Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Patrick Di Martino.
Polyurethanes (PURs) are versatile polymers used in a wide variety of fields, such as the medical, automotive, textile, thermal insulation, and coating industries as well as many everyday objects. Many PURs have applications that require a long service life, sometimes with exposure to aggressive conditions. They can undergo different types of physicochemical and biological degradation, but they are not compostable, and many of them constitute persistent waste in the environment. Although both bacteria and fungi can be involved in the degradation of PURs, fungi are often the main biodegradation agents.
- polyurethane
- polyester urethane
- polyether urethane
- deterioration
1. Introduction
Polyurethanes (PURs) are used in medical, automotive, textile, thermal insulative, coating, and everyday objects [1]. PURs can be found in car seats, furniture, mattresses, clothing, waterproof coatings, paints, and pipes. This presence in many fields of application is linked to the versatility of polymers with urethane functions. However, polyurethanes usually have other functions (urea, ether, ester, aromatic, hydroxide, amine, etc.) and, thus, present a wide variety of physical or chemical properties.
Generally, urethane bonds -O-(C=O)-NH- are obtained by reactions between alcohols -OH and isocyanates -N=C=O. Polyurethane is a polymer containing several urethane bonds in its chain. To form a PUR, polyols, and diisocyanates are used. PURs are composed of a succession of hard and soft segments, themselves composed, respectively, of isocyanates and polyols. The nature of the polyol and the isocyanate determines the final properties of the polymer, such as its softness, hardness, and flexibility. Thus, the flexibility of PUR is increased by lengthening the chain of the polyol. Isocyanates are molecules with short chains that constitute areas of increased crystallization, hence the name “hard segments”. Isocyanate can be aliphatic, aromatic, or cycloaliphatic. The most commonly used diisocyanates are isophorone diisocyanate, toluene diisocyanate, and hexamethylene diisocyanate. Polyurethanes can be synthesized without isocyanate. Synthesis can be achieved in an aqueous solvent via transurethanization and aminolysis of cyclic carbonate. It consists of a unique reaction of cyclic carbonates with amines to form hydroxyurethanes. Polyols can be polyether for foams, polyester for thermoplastics, or polycarbonate for implanted biomaterials.
Many PURs have applications that require a long service life, sometimes with exposure to aggressive conditions. They are thus subject to aging and even physicochemical and biological degradation phenomena. Sunlight, water, heat, organic and inorganic chemicals, and reactive oxygen species are all sources of degradation of PURs [3,4][2][3]. Like other synthetic polymers, PURs are significant environmental contaminants despite their usefulness for various human activities. It is essential to understand the mechanisms of their degradation in the environment or during waste treatment processes in order to ensure their functionality over time and limit their negative impact [5,6,7][4][5][6]. The mechanism and ease with which a PUR is biodegraded depend on its molecular composition and structure. The susceptibility of a polymer to biodegradation depends on its physical and chemical characteristics. The higher its molar mass and density, the less susceptible the polymer is to biodegradation. High hydrophobicity may limit the ability of some microorganisms to bind to the material, thus limiting their ability to degrade it. By limiting the accessibility of chains, crystallinity limits the biodegradability of a polymer [8][7]. A highly crosslinked polymer is also less prone to biodegradation. Some chemical bonds that make up a polymer may be more easily degradable than others and be a prime target for biodegradation initiation [9,10,11,12][8][9][10][11]. Hardness also influences the biodegradability of a polymer [13,14][12][13]. The biodegradation of a polymer takes place in several stages as follows: adhesion of cells or spores, growth of the biomass and biofilm development [15[14][15],16], fragmentation of the polymer, depolymerization by the action of enzymes and extracellular free radicals, intracellular metabolization, and finally mineralization. High-molecular-weight polymers cannot be transported across the cell membrane of microorganisms. Their degradation into low-molecular-weight polymers allows them to cross the cell membrane and be metabolized in microbial cells [11,17][10][16].
PURs are not biodegradable or compostable polymers according to NF EN 13432 and OECD (Organisation for Economic Co-operation and Development) 301B standards [18,19][17][18]. Thus, for a material to be considered biodegradable, it must be able to reach 90% biodegradation in less than 6 months, and after composting for 3 months, the total residue greater than 2 mm must be less than 10% of the initial mass. Alternatively, 60% of the material must be released as CO2 within 28 days of exposition to an inoculum from activated sludge, unchlorinated sewage effluents, surface waters, and/or soils. Nevertheless, some degree of biodegradation of PURs can be observed either with microbial consortia present in soil or with microorganisms in vitro in the laboratory.
2. Biodiversity of Fungi Involved in Polyurethane Degradation
Bacteria and fungi can be involved in the degradation of PURs, but fungi are often the main biodegradation agents. Pieces of PESTUR buried in the soil are mainly biodegraded by fungi such as Geomyces pannorum, Nectria gliocladioides, and Penicillium ochrochloron [20][19]. Although both are used to inoculate a waste treatment bioreactor, Fusarium solani is much more effective than Pseudomonas sp. in biodegrading polyurethane foam in vitro [6][5]. Various fungi such as Alternaria, Aspergillus flavus, Aspergillus fumigatus, Aspergillus tubingensis, Chaetobium globosum, members of the Cladosporium cladosporioides complex, Curvularia senegalensis, Penicillium chrysogenum, Papiliotrema laurentii, and Pestalotiopsis are capable of biodegrading PESTURS [10,21,22,23,24,25,26,27,28,29][9][20][21][22][23][24][25][26][27][28]. In addition to their activity towards polyester urethanes, Alternaria, Aspergillus fumigatus, Aspergillus niger, various species of Cladosporium, and Penicillium chrysogenum can also biodegrade PEURs [25,28,30,31][24][27][29][30]. Fungal biodegradation of PURs is linked to the production of enzymes inducible by the presence of the substrate. Esterase and protease are the two main families of enzymes involved in the biodegradation process, both of which are capable of hydrolyzing the urethane bond. Esterase can also degrade PESTUR by hydrolyzing the ester bond. Amidases are involved in PUR biodegradation by hydrolysis of amide bonds. Ureases can biodegrade PUR poly(ether urea) by mainly attacking urea bonds. Owen et al. described a urethane hydrolase produced by the soil fungus Exophiala jeanselenei involved in the biodegradation of urethane groups in Tolyl-carbamate urethane compounds [32][31]. Recent studies have explored the secretome of fungi capable of using PURs as their sole carbon source [33][32]. Proteases are the dominant enzyme group in Fusarium secretomes of different strains. The secretome of Fusarium oxysporum BPOP18 contains several hydrolases, such as acetylesterases, carboxypeptidases, cutinases, lipases, peptide hydrolases, and oxidoreductases. Oxidative enzymes can cleave C-C bonds. Laccases, peroxidases, and tyrosinases are thought to modify the structure of polyurethane compounds by forming carbonyl groups [33,34][32][33]. The fungal biodegradation process is increased when C=C double bonds are present in PUR [35][34]. The chemical structure of PURs determines their biodegradation under composting conditions [36][35]. As in other conditions of exposure to microbial attack, a PEUR is less susceptible to biodegradation than a PESTUR during composting. The increase in the number of hard segments or the presence of aromatic diisocyanates decreases the biodegradability of PUR, unlike that of aliphatic diisocyanates [5,36][4][35]. The biodegradation of PESTUR by thermophilic and thermotolerant fungi is observed at an increased rate during the thermophilic and early maturation phases of the composting process. Thus, the Thermomyces lanuginosus species, which produces numerous enzymes (thermostable proteases, amylases, xylanases, ureases, and lipases), are dominant on the surface of PESTUR coupons at 50 and 55 °C [5][4]. Bio-based PESTUR and, in particular, vegetable oil-based PESTUR can be engineered to be fully biodegradable [37,38,39][36][37][38]. Such polymers can be rapidly biodegraded in compost, soil, and natural ocean environments by depolymerization, resulting in the release of original monomers that are then consumed during microbial growth. In the study by Gunawan et al., SEM imaging showed progressive degradation over time of biodegradable PUR samples during immersion in the ocean (Figure 1) [39][38]. This biodegradation resulted in increased porosity, crumbling, and cracking of the foam surface (Figure 1B–D) compared to the surface of a foam sample not submerged in the ocean (Figure 1A). Biodegradation of Starch-PUR films in soil occurs in successive steps as follows: wetting and colonization by microorganisms, hydrolysis of the starch part, fractionation of the polymer into small structures, and slow degradation of the polyurethane part [40][39].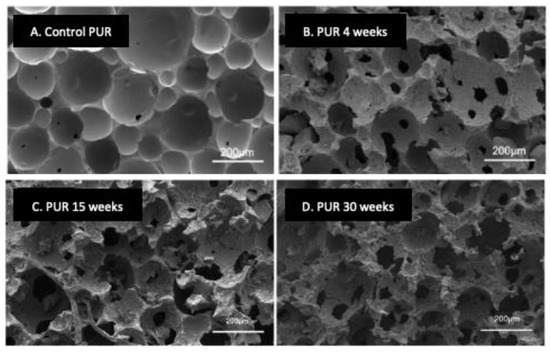
Figure 1. Scanning electron microscopy view of the biodegradation of PUR foam samples composed of linear polyester polyols based on aliphatic diacids and aliphatic diols incubated in a natural ocean environment over time. (A), the control sample was not immersed in the ocean. (B–D), samples were immersed in the ocean for 4, 15, or 30 weeks, respectively. Adapted from Ref. [39][38] which is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
3. Experimental Analysis of Polyurethane Biodegradation
The weight loss measurement is a global analysis, which constitutes the first global approach for studying the biodegradation of PURs [46,47][45][46]. This technique is not highly sensitive and requires significant biodegradation of the polymer to be efficient. In the study by Magnin et al., thermoplastic polyurethanes had a mass loss of a few percent (9% maximum) after exposure for two months at 30 °C to strains of Alternaria, Penicillium, or Aspergillus (Figure 2) [46][45]. In order not to underestimate biodegradation, it is essential to rid the material of all the biomass that has developed on its surface. For this, washes with mechanical action and chemical treatments with ethanol, a non-ionic surfactant, or sodium hypochlorite can be used [27,28,47][26][27][46].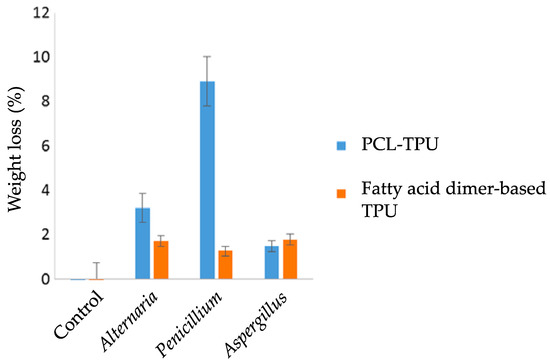
Figure 2. Weight loss measurements of thermoplastic polyurethane polycaprolactone (PLC-TPU) and TPU based on fatty acid dimers after 2 months of incubation at 30 °C with different molds. Control: material incubated in the absence of molds. Adapted from Ref. [46][45] which is an open access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/).
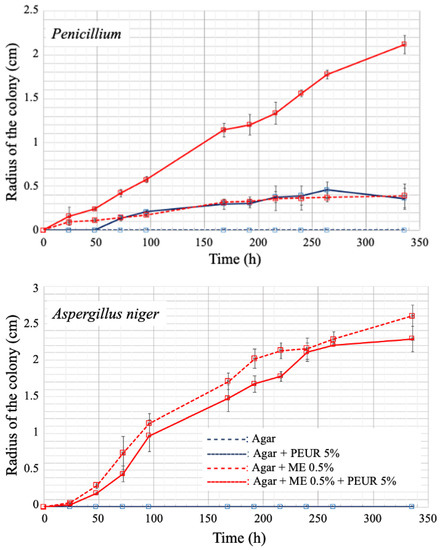
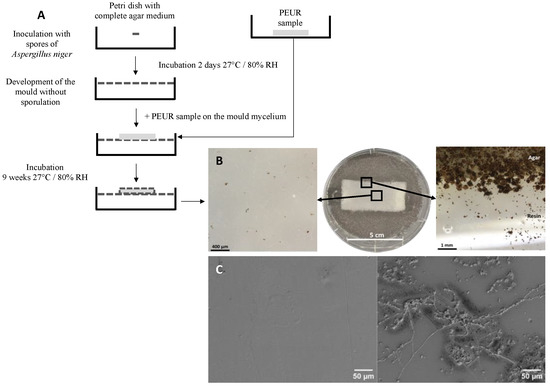
Figure 4. (A), Diagram of method B′ of the ISO 846 standard. In this method, the PUR sample is placed on the agar nutrient medium containing a carbon source when it is completely covered by mycelium. The test was performed in a 90 mm diameter Petri dish. (B), Growth of Aspergillus niger on polyether urethane after 9 weeks of contact according to the method described in A The magnification was obtained with a stereomicroscope. (C), scanning electron microscope views of a PUR coupon incubated on an agar medium containing no carbon source (left picture) and of a coupon of the same material incubated under the same conditions in the presence of A. niger (right picture).
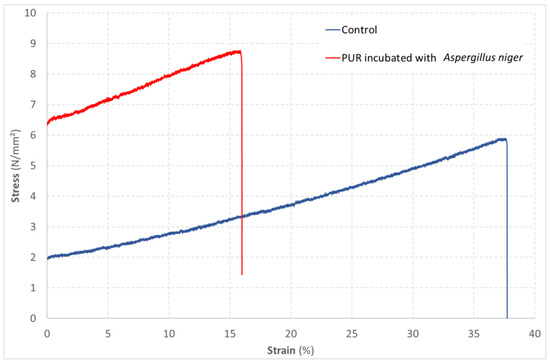
Figure 5. Evolution of the strain of a PUR material as a function of the stress applied in a tensile test. Comparison of an unexposed material (control) with a material exposed to the growth of Aspergillus niger according to method B′ of the ISO 846 standard.
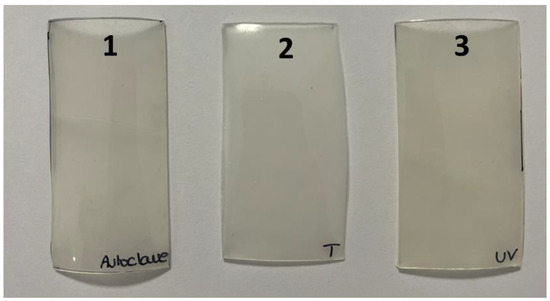
Figure 6. Macroscopic observation of PUR coupons (6 × 3 cm) after different physical treatments. 1, autoclaving 15 min at 121 °C. 2, no treatment (control). 3, UV-C exposure for 24 h at 254 nm.
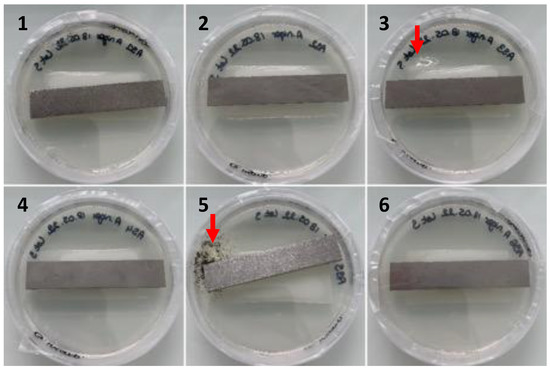
Figure 7. Observation of the growth of contaminants (red arrows, Petri dishes 3 and 5) developing from PUR coupons deposited on nutrient agar (Petri dish 9 cm in diameter) after cleaning with ethanol and disinfection with o-phenylphenol after 6 weeks of incubation at 27 °C/80% relative humidity according to ISO 846.
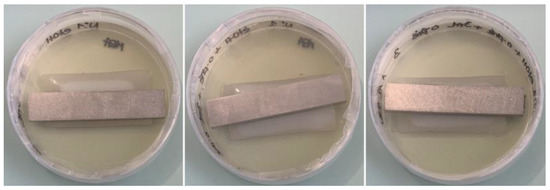
Figure 8. Observation of cleaned and disinfected PUR coupons placed on malt extract agar (Petri dish 9 cm in diameter), incubated for 6 weeks at 27 °C/80% relative humidity. Before depositing on agar plates, the coupons were cleaned with distilled water and disinfected with successive baths of 70% ethanol and 1% o-phenylphenol.
4. Conclusions
Molds are major agents in the biodegradation of PURs. They express this activity within complex communities in soils or composts, and many of them biodegrade polyurethanes in vitro in monoculture. The fungal biodegradation of PURs is mainly linked to the joint secretion of several enzymes that cleave the urethane bond or other bonds in the polymer either by hydrolysis or oxidation. Knowledge of the mechanisms of this bio-degradation can be used to develop materials resistant to fungal biodegradation that are intended for long-term exposure to microorganisms, like waterproofing coatings, or, on the contrary, to develop biodegradable PURs that are compostable or have anti-fouling properties.
Molds are major agents in the biodegradation of PURs. They express this activity within complex communities in soils or composts, and many of them biodegrade polyurethanes in vitro in monoculture. The fungal biodegradation of PURs is mainly linked to the joint secretion of several enzymes that cleave the urethane bond or other bonds in the polymer either by hydrolysis or oxidation. Knowledge of the mechanisms of this bio-degradation can be used to develop materials resistant to fungal biodegradation that are intended for long-term exposure to microorganisms, like waterproofing coatings, or, on the contrary, to develop biodegradable PURs that are compostable or have anti-fouling properties.
References
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101.
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268.
- Gao, Q.; Wang, L.; Luo, H.; Fan, H.; Xiang, J.; Yan, J.; Li, C.; Chen, Z. Photodegradation behavior and blocking strategy of waterborne polyurethane under UV and Xenon irradiation. Mater. Today Commun. 2023, 34, 105212.
- Zafar, U.; Nzeram, P.; Langarica-Fuentes, A.; Houlden, A.; Heyworth, A.; Saiani, A.; Robson, G.D. Biodegradation of polyester polyurethane during commercial composting and analysis of associated fungal communities. Bioresour. Technol. 2014, 158, 374–377.
- Trhlíková, O.; Vlčková, V.; Abbrent, S.; Valešová, K.; Kanizsová, L.; Skleničková, K.; Paruzel, A.; Bujok, S.; Walterová, Z.; Innemanová, P.; et al. Microbial and abiotic degradation of fully aliphatic polyurethane foam suitable for biotechnologies. Polym. Degrad. Stab. 2021, 194, 109764.
- Shashoua, Y.; Peydaei, A.; Mortensen, M.N.; Kanstrup, A.B.; Gregory, D.J. Real time degradation studies on polyurethane household sponges in Danish weather and marine environments. Mar. Pollut. Bull. 2022, 184, 114128.
- Huang, S.J.; Roby, M.S. Biodegradable Polymers Poly(amide-urethanes). J. Bioact. Compat. Polym. 1986, 1, 61–71.
- Nakajima-Kambe, T.; Shigeno-Akutsu, Y.; Nomura, N.; Onuma, F.; Nakahara, T. Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl. Microbiol. Biotechnol. 1999, 51, 134–140.
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480.
- Alshehrei, F. Biodegradation of Synthetic and Natural Plastic by Microorganisms. J. Appl. Environ. Microbiol. 2017, 5, 8–19.
- Hung, C.-S.; Barlow, D.E.; Varaljay, V.A.; Drake, C.A.; Crouch, A.L.; Russell, J.N.; Nadeau, L.J.; Crookes-Goodson, W.J.; Biffinger, J.C. The biodegradation of polyester and polyester polyurethane coatings using Papiliotrema laurentii. Int. Biodeterior. Biodegrad. 2019, 139, 34–43.
- Kawai, F. Biodegradation of Polyethers and Polyacrylate. In Studies in Polymer Science; Biodegradable Plastics and Polymers; Doi, Y., Fukuda, K., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 12, pp. 24–38.
- Mohan, S.K.; Srivastava, T. Microbial deterioration and degradation of Polymeric materials. J. Biochem. Tech. 2010, 2, 210–215.
- Delacuvellerie, A.; Benali, S.; Cyriaque, V.; Moins, S.; Raquez, J.-M.; Gobert, S.; Wattiez, R. Microbial biofilm composition and polymer degradation of compostable and non-compostable plastics immersed in the marine environment. J. Hazard. Mater. 2021, 419, 126526.
- Swiontek Brzezinska, M.; Walczak, M.; Kalwasińska, A.; Richert, A.; Świątczak, J.; Deja-Sikora, E.; Burkowska-But, A. Biofilm formation during biodegradation of polylactide, poly (3,4 hydroxybutyrate) and poly(ε-caprolactone) in activated sludge. Int. J. Biol. Macromol. 2020, 159, 539–546.
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265.
- NF EN ISO 846-Plastiques-Évaluation de l’action des Micro-Organismes. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-en-iso-846/plastiques-evaluation-de-laction-des-microorganismes/fa193962/83371 (accessed on 15 June 2023).
- OECD. Test No. 301: Ready Biodegradability; Organisation for Economic Co-operation and Development: Paris, France, 1992; Available online: https://www.oecd-ilibrary.org/environment/test-no-301-ready-biodegradability_9789264070349-en (accessed on 27 June 2023).
- Barratt, S.R.; Ennos, A.R.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungi are the predominant micro-organisms responsible for degradation of soil-buried polyester polyurethane over a range of soil water holding capacities. J. Appl. Microbiol. 2003, 95, 78–85.
- Darby, R.T.; Kaplan, A.M. Fungal Susceptibility of Polyurethanes. Appl. Microbiol. 1968, 16, 900–905.
- Pathirana, R.A.; Seal, J. Studies on polyurethane deteriorating fungi. Part 2. An examination of their enzyme activities. Int. Biodeterior. 1984, 20, 229–235.
- Crabbe, J.R.; Campbell, J.R.; Thompson, L.; Walz, S.L.; Schultz, W.W. Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int. Biodeterior. Biodegrad. 1994, 33, 103–113.
- Cosgrove, L.; McGeechan, P.L.; Robson, G.D.; Handley, P.S. Fungal Communities Associated with Degradation of Polyester Polyurethane in Soil. Appl. Environ. Microbiol. 2007, 73, 5817–5824.
- Matsumiya, Y.; Murata, N.; Tanabe, E.; Kubota, K.; Kubo, M. Isolation and characterization of an ether-type polyurethane-degrading micro-organism and analysis of degradation mechanism by Alternaria sp. J. Appl. Microbiol. 2010, 108, 1946–1953.
- Russell, J.R.; Huang, J.; Anand, P.; Kucera, K.; Sandoval, A.G.; Dantzler, K.W.; Hickman, D.; Jee, J.; Kimovec, F.M.; Koppstein, D.; et al. Biodegradation of Polyester Polyurethane by Endophytic Fungi. Appl. Environ. Microbiol. 2011, 77, 6076–6084.
- Mathur, G.; Prasad, R. Degradation of Polyurethane by Aspergillus flavus (ITCC 6051) Isolated from Soil. Appl. Biochem. Biotechnol. 2012, 167, 1595–1602.
- Álvarez-Barragán, J.; Domínguez-Malfavón, L.; Vargas-Suárez, M.; González-Hernández, R.; Aguilar-Osorio, G.; Loza-Tavera, H. Biodegradative Activities of Selected Environmental Fungi on a Polyester Polyurethane Varnish and Polyether Polyurethane Foams. Appl. Environ. Microbiol. 2016, 82, 5225–5235.
- Zhang, K.; Hu, J.; Yang, S.; Xu, W.; Wang, Z.; Zhuang, P.; Grossart, H.-P.; Luo, Z. Biodegradation of polyester polyurethane by the marine fungus Cladosporium halotolerans 6UPA1. J. Hazard. Mater. 2022, 437, 129406.
- Filip, Z. Polyurethane as the sole nutrient source for Aspergillus niger and Cladosporium herbarum. Eur. J. Appl. Microbiol. Biotechnol. 1979, 7, 277–280.
- Plancher, L.; Nguyen, G.T.M.; Hébert, R.; Maestri, C.; Mélinge, Y.; Ledésert, B.; Di Martino, P. Oxidative biodegradation of a solid-solid polyether-urethane phase change material by Penicillium and Aspergillus. Mater. Today Commun. 2021, 29, 102949.
- Owen, S.; Otani, T.; Masaoka, S.; Ohe, T. The Biodegradation of Low-molecular-weight Urethane Compounds by a Strain of Exophiala jeanselmei. Biosci. Biotechnol. Biochem. 1996, 60, 244–248.
- Taxeidis, G.; Nikolaivits, E.; Siaperas, R.; Gkountela, C.; Vouyiouka, S.; Pantelic, B.; Nikodinovic-Runic, J.; Topakas, E. Triggering and identifying the polyurethane and polyethylene-degrading machinery of filamentous fungi secretomes. Environ. Pollut. 2023, 325, 121460.
- Magnin, A.; Entzmann, L.; Pollet, E.; Avérous, L. Breakthrough in polyurethane bio-recycling: An efficient laccase-mediated system for the degradation of different types of polyurethanes. Waste Manag. 2021, 132, 23–30.
- Burelo, M.; Gaytán, I.; Loza-Tavera, H.; Cruz-Morales, J.A.; Zárate-Saldaña, D.; Cruz-Gómez, M.J.; Gutiérrez, S. Synthesis, characterization and biodegradation studies of polyurethanes: Effect of unsaturation on biodegradability. Chemosphere 2022, 307, 136136.
- Kim, Y.D.; Kim, S.C. Effect of chemical structure on the biodegradation of polyurethanes under composting conditions. Polym. Degrad. Stab. 1998, 62, 343–352.
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Degradation Study of Biobased Polyester–Polyurethane and its Nanocomposite Under Natural Soil Burial, UV Radiation and Hydrolytic-Salt Water Circumstances. J. Polym. Environ. 2018, 26, 1528–1539.
- Gunawan, N.R.; Tessman, M.; Schreiman, A.C.; Simkovsky, R.; Samoylov, A.A.; Neelakantan, N.K.; Bemis, T.A.; Burkart, M.D.; Pomeroy, R.S.; Mayfield, S.P. Rapid biodegradation of renewable polyurethane foams with identification of associated microorganisms and decomposition products. Bioresour. Technol. Rep. 2020, 11, 100513.
- Gunawan, N.R.; Tessman, M.; Zhen, D.; Johnson, L.; Evans, P.; Clements, S.M.; Pomeroy, R.S.; Burkart, M.D.; Simkovsky, R.; Mayfield, S.P. Biodegradation of renewable polyurethane foams in marine environments occurs through depolymerization by marine microorganisms. Sci. Total Environ. 2022, 850, 158761.
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Aerobic biodegradation of starch–polyurethane flexible films under soil burial condition: Changes in physical structure and chemical composition. Int. Biodeterior. Biodegrad. 2019, 145, 104793.
- Ma, C.; Xu, L.; Xu, W.; Zhang, G. Degradable polyurethane for marine anti-biofouling. J. Mater. Chem. B 2013, 1, 3099–3106.
- Ali, A.; Xiao, Y.; Song, L.; Hu, J.; Rao, Q.; Shoaib, M.; Amin, B.U.; Zhan, X.; Zhang, Q. Biodegradable polyurethane based clay composite and their anti-biofouling properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126946.
- Weintraub, S.; Harris, L.G.; Thevissen, K.; Lewitus, D.Y. Polyastaxanthin-based coatings reduce bacterial colonization in vivo. Materialia 2018, 3, 15–20.
- De La Franier, B.; Asker, D.; van den Berg, D.; Hatton, B.; Thompson, M. Reduction of microbial adhesion on polyurethane by a sub-nanometer covalently-attached surface modifier. Colloids Surf. B Biointerfaces 2021, 200, 111579.
- Sheikh, S.; Blaszykowski, C.; Nolan, R.; Thompson, D.; Thompson, M. On the hydration of subnanometric antifouling organosilane adlayers: A molecular dynamics simulation. J. Colloid Interface Sci. 2015, 437, 197–204.
- Magnin, A.; Hoornaert, L.; Pollet, E.; Laurichesse, S.; Phalip, V.; Avérous, L. Isolation and characterization of different promising fungi for biological waste management of polyurethanes. Microb. Biotechnol. 2018, 12, 544–555.
- Ciardelli, G.; Rechichi, A.; Cerrai, P.; Tricoli, M.; Barbani, N.; Giusti, P. Segmented Polyurethanes for Medical Applications: Synthesis, Characterization and in vitro Enzymatic Degradation Studies. Macromol. Symp. 2004, 218, 261–272.
- Biffinger, J.C.; Barlow, D.E.; Cockrell, A.L.; Cusick, K.D.; Hervey, W.J.; Fitzgerald, L.A.; Nadeau, L.J.; Hung, C.S.; Crookes-Goodson, W.J.; Russell, J.N. The applicability of Impranil®DLN for gauging the biodegradation of polyurethanes. Polym. Degrad. Stab. 2015, 120, 178–185.
- Liu, J.; He, J.; Xue, R.; Xu, B.; Qian, X.; Xin, F.; Blank, L.M.; Zhou, J.; Wei, R.; Dong, W.; et al. Biodegradation and up-cycling of polyurethanes: Progress, challenges, and prospects. Biotechnol. Adv. 2021, 48, 107730.
- Lattuati-Derieux, A.; Thao-Heu, S.; Lavédrine, B. Assessment of the degradation of polyurethane foams after artificial and natural ageing by using pyrolysis-gas chromatography/mass spectrometry and headspace-solid phase microextraction-gas chromatography/mass spectrometry. J. Chromatogr. A 2011, 1218, 4498–4508.
- Sarkar, D.; Lopina, S.T. Oxidative and enzymatic degradations of l-tyrosine based polyurethanes. Polym. Degrad. Stab. 2007, 92, 1994–2004.
- Oprea, S. Dependence of fungal biodegradation of PEG/castor oil-based polyurethane elastomers on the hard-segment structure. Polym. Degrad. Stab. 2010, 95, 2396–2404.
- Stepien, A.E.; Zebrowski, J.; Piszczyk, Ł.; Boyko, V.V.; Riabov, S.V.; Dmitrieva, T.; Bortnitskiy, V.I.; Gonchar, M.; Wojnarowska-Nowak, R.; Ryszkowska, J. Assessment of the impact of bacteria Pseudomonas denitrificans, Pseudomonas fluorescens, Bacillus subtilis and yeast Yarrowia lipolytica on commercial poly(ether urethanes). Polym. Test. 2017, 63, 484–493.
- Harussani, M.M.; Sapuan, S.M.; Firdaus, A.H.M.; El-Badry, Y.A.; Hussein, E.E.; El-Bahy, Z.M. Determination of the Tensile Properties and Biodegradability of Cornstarch-Based Biopolymers Plasticized with Sorbitol and Glycerol. Polymers 2021, 13, 3709.
- Andrzejewska, A. One Year Evaluation of Material Properties Changes of Polylactide Parts in Various Hydrolytic Degradation Conditions. Polymers 2019, 11, 1496.
- NF EN ISO 527-2-Plastiques-Détermination des Propriétés en Traction-Partie 2: Conditions d’essai des Plastiques Pour Moulage et Extrusion. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-en-iso-5272/plastiques-determination-des-proprietes-en-traction-partie-2-conditions-des/fa154013/39223 (accessed on 20 June 2023).
- Rafiemanzelat, F.; Jafari, M.; Emtiazi, G. Study of Biological Degradation of New Poly(Ether-Urethane-Urea)s Containing Cyclopeptide Moiety and PEG by Bacillus amyloliquefaciens Isolated from Soil. Appl. Biochem. Biotechnol. 2015, 177, 842–860.
- Kuka, E.; Cirule, D.; Andersone, I.; Andersons, B.; Antons, A.; Kevers, M.; Danieks, M. Photodegradation risk evaluation of polyurethane gluelines in wood products by infrared spectroscopy and mechanical tests. Constr. Build. Mater. 2023, 379, 131251.
- Best, M.; Springthorpe, V.S.; Sattar, S.A. Feasibility of a combined carrier test for disinfectants: Studies with a mixture of five types of microorganisms. Am. J. Infect. Control. 1994, 22, 152–162.
- Penna, T.C.V.; Mazzola, P.G.; Silva Martins, A.M. The efficacy of chemical agents in cleaning and disinfection programs. BMC Infect. Dis. 2001, 1, 16.
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179.
More
