Acute respiratory distress syndrome (ARDS) is a serious clinical illness, defined by severe hypoxemic respiratory failure, which continues to be associated with significant morbidity, mortality, and healthcare resource utilization.
- ARDS
- ventilatory support
- COVID-19
- ARDS, COVID-19, ventilatory support
definition
Acute respiratory distress syndrome (ARDS) is a serious clinical illness, defined by severe hypoxemic respiratory failure, which continues to be associated with significant morbidity, mortality, and healthcare resource utilization.
1. Introduction
ARDS comprises 7–10% of admissions and 15–25% of mechanically ventilated patients in the intensive care unit (ICU), is fatal in 30–50% of patients, and costs on average over USD 90,000 per patient’s ICU stay[1][2][3][4][5]. [1–5]
ARDS has been intensively investigated for more than 50 years, resulting in our current understanding of a clinical-physiologic syndrome of lung inflammation and injury, biologically driven by a plethora of inflammatory cells and soluble molecules (i.e., cytokines). Despite greater understanding and multiple international clinical practice guidelines, ARDS remains under-recognized, the clinical importance is under-appreciated, and management is sub-optimal[2] [2]. As such, many patients continue to suffer more severe, prolonged ARDS and worse clinical outcomes including higher mortality. Moreover, novel causes of ARDS, like coronavirus disease 2019 (COVID-19) are contributing to significant human disease and will undoubtedly continue to do so in the future. The global COVID-19 pandemic offers an important opportunity for all physicians to update their understanding of ARDS.
2. Causes of ARDS
The most common clinical conditions associated with development of ARDS include severe pneumonia (30–50%) and sepsis (25–30%; Figure 1), as confirmed in many large single- and multi-centred cohorts, including the Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNGSAFE) registry, the largest cross-sectional study of ARDS patients admitted to intensive care units (ICUs)[2][6] [2,6].
Pneumonia-associated ARDS is frequently due to bacterial infection (e.g., Streptococcus pneumoniae, Staphylococcus aureus), but also develops with viral (e.g., influenza A) and fungal (e.g., Pneumocystis jirovecii) infections[6] [6]. Coronavirus (CoV) causes of pneumonia and resulting ARDS have been recognized since the 2003 pandemic of severe acute respiratory syndrome (SARS). SARS-CoV-2 is a novel human coronavirus responsible for the pandemic known as COVID-19, first described in Wuhan, China in December 2019[7] [7]. Since then, more than 40 million COVID-19 cases globally have resulted in over 1 million deaths[8] [8]. Although most infected individuals are asymptomatic or exhibit only mild symptoms, a significant minority of COVID-19 patients develop severe illness requiring hospitalization (10–14%), typically manifested as pneumonia[9][10] [9,10].
Sepsis has long been recognized as a common and clinically important cause of ARDS. For example, sepsis was the primary cause of ARDS in 16% of cases in the LUNGSAFE registry and approximately 18% of patients with septic shock developed ARDS[2] [2]. Moreover, sepsis-induced ARDS can have a worse prognosis than other causes of ARDS, typically because of the presence of co-morbid illnesses and higher risk of multiple organ dysfunction syndrome (MODS)[11][12] [11,12].
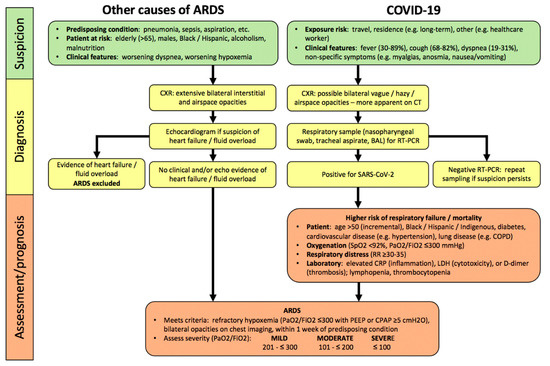
Figure 1. Algorithm for hospitalized patients at risk for acute respiratory distress syndrome (ARDS) and coronavirus disease 2019 (COVID-19). In contrast, to COVID-19, there are no specific lab abnormalities which adequately assess severity or predict prognosis in other causes of ARDS. Abbreviations: PaO2, partial pressure of oxygen in arterial blood; FiO2, inspired oxygen fraction; RR, respiratory rate; CRP, C-reactive protein; LDH, lactate dehydrogenase; PEEP, positive end-expiratory pressure; CPAP, continuous positive airway pressure.
3. Clinical Pathophysiology of ARDS
ARDS is characterized by the rapid development of severe lung inflammation causing damage to alveolar epithelial cells (AEC) and pulmonary microvascular endothelial cells (EC). Dysfunction of the alveolar-capillary endothelial barrier results in diffuse alveolar damage (DAD), which includes an initial exudative phase characterized by high-permeability, proteinaceous pulmonary interstitial and alveolar edema associated with the injury and death of EC as well as AEC desquamation, and a delayed fibroproliferative phase comprising fibrosis in intraluminal and interstitial compartments, and type II AEC proliferation (Table 1)[13][14] [13,14]. Pathologic DAD is found in about half of patients with ARDS, and is associated with more severe hypoxemia and higher mortality[13] [13].
Severe hypoxemia in ARDS is exacerbated by concomitant pathophysiologic disturbances, including surfactant dysfunction (reducing lung compliance causing atelectasis), pulmonary microvascular thrombosis (due to EC injury), higher physiological dead space, and increased shunt fraction due to impairment of hypoxic pulmonary vasoconstriction[15][16][17][18] [15–18]. In addition, patients’ respiratory distress and strong inspiratory efforts can increase negative pleural pressure swings, increasing lung inflation stress, pulmonary blood flow and vascular pressures, potentially worsening pulmonary edema, collectively termed patient self-induced lung injury (P-SILI)[19] [19].
The pathology of COVID-19-associated ARDS (COVID-ARDS) is also largely characterized by DAD, with some key differences such as lymphocyte rather than neutrophil predominance (Table 1). Some studies have highlighted more severe pulmonary microvascular EC injury associated with extensive microvascular thrombosis[20][21] [20,21], for example, pulmonary vascular clot burden was up to nine times greater in COVID-ARDS versus influenza-associated ARDS[20] [20]. However, such pulmonary microvascular findings have not been consistently observed in other pathologic descriptions[22][23][24] [22–24]. The pathophysiology of respiratory failure in most patients with COVID-ARDS is also similar to other causes of ARDS, including atelectasis, low respiratory compliance, and intrapulmonary shunt[25][26] [25,26]. It has been suggested that some COVID-ARDS patients manifest a different phenotype characterized by consolidation without atelectasis, preserved respiratory compliance, and more striking perfusion dysregulation, which may have treatment implications[27][28][29] [27–29]. This remains an area of controversy and active clinical and physiologic research.
Table 1.
Pulmonary pathology features of COVID-19-associated ARDS (COVID-ARDS) versus other causes of acute respiratory distress syndrome (ARDS).
|
Pathology |
ARDS |
COVID-ARDS |
|
Diffuse Alveolar |
Early/Exudative: - interstitial/alveolar edema - “hyaline” membranes - neutrophil infiltration - AEC desquamation - pulmonary microvascular thrombosis Late/Fibroproliferative: - alveolar/interstitial fibrosis - type II AEC hyperplasia
|
Similar to ARDS except: - paucity of neutrophils - interstitial/alveolar lymphocytic infiltration - possibly increased pulmonary microvascular thrombi relative to other causes
|
|
Other features |
- organizing pneumonia (fibrosis) - alveolar haemorrhage - viral pneumonia |
- occasional viral cytopathic changes (multinucleated syncytial cells, atypical enlarged AEC) - viral inclusions in AEC |
Abbreviations: AEC, alveolar epithelial cells.
4. Diagnosis of ARDS
- Diagnosis of ARDS
43.1. Clinical Assessment
By definition, ARDS develops within one week of onset or worsening of a predisposing condition (Figure 1), most commonly (>90%) within 48 h[1][2] [1,2]. Demographic risks for developing ARDS are recognized (e.g., greater age, male sex, non-Caucasian ethnicity)[30][31] [30,31]. ARDS diagnosis in hospitalized patients requires a clinical suspicion, based upon predisposing conditions, worsening oxygenation and dyspnea, bilateral interstitial and/or alveolar opacities consistent with pulmonary edema on chest radiograph (CXR), and exclusion of common causes of pulmonary edema (e.g., heart failure, fluid overload) clinically or with echocardiography[1] [1].
A significant care-gap exists in the diagnosis of ARDS, especially mild ARDS which was unrecognized in 50% of patients in the large, global LUNGSAFE registry [2]. Indeed, mild ARDS is not a benign illness, as less than 20% of patients recovered within a week and overall in-hospital mortality was 29.7%. In addition, more than 40% of mild ARDS progressed to moderate–severe ARDS which was associated with higher mortality of 35–42.9%[32] [32].
Most COVID-19 patients develop symptoms of fever, cough, and dyspnea within 5 days of infection (Figure 1). Hospitalized patients can deteriorate quite rapidly within hours to a few days, manifesting worsening hypoxemia and respiratory distress as features of severe pneumonia, and 20–30% develop COVID-ARDS[9][10][33][34] [9,10,33,34]. Compared to patients with other causes of ARDS, pulmonary opacities are less obvious on CXR (54–76%) in COVID-ARDS patients[35][36] [35,36]. Chest CT scan is clearly more sensitive to the presence of abnormalities in patients with confirmed COVID-19, with a sensitivity of 93.1% (95% CI: 90.2–95.0) in a meta-analysis (65 studies; 5759 patients)[37] [37], but abnormalities are poorly specific for a diagnosis of COVID-19 compared to other respiratory infections.
43.2. Assessment of Severity
ARDS severity is assessed by the degree of hypoxemia, quantified by the ratio of arterial partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) as per the Berlin criteria, which is strongly predictive of worsening survival (Figure 1)[1] [1]. In addition, the presence of hypercapnia (PaCO2 >50 mmHg) was independently associated with more organ dysfunction and higher mortality[38] [38]. Other laboratory abnormalities have not been shown to assess severity or predict prognosis in ARDS, but key investigations can identify prognostically-important complications of non-pulmonary organ dysfunction, e.g., cardiac, renal, and potentially MODS[39] [39]
In patients with COVID-19, severity of pneumonia and respiratory failure is also assessed by the degree of hypoxemia, including arterial oxygen saturation by pulse oximetry (SpO2), and the PaO2/FiO2 ratio[26][40] [26,40] . COVID-19 is associated with distinct laboratory abnormalities which predict greater risk of respiratory failure and worse clinical outcomes including higher mortality, independent of the severity of ARDS. These include markers of inflammation (elevated C-reactive protein (CRP)), cytotoxicity (increased lactate dehydrogenase (LDH)), and both macrovascular and microvascular thrombosis in systemic and pulmonary circulations (higher D-dimer levels), as well as lymphopenia (Figure 1)[41][42] [41,42]. It has been suggested that these markers should be assessed at baseline in hospitalized COVID-19 patients[40][43] [40,43], however the clinical utility of serial monitoring has not yet been established.
Given the presence of multiple physiologic and laboratory abnormalities in COVID-19, there may be more robust prognostic value in assessing a combination of parameters. For example, in a multicentre, observational retrospective study of patients being assessed in the ED, a model developed through machine-learning, the Quick COVID-19 Severity Index comprising three respiratory parameters (FiO2, SpO2, respiratory rate (RR)) was predictive of the risk of respiratory failure within the first 24 h of admission[44] [44]. Following hospital admission, another machine-learning composite score, which included age, lymphocyte count and levels of inflammatory markers (e.g., LDH, CRP), was found to best predict the risk of severe hypoxemic respiratory failure, need for ICU admission and/or invasive respiratory support, and mortality in hospitalized COVID-19 patients[45] [45]. Finally, in COVID-19 patients with ARDS, a multicentre, observational study identified the highest risk of mortality was associated with both reduced respiratory compliance and higher D-dimer levels[46] [46].
4. Management of Patients with ARDS
4.1. General Approach
Management of ARDS remains largely supportive, including treatment of the predisposing condition, as there are no specific medical therapies that address the lung inflammation and alveolo-capillary injury. Standard care for ICU-admitted patients includes early nutritional support, appropriate analgesia, sedation, thromboprophylaxis, semi-recumbent position, gastric ulcer prophylaxis, and glycaemic control (FASTHUG)[47] [47]. In ARDS patients, the frequent presence of non-pulmonary organ dysfunction or development of MODS contributes to severity of illness, intensity of required care, and mortality[12][48] [12,48]. Similarly, thirty-to-fifty percent of critically-ill COVID-19 patients will develop non-pulmonary organ dysfunction leading to MODS, which is the most common cause of mortality[34][36][49] [34,36,49].
Many respiratory support modalities are high-risk aerosol-generating medical procedures requiring specific attention, during the care of COVID-19 patients, to minimization of unnecessary staff exposure, appropriate contact precautions, and airway management expertise. Physicians are encouraged to follow local guidelines for safe application and monitoring of all respiratory support and associated procedures, e.g., high-flow nasal-cannula O2 (HFNO), non-invasive positive pressure ventilation (NIPPV), intubation, mechanical ventilation (MV), bronchoscopy[40] [40].
4.2. Respiratory Support of Mild ARDS
Initial respiratory support of patients with hypoxemia consists of supplemental O2 [[50]50]. Specific SpO2 targets in various patient populations remain uncertain, given competing goals of addressing persistent hypoxemia as well as avoiding hyperoxia, both of which may be associated with increased mortality[51][52] [51,52]. In ARDS, permissive hypoxemia is not recommended[53][54][55][56] [53–56]. For example, conservative O2 (SpO2 88–92%) was associated with a non-significant higher risk of 28-day mortality, but higher 90-day mortality and more intestinal ischemia than more liberal O2 (SpO2 ≥ 96%) [53]. In persistent hypoxemic respiratory failure despite maximal supplemental O2 by facemask, various non-invasive respiratory support modalities may be considered, and clearly are being commonly employed recently in COVID-19 patients[57][58][59] [57–59].
4.2.1. High-Flow Nasal-Cannula O2 (HFNO)
This is a novel technique which can improve oxygenation in hypoxemic respiratory failure (Figure 2), through several mechanisms including higher inspired O2 concentrations ≤90%, decreased dead space, and increased lung volume through generation of a low-level of continuous positive airway pressure (CPAP)[60][61] [60,61]. In the largest randomized controlled trial (RCT) of HFNO vs. standard O2 therapy in patients with hypoxemic respiratory failure (the absence of use of CPAP meant that ARDS could not be formally diagnosed based on Berlin criteria), HFNO reduced 90-day mortality by 50% but there was no difference in the need for invasive respiratory support through intubation/MV[60] [60]. A retrospective review and two meta-analyses have concluded that HFNO was associated with 15–24% reduced risk of subsequent intubation in hypoxemic respiratory failure, but did not reduce duration of hospital or ICU admission or improve survival[62][63][64] [62–64].
HFNO has more commonly been used in the management of hypoxemic respiratory failure in COVID-19 patients, depending on geography and access to other respiratory support measures[57][65][66] [57,65,66]. For example, 5–64% of moderate–severe hypoxemic COVID-19 patients in Italy, China, and the US were initially supported with HFNO[42][67][68][69]. [42,67–69] In a retrospective review of the largest single-centre series of 104 COVID-19 patients with moderate–severe hypoxemia, 64% of those treated with HFNO avoided intubation and had mortality of 2.9%, compared to 14.4% in those requiring subsequent intubation/MV[63] [63].
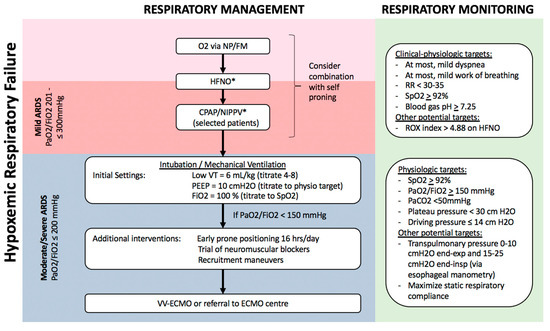
Figure 2. Algorithm for respiratory management of patients with hypoxemic respiratory failure. Patients with hypoxemia despite supplemental O2, including those who meet criteria for mild ARDS, can potentially be managed with non-invasive respiratory support, including HFNO and NIPPV, possibly in combination with self-proning. Sequential escalation of non-invasive respiratory support modalities should be considered unless clinical-physiologic targets are met, depending on local critical care expertise and resources. Patients with moderate–severe ARDS require invasive mechanical ventilation with monitoring and adjustment of ventilatory parameters to minimize ventilator-induced lung injury (VILI), and may benefit from additional measures to improve oxygenation such as prone positioning, recruitment manoeuvres, and potentially veno-venous extra-corporeal membrane oxygenation (VV-ECMO). Notes: a. Non-invasive respiratory support with HFNO/CPAP/NIPPV requires careful monitoring for lack of improvement or persistent respiratory distress, and consideration of intubation/mechanical ventilation. b. All non-invasive and invasive respiratory support modalities are high-risk aerosol-generating medical procedures which should be carried out by experts in airway management, with appropriate precautions (e.g., minimal staff in room, N95, negative pressure room). c. Tidal volume is referenced to predicted body weight. d. Recruitment manoeuvres requires sustained inflation, e.g., inspiratory hold at 35–40 cm H2O for set time (e.g., 40 s). Stepwise recruitment (with incremental levels of PEEP) is not recommended. e. ROX index = SpO2/FiO2/Respiratory Rate. f. Plateau pressure = airway pressure after 0.5 s pause at end-inspiration. g. Driving pressure = plateau pressure – PEEP. h. Transpulmonary pressure = airway pressure – pleural pressure (under zero flow conditions). i. Static respiratory compliance = tidal volume/(plateau pressure – PEEP). Abbreviations: NP, nasal prongs; FM, facemask; HFNO, high-flow nasal oxygen; CPAP, continuous positive airway pressure; NIPPV, non-invasive positive pressure ventilation; RR, respiratory rate; VT, tidal volume; PEEP, positive end-expiratory pressure; ECMO, extra-corporeal membrane oxygenation. * These interventions, while increasingly being used globally, especially during the COVID-19 pandemic, are not yet supported by robust evidence in patients with ARDS.
4.2.2. Continuous Positive Airway Pressure (CPAP)/Non-Invasive Positive Pressure Ventilation (NIPPV)
In patients with persistent hypoxemia despite maximal supplemental O2 by either facemask or HFNO, a trial of either CPAP (via nasal/facemask or hood/helmet) or NIPPV via facemask can be considered. CPAP/NIPPV may be beneficial in improving oxygenation and respiratory distress, decreasing FiO2 requirements, and possibly reducing the need for invasive support through intubation/MV[70][71] [70,71]. For example, the above-cited network meta-analysis of non-invasive respiratory support in hypoxemic respiratory failure reported that both helmet NIPPV (RR 0.26, 95%CI 0.14–0.46) and facemask NIPPV (RR 0.76, 95%CI 0.62–0.90) reduced the risk of subsequent intubation and were both associated with reduced risk of death compared to supplemental O2 [62][72][62,72].
CPAP/NIPPV is commonly being used globally for patients with hypoxemic respiratory failure including ARDS, e.g., 15.5% of ARDS patients in the global LUNGSAFE registry[70] [70]. However, there is a clear risk of failure of such non-invasive respiratory support, as 22.2% of mild and 42–47% of moderate–severe ARDS patients failed CPAP/NIPPV trial within 2 days, experiencing lack of improvement or worsening of respiratory distress and/or hypoxemia[70] [70]. During NIPPV trials, careful respiratory monitoring is essential because clinical outcomes are worse in patients who fail NIPPV, possibly because of delayed definitive management of respiratory failure with intubation and MV[73][74] [73,74]. For example, patients with hypoxemic respiratory failure who failed NIPPV had longer ICU and hospital stay, as well as more than four-fold higher mortality[74] [74]. Thus, NIPPV may be beneficial in patients with mild ARDS (Figure 2), but this specific respiratory support measure has not been specifically recommended in recent guidelines[48][75] [48,75].
Non-invasive respiratory support with CPAP/NIPPV is also being increasingly instituted in COVID-19 patients, especially under local conditions of constrained ICU resources[59] [59]. For example, 3–56% of hypoxemic COVID-19 were treated with CPAP/NIPPV, with higher rates of usage in critically ill and moderate–severe patients[7][10][33][49][66][67][68][69][76][77] [7,10,33,49,66–69,76,77]. Several uncontrolled reports suggested a reduced need for intubation, but only a single controlled study has addressed this, using a retrospective, historical time period-controlled cohort design, reporting significantly higher intubation-free survival at 7 days with CPAP[78] [78]. While such non-invasive respiratory support measures may be appropriate in some COVID-19 patients with hypoxemic respiratory failure, specifically those who either do not yet meet criteria for ARDS or have mild ARDS (Figure 2), current guidelines do not provide any specific recommendations in the absence of more robust data[40][44][59][79] [40,43,59,79].
4.2.3. Prone Positioning
Based on strong evidence for improved clinical outcomes in ARDS patients who are intubated and ventilated (see Section 3.3. Respiratory Support of Moderate–Severe ARDS below[80] [80]), prone positioning is being increasingly used to improve oxygenation in spontaneously-breathing non-intubated patients with hypoxemic respiratory failure, including COVID-19. For example, in a small prospective cohort study of 20 patients with ARDS, prone positioning combined with either HFNO or NIPPV was associated with reduced need for intubation/MV only in patients with moderate ARDS, not in those with severe ARDS[81] [81].
Several uncontrolled series have reported that self-proning may improve oxygenation in spontaneously breathing COVID-19 patients receiving supplemental O2 or other non-invasive respiratory support (e.g., HFNO, CPAP/NIPPV)[65][82][83][84][85] [65,82–85]. In the first reported series of 50 COVID-19 patients managed in the emergency department, oxygenation improved from an average of 84% on supplemental O2 to 94% after self-proning for 5 min[82] [82]. In addition, 64% of patients with unspecified repeated self-proning sessions recovered to hospital discharge without intubation/MV. Self-proning was not tolerated, including worsening oxygenation and/or respiratory distress, in 13–25% of patients[84][86][87] [84,86,87]. Moreover, although oxygenation improves in most patients when prone, the improvement is maintained in only about 50% of patients when resuming the supine position, with some evidence that proning may be more effective earlier in the hospital course and specifically in patients with higher inflammatory markers (e.g., CRP, LDH)[84] [84]. Early oxygenation improvement has been associated with reduced need for subsequent intubation/MV in some studies[87][88] [87,88] but not in others[84] [84]. In summary, self-proning is currently widely employed in the management of COVID-19 patients globally in the absence of strong evidence of improved outcomes and there are no clear recommendations regarding specifics of patient selection, duration and frequency of proning sessions. Self-proning is not feasible or tolerable for all patients, is associated with clear risks, including inadequate respiratory support in patients with respiratory distress and/or high work of breathing which are associated with higher risk of P-SILI and progressive lung injury[19][89][90] [19,89,90]. As such, prone positioning in spontaneously breathing patients mandates rigorous clinical and respiratory monitoring for lack of improvement and/or persistent respiratory distress in order to facilitate timely intubation/MV.
4.3. Respiratory Support of Moderate–Severe ARDS
In moderate–severe ARDS patients, respiratory management is similar for ARDS from all causes including COVID-19 (Figure 2)[26][40][48][75][79][91] [26,40,48,75,79,91]. Invasive respiratory support through endotracheal intubation and MV is strongly recommended for worsening or persistent respiratory distress, persistent hypoxemia (SpO2 < 92%), or progressive hypercapnia. In patients requiring MV, specific ventilatory modalities and parameters are guidelines-recommended based on improved outcomes in multiple RCTs (Figure 2)[48][75][91] [48,75,91]. The goal is to use a lung protective strategy to prevent excessive lung tidal-inflation stress (volutrauma) and cyclic atelectasis-recruitment (atelectrauma), reducing the risk of ventilation-induced lung injury (VILI)[92] [92]. The most important measure is MV using low-tidal volumes, specifically a target of 4–8 mL/kg predicted body weight[91][93] [91,93].
The application of positive end-expiratory pressure (PEEP) is essential in order to reduce atelectasis and maximize respiratory compliance, and PEEP is optimally selected to avoid excessive plateau and driving pressures (Figure 2)[91][93][94] [91,93,94]. Novel physiologic monitoring using oesophageal manometry may allow optimization of PEEP in individual patients, although the benefit of such an approach in terms of clinical outcomes remains uncertain[95][96] [95,96]. Additionally, early prone positioning should be implemented as a lung protective measure, as it has been shown to reduce 28-day mortality by 16% when implemented 12–24 h after initiation of MV[80] [80].
Several weak recommendations suggest approaches for management of persistent hypoxemia, patient-ventilator dyssynchrony, or low lung compliance with high plateau or driving pressures (Figure 2). These include short courses of neuromuscular blockade-induced paralysis, and specific recruitment manoeuvres[91][97][98] [91,97,98]. Refractory hypoxemia not responding to conventional therapy warrants consideration of veno-venous extra-corporeal membrane oxygenation (VV-ECMO). Besides directly improving hypoxemia and related multiple organ dysfunction, ECMO may offer more homogeneous, ultraprotective ventilation. In brief, ECMO should be considered when patients have (a) persistent PaO2/FiO2 <50 mmHg for >3 hrs or <80 mmHg for >6 h despite FiO2 >80% and PEEP >10, or (b) pH < 7.25 with PaCO2 > 60 mmHg for >6 h. If ECMO is not available locally, patients with severe respiratory failure should be considered for transfer to a high-volume facility with ECMO expertise, if clinically feasible. VV-ECMO achieves similar outcomes in all causes of ARDS, including COVID-ARDS[99][100] [99,100].
4.4. Medical Approaches to ARDS Therapy
Given the central contribution of alveolo-capillary injury and high-permeability pulmonary edema to refractory hypoxemia in ARDS, conservative fluid management after initial resuscitation may reduce edema, improve gas-exchange, and improve clinical outcomes such as decreased duration of MV and ICU length of stay (Table 2)[91][101] [91,101]. Regardless of the primary cause of ARDS, the presence of concomitant bacterial infection should be investigated, and broad-spectrum antibiotic therapy considered. Limited evidence indicates early systemic steroids may reduce duration of MV and mortality, but there are conflicting recommendations regarding dose, timing, and consideration in individual patients (Table 2)[91][102] [91,102]. A multitude of RCTs of various anti-inflammatory and pathophysiology-based therapies have failed to improve clinical outcomes, such that there is no specific medical therapy currently indicated or recommended for lung inflammation and injury in ARDS patients.
There is active research into various anti-viral and anti-inflammatory therapies specifically for SARS-CoV-2 infection resulting in COVID-19 pneumonia and/or ARDS (Table 2)[79][103] [79,103]. Strong evidence supports that corticosteroids (i.e., dexamethasone) reduce the need for ICU admission and intubation/MV in hospitalized, hypoxemic COVID-19 patients. Moreover, in COVID-ARDS patients, corticosteroids shorten the duration of MV and reduce mortality[104] [104]. As such, corticosteroids are strongly recommended for hypoxemic COVID-19 patients[104][105] [104,105]. Remdesivir is the first antiviral drug found to have some clinical benefit, namely in reducing time to recovery[106] [106]. Many putative therapies are in ongoing clinical trials with some promise of preventing or treating COVID-19, including human convalescent plasma, systemic anticoagulation, and 25-hydroxy vitamin D[107][108][109] [107–109]. A number of other medical therapies have been considered but have shown no benefit, including lopanivir/ritonavir and hydroxychloroquine[110][111] [110,111]. There is concern around the routine clinical use of unproven experimental therapies, including high risk of drug–drug interactions given that the majority of hospitalized COVID-19 patients are older with multiple co-morbidities requiring treatment with many other medications[112] [112].
Table 2.
Medical treatment approaches for ARDS and specifically for COVID-19-associated ARDS.
|
Intervention |
ARDS |
COVID-ARDS |
|
Fluid management |
||
|
Conservative fluid strategy |
Weak recommendation post initial resuscitation (SCCM[48], FICM-ICS[75]) |
Weak recommendation (SSC [40]) |
|
Anti-inflammatory therapy |
||
|
Steroid |
Weak recommendation - Methylprednisolone 1–2 mg/kg/d with 14 d taper (FICM-ICS[75], SCCM-ESICM[102] |
Recommended - Dexamethasone 6 mg/d for 10 d (WHO[33], IDSA[79], CMAJ[103]) |
|
Other (Physiologic/Biologic) |
Not recommended - β2-agonists - Exogenous surfactant - Anti-IL1β - Statins |
Not recommended - Hydroxychloroquine/chloroquine - Lopanivir/ritonavir |
|
Experimental |
Current trials - Anti-tissue factor antibody fragment - MAPK inhibitor - Stem cell therapies - Complement inhibitor - JAK inhibitor |
Current trials - Intravenous Immunoglobulin - IL-6 inhibitor (e.g., tocilizumab) - IL-1 inhibitor (e.g., anakinra) - Anti-GM-CSF (e.g., mavrilimumab) - Anticoagulants (e.g., Low molecular weight heparin) - Fibrinolytics (e.g., tPA) - 25-OH vitamin D |
|
Anti-microbials |
||
|
Antibiotics |
Strong recommendation - If ARDS due to pneumonia or sepsis (SCCM[48]) - If evidence of ventilator-associated pneumonia (SCCM[48]]) |
Weak recommendation - In patients requiring MV (SSC[40], IDSA[79]) - If concomitant bacterial pneumonia (SSC[40], IDSA[79])) |
|
Antivirals |
Specific viral targeted therapy indicated - If viral infection identified (e.g., influenza, RSV) |
Specific viral targeted therapy indicated - If evidence of concomitant viral pneumonia (e.g., influenza, RSV) SARS-CoV-2 targeted therapy - Remdesivir (IDSA[79]) |
|
Intervention |
ARDS |
COVID-ARDS |
|
Fluid management |
||
|
Conservative fluid strategy |
Weak recommendation post initial resuscitation (SCCM [48], FICM-ICS [75]) |
Weak recommendation (SSC [40]) |
|
Anti-inflammatory therapy |
||
|
Steroid |
Weak recommendation - Methylprednisolone 1–2 mg/kg/d with 14 d taper (FICM-ICS [75], SCCM-ESICM [102] |
Recommended - Dexamethasone 6 mg/d for 10 d (WHO [33], IDSA [79], CMAJ [103]) |
|
Other (Physiologic/Biologic) |
Not recommended - β2-agonists - Exogenous surfactant - Anti-IL1β - Statins |
Not recommended - Hydroxychloroquine/chloroquine - Lopanivir/ritonavir |
|
Experimental |
Current trials - Anti-tissue factor antibody fragment - MAPK inhibitor - Stem cell therapies - Complement inhibitor - JAK inhibitor |
Current trials - Intravenous Immunoglobulin - IL-6 inhibitor (e.g., tocilizumab) - IL-1 inhibitor (e.g., anakinra) - Anti-GM-CSF (e.g., mavrilimumab) - Anticoagulants (e.g., Low molecular weight heparin) - Fibrinolytics (e.g., tPA) - 25-OH vitamin D |
|
Anti-microbials |
||
|
Antibiotics |
Strong recommendation - If ARDS due to pneumonia or sepsis (SCCM [48]) - If evidence of ventilator-associated pneumonia (SCCM [48]) |
Weak recommendation - In patients requiring MV (SSC [40], IDSA [79]) - If concomitant bacterial pneumonia (SSC [40], IDSA [79])) |
|
Antivirals |
Specific viral targeted therapy indicated - If viral infection identified (e.g., influenza, RSV) |
Specific viral targeted therapy indicated - If evidence of concomitant viral pneumonia (e.g., influenza, RSV) SARS-CoV-2 targeted therapy - Remdesivir (IDSA [79]) |
