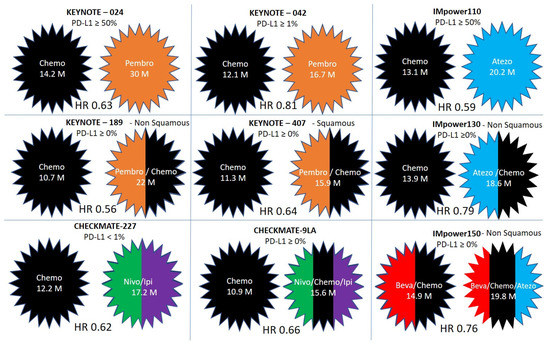
|
|
Pathology |
PDL-1 |
Arm I (OS) |
Arm II (OS) |
HR |
|
KEYNOTE-024 |
squamous (18%) and nonsquamous (82%) |
≥50% |
Pembrolizumab |
Investigator’s choice of platinum-based chemotherapy |
|
|
30 months |
14.2 months |
0.63 |
|||
|
KEYNOTE-042 |
squamous (38%) and nonsquamous (62%) |
≥1% |
Pembrolizumab |
Investigator’s choice of platinum-based chemotherapy doublet |
|
|
16.7 months |
12.1 months |
0.81 |
|||
|
KEYNOTE-189 |
nonsquamous |
Any level |
Pembrolizumab & Pemetrexed + Cisplatin/Carboplatin |
Pemetrexed + Cisplatin/Carboplatin |
|
|
22 months |
10.7 month |
0.56 |
|||
|
KEYNOTE-407 |
squamous |
Any level |
Pembrolizumab & Carboplatin + paclitaxel or nab–paclitaxel |
Carboplatin + paclitaxel or nab–paclitaxel |
|
|
15.9 months |
11.3 months |
0.64 |
|||
|
CHECKMATE-227 |
squamous (28%) and nonsquamous (72%) |
Any level
≥1% <1% |
Nivolumab and Ipilimumab |
Cisplatin/Carboplatin + Gemcitabine (for squamous) or pemetrexed (nonsquamous) |
|
|
17.1 months |
14.9 months |
0.79 |
|||
|
17.2 months |
12.2 months |
0.62 |
|||
|
CHECKMATE 9LA |
squamous and nonsquamous |
Any level |
Nivolumab & Ipilimumab + Cisplatin/Carboplatin + Pemetrexed/Paclitaxel |
Cisplatin/Carboplatin + Pemetrexed/Paclitaxel |
|
|
15.6 months |
10.9 months |
0.66 |
|||
|
IMpower110 |
squamous (25%) and nonsquamous (75%) |
≥50% |
Atezolizumab |
Cisplatin/Carboplatin + Gemcitabine (for squamous) or pemetrexed (nonsquamous) |
|
|
20.2 months |
13.1 months |
0.59 |
|||
|
IMpower130 |
non-squamous |
Any level |
Atezolizumab & Carboplatin +nab-paclitaxel
|
Carboplatin +nab-paclitaxel |
|
|
18.6 months |
13.9 months |
0.79 |
|||
|
IMpower150 |
non-squamous |
Any level |
Atezolizumab + Bevacizumab + Carboplatin, and Paclitaxel |
Bevacizumab + Carboplatin, and Paclitaxel |
|
|
19.8 months |
14.9 months |
0.76 |
4.1. Keynote-024
- (1)
-
Median OS in this study is 30 months, to our knowledge the longest among first line studies of NSCLC.
- (2)
-
Interestingly, females benefited much less than males with pembrolizumab compared to chemotherapy. HR for benefit among men was 0.54, and among women was 0.95. Absolute survival numbers among sexes were not published in the original [33] or updated analysis [32]. This was not a preplanned analysis, and interpretation of the results regarding the patients’ sex should be taken with caution.
- (3)
-
Never smokers had less benefit from Pembrolizumab versus chemotherapy (HR 0.9) compared to smokers (HR 0.59).
4.2. Keynote-042
- (1)
-
Compared to KEYNOTE-024, OS in KEYNOTE-042 was less even in patients with PD-L1 ≥ 50%.
- (2)
-
The similar OS in the initial months of the study between the chemotherapy and Pembrolizumab arms, trending initially to better results with chemotherapy before the curves crosses, indicates that combination therapy could provide better outcomes in a subset of patients.
- (3)
-
Female patients had less benefit compared to male patients. HR for benefit among men was 0.71 and among women was 1.01. This is consistent with KEYNOTE-024 that showed no improved survival for women with Pembrolizumab compared to chemotherapy. This was not a preplanned analysis, and interpretation of the results regarding the patients’ sex should be taken with caution.
- (4)
-
Never smokers did worse with Pembrolizumab versus chemotherapy, with HR of 1.1, compared to 0.6 and 0.71 in former and current smokers, as reported in the publication supplementary appendix [103].
4.3. Keynote-189 and Keynote-407
4.3.1. Keynote-189
4.3.2. Keynote-407
4.4. IMpower110
4.5. IMpower130
4.6. IMpower150
4.7. Checkmate-227
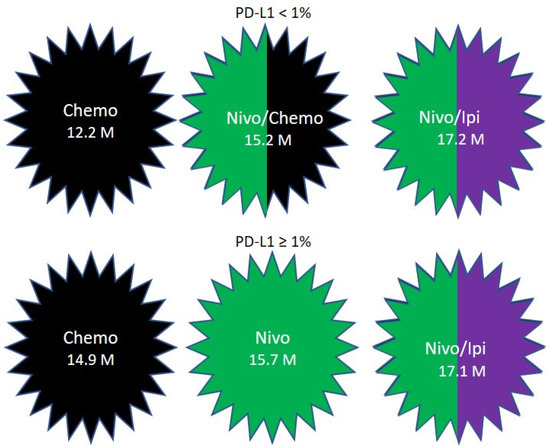
-
Patients who never smoked had OS of 15.3 months with nivolumab plus ipilimumab compared to 16.1 months with chemotherapy alone [111].
-
Patients with PD-L1 < 1% and liver metastasis had a statistically significant benefit from nivolumab plus ipilimumab compared to chemotherapy with survival of 11.7 versus 7.8 months, respectively. This significance was not maintained in patients with PD-L1 ≥ 1 and liver metastasis. For patients, regardless of PD-L1, with liver metastasis, survival was 10.3 months with nivolumab plus ipilimumab compared to 10.4 months with chemotherapy [111].
-
Nivolumab plus ipilimumab was beneficial compared to chemotherapy in patients above and below the age of 65 years.
4.8. Checkmate-9LA
- (1)
-
Clinical benefit for the combination of immunotherapy-chemotherapy was seen over chemotherapy only, regardless of PD-L1 expression.
- (2)
-
Never smokers had worse survival outcome with the immunotherapy-chemotherapy combination compared to chemotherapy only, with a median OS of 14.1 versus 17.8 months, respectively.
- (3)
-
Patients ≥ 75-year-old did worse with the immunotherapy-chemotherapy compared to chemotherapy only, with median OS of 8.5 versus 11.5 months, respectively.
5. Discussion