The retinal pigment epithelium (RPE) performs a range of necessary functions within the neural layers of the retina and helps ensure vision. The regulation of pro-oxidative and antioxidant processes is the basis for maintaining RPE homeostasis and preventing retinal degenerative processes. Long-term stable changes in the redox balance under the influence of endogenous or exogenous factors can lead to oxidative stress (OS) and the development of a number of retinal pathologies associated with RPE dysfunction, and can eventually lead to vision loss. Reparative autophagy, ubiquitin–proteasome utilization, the repair of damaged proteins, and the maintenance of their conformational structure are important interrelated mechanisms of the endogenous defense system that protects against oxidative damage. Antioxidant protection of RPE cells is realized as a result of the activity of specific transcription factors, a large group of enzymes, chaperone proteins, etc., which form many signaling pathways in the RPE and the retina.
- retinal pigment epithelium
- redox homeostasis
- reactive oxygen species
- oxidative stress
- endogenous cell defense
1. Introduction
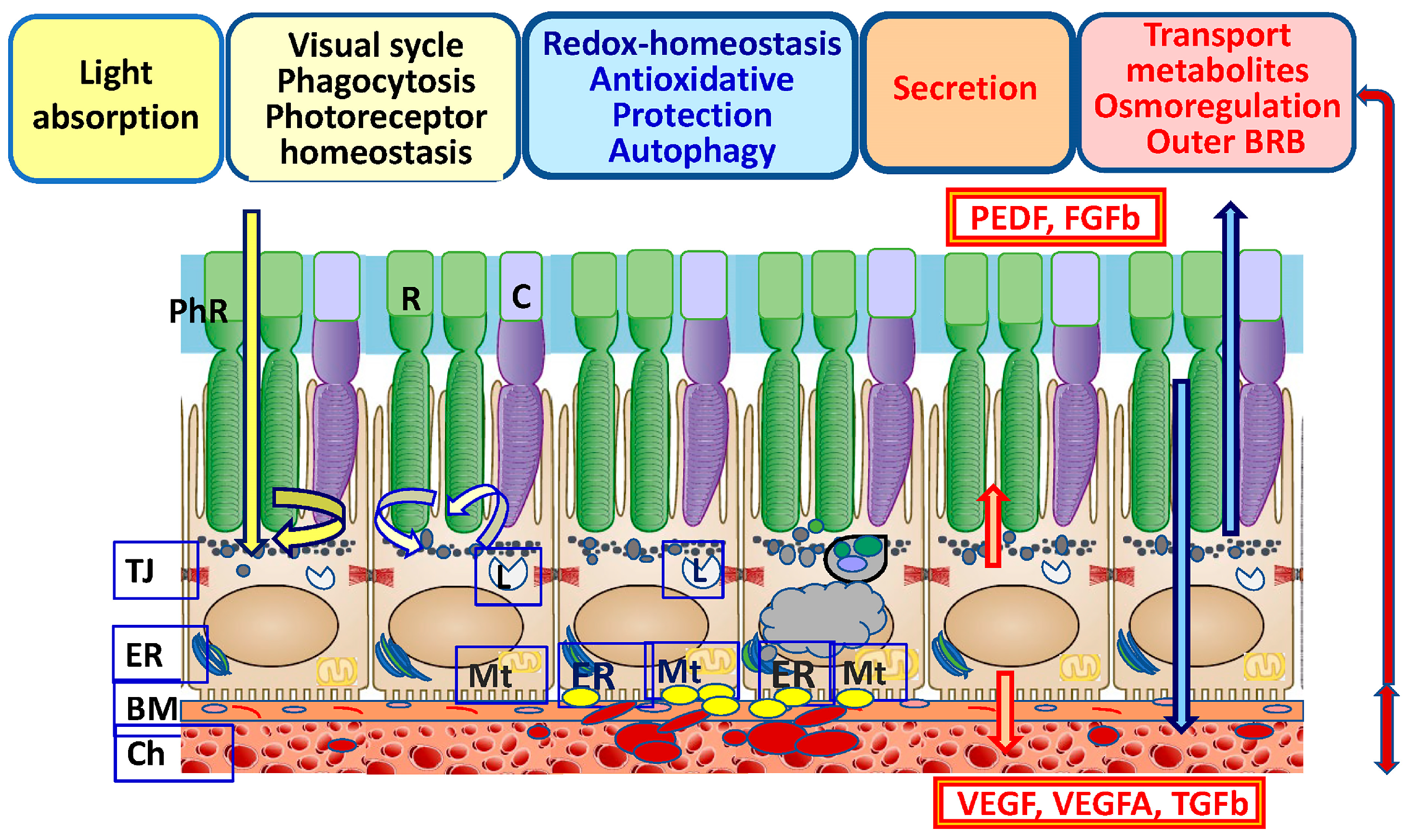
2. Functions of the RPE
The RPE is a single-row hexagonal layer of polarized pigmented cells. RPE cells on the apical side form tight contacts with the photoreceptors’ outer segments (POSs), and on the basal side they interact with the MB, which is closely associated with the choroid [2,20][2][20]. The physiological functions of RPE cells are associated with protecting (shielding) photoreceptors from excess light (Figure 1). Additionally, the RPE maintains homeostasis, pH, and circulating liquid volume in the subretinal space by transporting metabolites to the choroid [1,36][1][23]. As a result of the reactions of the visual cycle and the metabolism of rhodopsin, during the isomerization from 11-cis-retinal to trans-retinal, a large amount of ROS is produced, causing lipid peroxidation (LPO) [37,38][24][25]. In the retina, there is a constant renewal of photoreceptor disks. RPE cells phagocytize used POSs, which subsequently leads to autophagy-lysosomal degradation. The RPE renews “exhausted” photoreceptor disks enriched in ROS and lipofuscin (LF), the main product of LPO [39][26]. Changes in the light regime affect intracellular pH and the concentration of Ca2+ and K+ ions and increase oxygen consumption, causing the generation of CO2 and H2O in the RPE [40][27]. BM underlies the RPE from the choroid side and plays an important role in regulating the transport of biomolecules (proteoglycans, chemokines, cytokines, growth factors, and toxic waste products) between photoreceptors, the RPE, and the choroid [52][28]. BM is a dense, cell-free fibrillar layer of the proper RPE basal lamina that is rich in collagen and elastin, with a predominance of heparan sulfates. BM also includes endothelial components of the capillary-rich choroid [53][29]. The formation of excessive amounts of basement membrane components may be a general epithelial cell response to stress in order to remain adherent. The disruption of ECM homeostasis likely results in an environment of increased OS that contributes to disease onset and progression [54][30]. BM provides a mechanical function, enables cell adhesion, acts as a barrier that limits the migration of choroid cells, and ensures the migration and differentiation of RPE cells in embryogenesis. In MB, these processes are strictly regulated for maintaining local RPE and retinal homeostasis, which depends both on the genetically determined state of tissues and on the influence of exogenous factors [23,55,56][31][32][33].3. Functional Prerequisite of RPE Cells for Oxidative Stress
The RPE is characterized by its tendency to have high oxygen tension due to its close proximity to the choriocapillaris and is considered to be exposed to the most intensive O2 pressure of any human tissue. The high metabolism rate of the RPE and retinal photoreceptors, the constant renewal of the membrane disks of POSs by phagocytosis, the high levels of polyunsaturated fatty acids and exposure to light, and the high oxygen consumption and intensity of energy metabolism processes are necessary to maintain normal physiological function [62][34]. ROS occur in photoreceptors as a result of the activity of mitochondria (as a byproduct of the oxidative phosphorylation chain) and due to the process of phototransduction under the action of light on the light-sensitive pigments of rods and cones. In turn, the peroxidation of polyunsaturated fatty acids (docosahexaenoic acid, etc.) that enrich the plasma membranes of photoreceptor discs occurs under the action of ROS. Intense mitochondrial metabolism, the phagocytosis of POSs, the phototoxic activity of LF, and the photosensitization of hemoglobin precursor are the sources of free radicals not only in the RPE but also in photoreceptors [63,64][35][36] (Figure 2).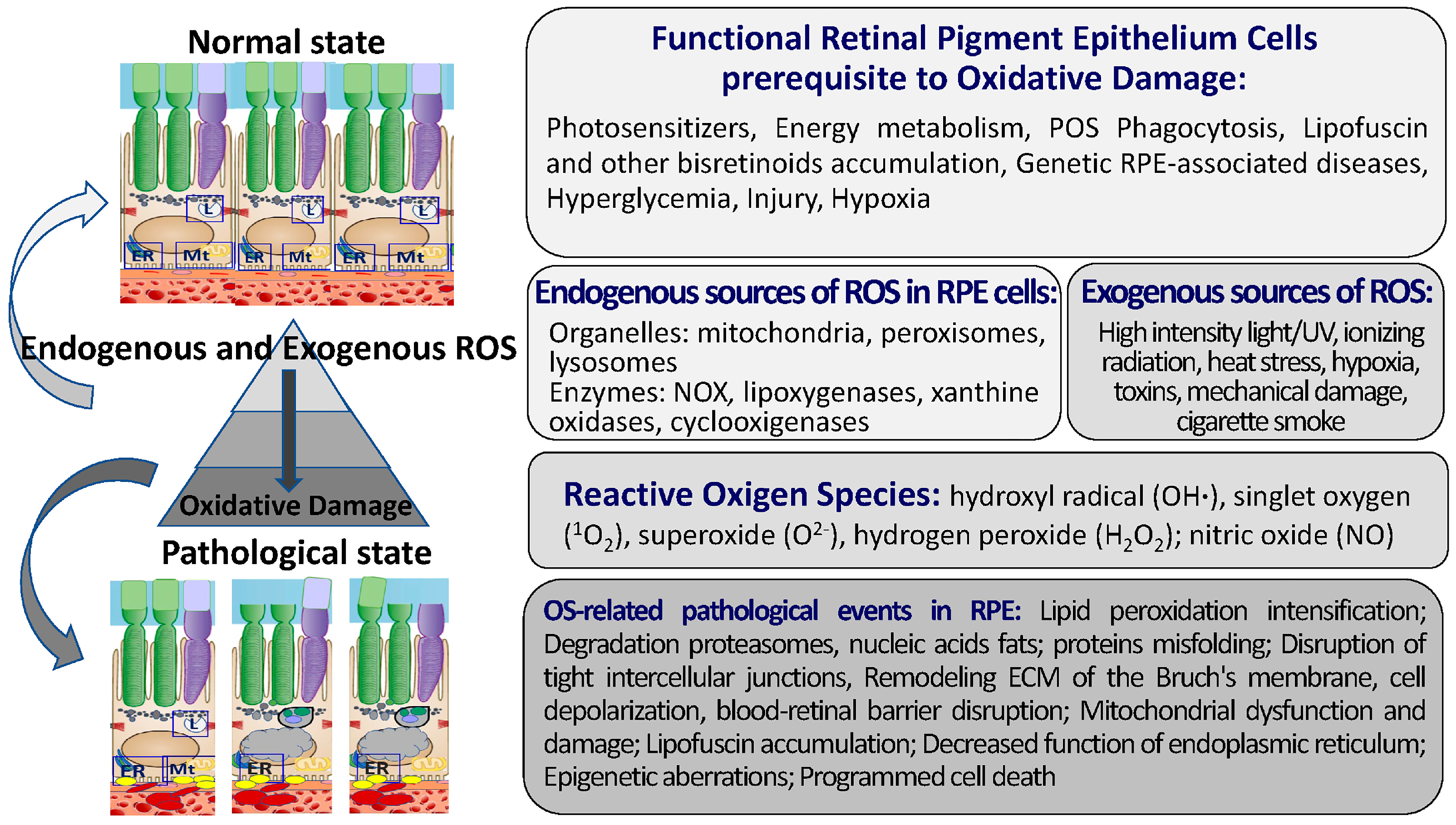
4. The Maintenance of Redox Homeostasis in RPE Cells
4.1. Key Components of the Pro-Oxidant System in the RPE
Here, the main elements of the pro-oxidant system in RPE cells are briefly described (Figure 2). The pro-oxidant system of RPE cells includes ROS [87][49]: hydroxyl radical (OH∙), singlet oxygen (1O2), superoxide (O2−), hydrogen peroxide (H2O2), and specialized enzymes, including NADP oxidase, nitric oxide synthase, and xanthine oxidase [88,89,90,91,92][50][51][52][53][54].ROS Sources in RPE
The main sources of ROS in the body are phagocytes: granulocytes, monocytes, macrophages, neutrophils, and eosinophils [93][55]. ROS (superoxide radicals) appear in the RPE during ER stress as a byproduct of oxidative phosphorylation, which occurs largely in the mitochondria. Superoxide radicals are massively generated in dark processes of the mitochondrial respiratory chain, as well as by exposure to visible light [94,95][56][57]. The production of ROS in mitochondria is highest at high mitochondrial membrane potentials. The increased ROS level activates uncoupling proteins located in the mitochondrial inner membrane and increases the transport of protons from the mitochondria, thereby reducing its membrane potential and the production of ROS [96][58]. Uncoupling proteins (UCPs) are a part of the large family of mitochondrial solute carriers. In addition to UCP2, the 2-oxoglutarate and dicarboxylate carriers were recently identified in RPE mitochondria [97][59]. It is difficult to estimate the relative contributions of ROS due to the environment versus those produced due to the high metabolic flux through the electron transport chain. ROS production is dependent on proton leaks that consist of a basal proton leak and induced proton leak. The basal proton leak is unregulated and correlates with the metabolic rate. The induced proton leak is precisely regulated in stress conditions and is induced by superoxide or peroxidation products through UCPs [99,100][60][61]. Mitochondrial OS in the RPE leads to metabolic dysfunction in both the RPE and retinal photoreceptors [95,101][57][62]. Mitochondrial DNA is more susceptible to oxidative damage in neuropathologies [102][63]. The excessive production of ROS in mitochondria correlates with age-related DNA disorders [103][64]. The action of ROS causes LPO of, for example, docosahexaenoic acid, among others, which can be enriched in the plasma membranes of photoreceptor disks [94,104][56][65]. LF granules also serve as a source of photo-induced generation of superoxide radicals in RPE cells; LF exposed to visible light (largely in the blue region) reduces oxygen to superoxide radicals [105][66]. Other endogenous sources of ROS formation are the enzyme system of the transmembrane complex of NADPH oxidases (NOXs), monoamine oxidases, and NO synthases, which generate superoxide anions and hydrogen peroxide and are involved in the OS reaction aimed at destroying pathogens [106,107,108][67][68][69].4.2. Key Components of the ADS in RPE Cells
The regulatory hierarchy of the main levels of the ADS is universal and responsible for the effective neutralization of free ROS, as well as for the reduction in oxidized molecules. The endogenous system of antioxidant defense in RPE cells also includes several regulatory levels (Figure 3). The first level is largely provided by the coordinated activity of redox- and OS-sensitive transcription factors.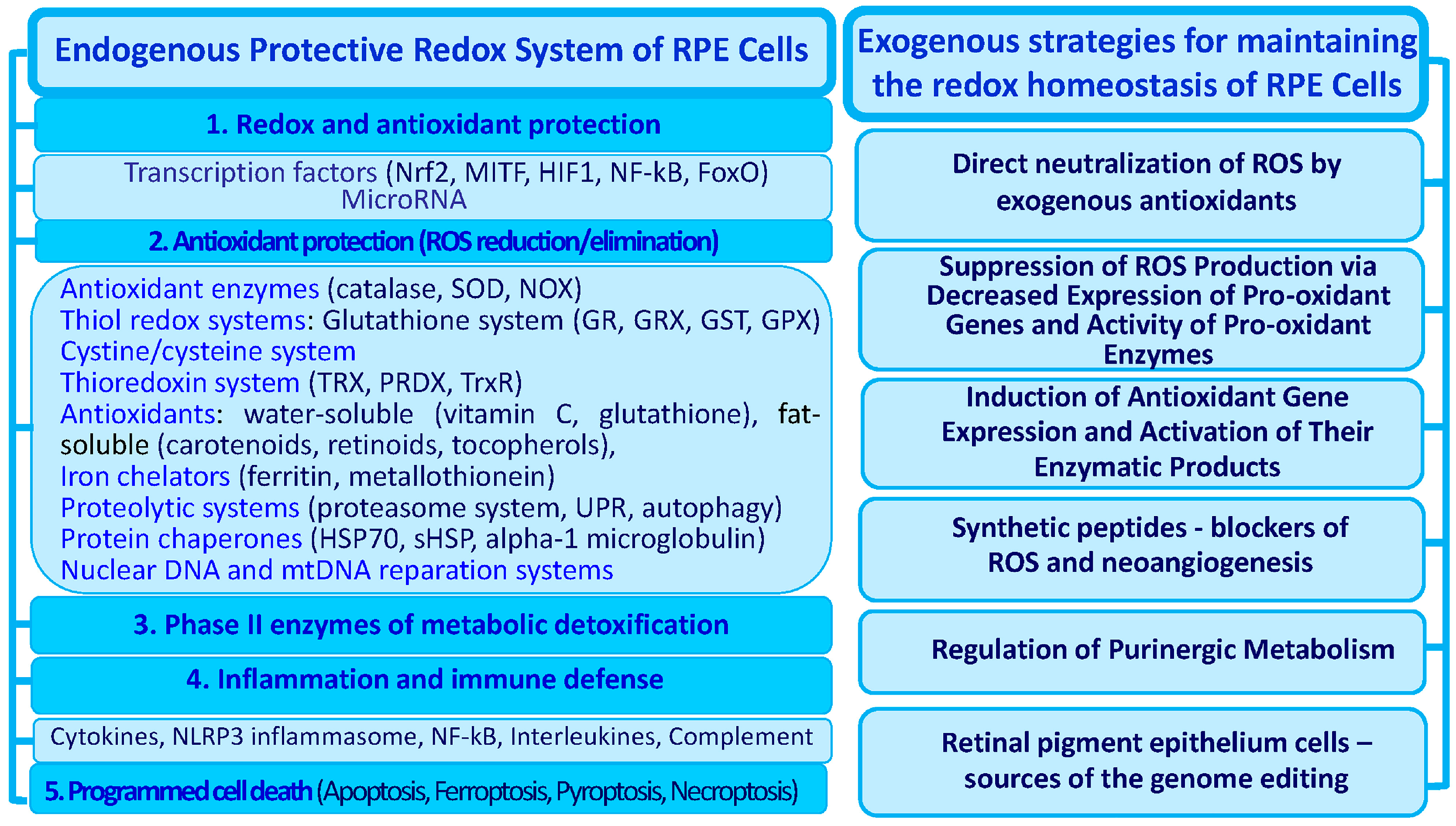
4.2.1. Transcription Factors
4.2.2. Melanin
The pigment melanin performs the function of “neutralization” of ROS (singlet oxygen) [135[83][84],136], causes the oxidation of superoxide radicals into molecular oxygen, reduces superoxide to hydrogen peroxide [105[66][85],137], binds redox-active metal ions, and performs the function of photoprotection [138][86].4.2.3. Enzyme Systems That Neutralize and Reduce ROS
Enzyme systems that protect RPE cells from OS include OS-neutralizing and OS-reducing enzymes. OS induces the expression of phase II enzymes including NADPH quinine oxidoreductase 1, HO-1, and catalytic (GCLC) and modulatory (GCLM) subunits of glutamate-cysteine ligase [139,140][87][88]. The RPE and retina of a rat demonstrated activation of the antioxidant protection enzymes Cu/Zn superoxide dismutase (SOD), Mg SOD, catalase, glutathione peroxidase (GPX), and the catalytic subunit of glutamate-cysteine ligase that is linked to the regulation of endogenous cyclophilin B levels [141][89]. SOD is a metalloenzyme whose synthesis increases under OS. This enzyme catalyzes O2- and transforms it into H2O2. SOD is expressed in mitochondria and protects cellular and mitochondrial structures from superoxide [142,143][90][91]. It has been shown in adult mice that mutations in the SOD1−/− and SOD2−/− genes lead to the development of signs of neovascularization and the death of retinal neurons, resembling human AMD [144,145][92][93]. Exposure to light and oxygen and oxidative phosphorylation generate OS via the electron transport chain. These ROS can be reduced by employing the systems of NADPH, glutathione, and antioxidant enzymes. NADPH is the key reductive equivalent generated via the pentose phosphate pathway in glucose oxidation by serine synthesis from 3-phosphoglycerate and by the activities of the NADP+-malic enzyme or NADP+-isocitrate dehydrogenase [109,134][76][94]. GPX catalyzes the breakdown of H2O2 into water in the cytosol using GSH as a reducing agent [150][95]. A decrease in GPX activity in RPE cells and photoreceptors increases their sensitivity to OS [97,151][59][96]. GPX, structurally a selenium-containing glycoprotein, is a key enzyme that protects membrane cells under low OS from oxidative damage [146][97].4.2.4. Iron Chelators
Endogenous proteins with iron-binding properties in the RPE include ferritin, metallothionein, and some heat shock proteins (HSPs) [152,153][98][99]. Under physiological conditions, the levels of iron-binding proteins and HSP70 proteins in RPE cells are usually low, but they increase in response to OS and changes in intracellular pH, participating in the chelation of iron cations and the regulation of proteostasis [153,154][99][100]. The violation of iron metabolism and its accumulation in cells, which has a toxic effect, is associated with a decrease in autophagy and the work of chelating agents. Disturbed iron metabolism and toxicity due to accumulation in the cell is an important destabilizing factor of RPE differentiation and degeneration in retinal neurons. These processes relate to disturbed autophagy and the activity of chelating agents in the RPE.4.2.5. Chaperone Proteins
RPE cells constitutively contain the small HSP αB-crystallin, which can function as an antiapoptotic protein induced during OS [158][101]. The role of HSPs is to prevent the intracellular accumulation of cytotoxic proteins, regulate protein folding, and recover damaged proteins in lysosomes and peroxisomes [159,160][102][103]. Under OS conditions, when the level of ATP in RPE cells decreases, the ATP-independent chaperone HspB1 is among the first to be activated. In this case, the external receptor-dependent pathway of cell death is blocked with the participation of tumor necrosis factor receptors (TNFRs) and the internal mitochondrial signaling pathway. Hsp27 can maintain mitochondrial stability and redox homeostasis in cells and interacts with the apoptotic signaling pathways at many stages. It can inhibit apoptosis through the sequestration of Bax and Bcl-xS in the cytoplasm and is also involved in the stabilization of Akt [159,160][102][103]. Functions of HspB1 in the RPE consist of the blocking signaling pathways that trigger caspase-dependent apoptosis [161][104]. The activation of the low-molecular-weight chaperone Hsp27 leads to the blocking of Ca2+-induced apoptosis, which is a result of the suppression of caspase-3 functions and the prevention of cytochrome C release from mitochondria into the cytoplasm [159,160][102][103]. OS induces the expression of redox-dependent antioxidants and DJ-1 chaperones in the RPE [165][105], such as alpha-1 microglobulin, that binds to ROS [166][106].4.2.6. Low-Molecular-Weight Antioxidants
Water-soluble antioxidants such as ascorbic acid (vitamin C) and GSH act as ROS scavengers in the cytosol [169][107]. Fat-soluble antioxidants that include α-tocopherol (vitamin E) and carotenoids (β-carotene, lutein, zeaxanthin, and lycopene) are associated with lysosome and mitochondrial membranes and protect cells from LPO. α-Tocopherol is one of the strongest antioxidants due to its capacity to arrest the autocatalytic chain reaction of LP. Carotenoids rely on a similar mechanism to neutralize peroxyl radicals and 1O2 is able to interrupt the autocatalytic chain reaction of LPO [170,171][108][109].4.2.7. Additional Endogenous Neuroprotectors
The RPE synthesizes biomolecules such as neuprotectin D1, which can play a protective role [172][110]. The synthesis of neuprotectin D1 by RPE cells during phagocytosis ensures the resistance of these cells to OS [173][111]. Purinergic signaling cascades also contribute to the regulation of redox homeostasis in the RPE. The balance between extracellular ATP and adenosine maintains the pH of the lysosomes, and its disturbance changes the lysosomal activity of RPE cells and stimulates excess LF production [174,175][112][113].4.2.8. Autophagy
Cellular and molecular processes that control the production and elimination of ROS ensure the implementation of the main functions of RPE cells and their survival [12,177][12][114]. Redox homeostasis is a critical persistence factor for RPE cells to maintain the balance between their pro- and antioxidant systems. The regulation and maintenance of redox homeostasis make it possible to prevent or restore ROS-mediated damage to RPE cells. ROS, along with other cellular stress factors (inflammation and exposure to toxins), cause an increase in reparative autophagy in the RPE, which protects RPE cells from oxidative damage and is aimed at restoring altered cellular structures [11]. Metabolically active RPE cells normally demonstrate a high basal rate of autophagy compared to retinal neurons. However, this process becomes less efficient with age, which was found in human and mouse RPE [135,179][83][115]. An inverse relationship was found between a decrease in the rate of autophagy and the formation of drusen in RPE pathologies [180][116].5. Key Components in the Mechanisms of Oxidative Stress Realization in the RPE—Potential Molecular Targets
5.1. Intracellular and Molecular Targets of OS
OS is an important part of overall cellular stress, which is influenced by various endogenous factors. The destructive activity of ROS in cells is manifested in the damage and oxidation of molecular targets, such as DNA, proteins, and membrane lipids [8,185,186,187][8][117][118][119]. OS disturbs the homeostasis of RPE cells (including redox homeostasis) and their interactions with neighboring tissues and affects the majority of intracellular processes (phagocytosis, autophagy, metabolite transport, proliferation, cell death, etc.). High levels of ROS lead to the accumulation of oxidized lipoproteins, which inhibit the degradation of POS in RPE cells during phagocytosis [188][120]. Ferroptosis, a new form of programmed cell death, was characterized by LPO and GSH depletion that was mediated by iron metabolism [192][121]. The molecular mechanisms underlying the interplay between OS and ferroptosis in RPE cells are the subject of active study. There is experimental evidence that the phenomenon of ferroptosis involves the disruption of the signaling pathways of genetic changes in iron homeostasis [193][122], the GSH metabolic pathway, and LPO metabolism [171][109]. Long-term stable changes in redox homeostasis that are influenced by exogenous or endogenous factors, in violation of interactions between RPE cells and adjacent tissues, can lead to the prevalence of oxidative processes and OS. Chronic OS leads to global changes in RPE cell metabolism and disruption of the ADS [102,194][63][123]. A strong OS ultimately leads to the death of RPE cells and neurons [119][73]. The cascades of protective reactions are launched, which are mediated by calcium ions, ATP, and ROS released into the intercellular space from damaged cells [195,196][124][125]. When the RPE and photoreceptors are damaged or exposed to OS (H2O2), ATP secretion, activation of ATP-dependent Ca2+ channels (P2X7R), the release of Ca2+ from cell storage, and increased Ca2+ transport to the cell are promoted [197,198][126][127].5.2. The Role of Transcription Factors in RPE Response to OS
OS action is mediated by transcription factor-1 activator and transcription factors Fos and ATF, which control cell proliferation, autophagy, and apoptosis [125][77]. Transcription factors of the BCL-2, BAX, BAK, and BIM families control changes in mitochondrial membrane permeability, calcium release from the ER, and its entry into mitochondria [204][128]. An important role in the regulation of redox reactions in the RPE is played by apurine endonuclease-1, which is involved in the activation of activator protein-1, as well as that of transcription factors NF-κB and HIF-1α [204,205][128][129]. OS also induces the expression of phase II enzymes: NADPH-quinine oxidoreductase-1, HO-1, the modifier subunit, and the catalytic subunit of glutamate-cysteine ligase [139,140][87][88]. ROS-producing NOXs are considered targets for the action of inhibitors of ROS formation, N-acetylcysteine (NAC), apocynin, and diphenylene iodonium [208,209,210][130][131][132].5.3. OS-Dependent Secretion of Growth Factors
The accumulation of oxidized phospholipids in the RPE stimulates the ATF4-dependent secretion of angiogenic factor VEGF, mediated by protein kinase CK2 [128][133]. Under conditions of OS, the secretion of growth factors by RPE cells is disrupted, which contributes to the cellular response of the RPE and can trigger signaling pathways in the RPE that mediate the development of angiogenesis and degenerative processes. It has been shown that OS induces the increased secretion of the transforming growth factor TGFβ in the RPE [211][134]. TGFβ signals and their associated effector Smad proteins, through de novo protein synthesis, enhance the secretion of angiogenic vascular growth factor VEGF in the RPE [45,212,213,214][135][136][137][138].5.4. Changes in ATP Metabolism
The concentration of ATP decreases inside the cells and increases outside the cells during OS [174][112]. High concentrations of extracellular ATP cause neuronal death, while maintaining the physiological level of adenosine is necessary for the functioning of the RPE and retinal neurons [175][113]. Enhanced production of ROS in the RPE, associated with the disruption of the integrity of cell membranes, increases the activity of calcium ATPase. Metabolic disorders, hyperglycemia, and OS can lead to profound changes in intracellular and extracellular nucleotide levels in the RPE and neurons [227][139].5.5. Endoplasmic Reticulum Stress
With the accumulation of LPO products in the RPE from the ER, a reaction with a non-structured protein occurs with the aim of restoring homeostasis [234][140]. The ER maintains cellular calcium homeostasis through a complex set of calcium-dependent molecular chaperones required for protein folding [235][141]. The suppression of proteasome function inhibits the ER-associated protein degradation pathway, which enhances their misfiling in the ER and triggers ER stress [236][142]. Proteotoxic stress enhances OS, inflammation, and hypoxia. Proteasome inhibition induces ER stress and stimulates the expression of hypoxia-inducible factors (HIFs). HIFs regulate the expression of multiple growth factors and cytokines involved in angiogenesis and inflammation in retinal degeneration [237][143]. The stimulation of VEGF expression by transcription factor HIF-1α in human RPE cells has been shown [238][144]. The inhibition of proteasome degradation enhances the accumulation of LF [73][43]. ER stress induces an unfolded protein response (UPR) via the transducers IRE1 (inositol-requiring protein-1), PERK (protein kinase, RNA-like kinase ER), and ATF6 (activating transcription factor-6) [234][140].5.6. Reorganization of the Cytoskeleton
The role of nitric oxide in the modulation of cytoskeletal reorganization was demonstrated in the model of retinal degeneration 1 (rd1). Proteomic analysis showed that the expression levels of vimentin and serine/threonine protein phosphatase 2A (PP2A) are significantly increased when mice are exposed to continuous light exposure for 7 days compared to 12 h light/dark cycling conditions. Simultaneously, nitric oxide inactivates the PP2A catalytic subunit, which leads to increased phosphorylation of vimentin, which is a substrate for this phosphatase [248][145]. OS-mediated accumulation of Aβ induces inflammatory activity, oxidative phosphorylation dysregulation, angiogenesis, and cytoskeleton destabilization, causing a large amount of damage in the subretinal region, which is associated with the pathogenesis of AMD [219,249][146][147].5.7. Communication between Mitochondria and Lysosomes in the RPE Cellular Response to OS
Molecular processes in the RPE working against OS are associated with the dysregulation of mitochondrial function. They lead to a decrease in the activity of glyceraldehyde-3-phosphate dehydrogenase and the accumulation of advanced glycation end products and polyols [112,251,252][148][149][150]. High concentrations of ROS cause the destruction of mitochondrial membranes and the disruption of membrane transporters, which leads to apoptosis, necrosis, or ferroptosis [13,253][13][151]. Depolarization of mitochondrial membranes and the accumulation of LPO products (acrolein, etc.) further stimulate ROS production by damaged mitochondria [254,255][152][153].5.8. Apoptosis Signals
OS in the RPE and photoreceptors disturbs the signaling pathways of antioxidant defense and triggers signaling pathways of cell death in a certain form (apoptosis, necrosis, or toxic cell damage), which allows for particular levels of OS severity and cell destruction [94,119][56][73]. Mitochondrial calcium overload leads to swelling and can subsequently cause an outflow of apoptogenic cytochrome c factors and apoptosis-inducing factors from the mitochondria into the cytoplasm, where they activate caspase cascades and ultimately lead to apoptosis of the RPE and neurons [258,260][154][155]. OS triggers key apoptosis signaling in RPE cells via c-Jun N-terminal kinase (JNK)/stress-activated protein kinase, p38 and ASK1, NF-κB signaling pathways, and mitochondrial activation of the PKC signaling pathway [112,194,261][123][148][156]. NF-κB stimulates excessive production of the pro-inflammatory interleukin IL-8 and HIF-1 [262][157]. An in vitro system was used to show that human RPE cells treated with hydrogen peroxide for 24 h respond with increased production of pro-inflammatory cytokines NF-κB and IL-6 and the phosphorylation of p38, MAPK, ERK, JNK, and intercellular adhesion molecule 1 (ICAM- 1) [263][158].5.9. The Influence of OS on the State of Chromatin in RPE
OS affects the activity of epigenetic proteins and chromatin remodeling. Currently, attempts are being made to map associated epigenetic and transcriptional changes in the RPE in normal and pathological conditions (AMD, PVR, retinitis pigmentosa, etc.) [268,269][159][160]. It has been shown that epigenomic changes associated with the development of these pathologies affect the functioning of transcription factors. Epigenomic changes lead to an increase in active chromatin (euchromatin) marks on the regulatory sites of DNA enhancers of putative targets for binding to transcription factors [270][161].6. The Exogenous Regulation of Redox Homeostasis of RPE Cells
6.1. The Aging of RPE Cells as an Endogenous Factor in the Development of Retinal Pathologies
The RPE displays several specialized functions essential for retinal homeostasis (see Figure 1). The disturbed structure and dysfunction of an aging RPE lead to degenerative retinal diseases, such as AMD. AMD primarily affects RPE cells with the subsequent degeneration of photoreceptors. OS is considered to be a major AMD risk factor. The aging of RPE cells is related to a progressive decline in their functions that can be coupled with ROS overproduction [283][162]. The RPE undergoes several structural changes during aging. These include the loss of melanin, the formation of drusen, the thickening of BM, the accumulation of LF, decreased mitochondrial mass, a disturbed mitochondrial network, and microvilli atrophy [284][163]. Cellular senescence, initially an irreversible inhibition of cellular division, is associated with a decline in basic cellular functions, such as differentiation, phagocytosis, and autophagy. Senescence-associated secretory phenotypes (SASPs) are associated with the release of ROS, selective growth factors, and inflammatory cytokines, chemokines, and proteases [285][164]. An age-dependent phagocytosis activity reduction may not only disrupt retinal homeostasis but also can affect RPE survival, leading to RPE apoptotic loss and photoreceptor degeneration. An age-related decrease in lysosomal enzymatic activity inhibits the autophagic clearance of outer segments, mitochondria, and protein aggregates, thereby accelerating the accumulation of LF and products of LPO in RPE cells [286][165]. Decreased autophagy is associated with the cell phenotype shifting to senescent cells that contribute to a loss of tissue homeostasis. The accumulation of LF in the RPE is a sign of senescence in AMD [287][166]. The contents of antioxidant proteins and small-molecular-weight antioxidants decline in an aging RPE [291][167]. An age-related decrease in the activity of many proteins pivotal to the antioxidant system has been observed, including Nrf2, SOD1-2, CAT, and glutathione peroxidase (GPX) [292,293,294][168][169][170]. Additionally, DNA repair, both in mitochondria and the nucleus, decreases with age [295][171].6.2. Strategies for the Exogenous Protection of RPE against Oxidative Stress
6.2.1. Direct Neutralization of ROS with the Help of Exogenous Antioxidants
Putative exogenous OS protectors include resveratrol, curcumin, acetylcysteine, multivitamins, metal oxide nanoparticles, polyphenolic compounds, spermidine, nucleosides, agonists of serotonin receptors, and agonists and antagonists of the purinergic system [298,299,300][172][173][174]. An example is the use of spermidine, a free-radical scavenger that inhibits the degradation of singlet oxygen and reduces the production of ROS and reactive nitrogen species, mainly through the activation of MAPK, ASK-1, and p38 [301,302][175][176]. Studies have confirmed that some natural antioxidant compounds can act as ROS scavengers, enhance antioxidant enzymes, and induce or inhibit signaling pathways and gene expression related to stress response and cell death. The most common fat-soluble antioxidants include α-tocopherol (vitamin E) and carotenoids (β-carotene, lutein, and zeaxanthin). These biomolecules, being lipophilic, are predominantly associated with the membranes of lysosomes and mitochondria. The action of α-tocopherol, which is one of the most powerful antioxidants, is based on its ability to quickly interrupt the autocatalytic LPO reaction [170,171,303][108][109][177].6.2.2. The Suppression of ROS Production via the Decreased Expression of Pro-Oxidant Genes and the Activity of Pro-Oxidant Enzymes
Among the biologically active antioxidant molecules, much attention has been focused on studying the mechanisms of action of red wine polyphenol resveratrol, as evidenced by a large number of studies. Using different models in vivo and in vitro, many aspects of the action of resveratrol have been identified. Using a model of ultraviolet A (400–315 nm)-induced OS, it was shown that resveratrol is able to suppress the production of H2O2 in RPE cells by the intracellular activation of p38 and kinase, which increases the viability of these cells [308][178]. An important aspect of the action of resveratrol is the weakening of ROS production in POSs, as well as the neutralization of the toxic effect of A2E in human RPE cells [309][179]. Resveratrol has been shown to impact several transcription factors (AP-1 and Egr-1), cell cycle regulators (p21Cip1/WAF1), and apoptosis (p53, Bcl-2, Bax, and survivin) [310][180]. Resveratrol suppresses hypoxia-inducible factor-1α accumulation and vascular endothelial growth factor (VEGF) secretion, while in endothelial cells, it inhibits VEGF-R2 phosphorylation, suggesting a role of resveratrol in the inhibition of angiogenesis and choroidal neovascularization [311][181]. Resveratrol is a potent inhibitor of multiple signaling pathways related to fibrosis development, e.g., TGFβ/SMAD, NF-κB signaling, and ERK signaling pathways [312][182]. Resveratrol not only protects ARPE-19 cells from H2O2-induced death by inhibiting oxidation processes but also prevents the migration and hyperproliferation of RPE cells by activating the phosphatidylinositol-3 kinase/Akt pathway and MAPK)/ERK 1/2 cascade [313,314][183][184]. The RPE cell line ARPE19 model showed that its inhibitory effect is mediated by the suppression of PDGF receptor β, phosphatidylinositol-3 kinase/Akt pathway activation, and the MAPK signaling cascade [314][184].6.2.3. The Induction of Antioxidant Gene Expression and the Activation of their Enzymatic Products
Nrf2 activation as well as NF-κB inhibition are the main ways to prevent ROS-induced processes in the RPE and photoreceptors [340,341][185][186]. Strategies aimed at activating the components of the ADS are associated with the use of exogenous carotenoids. Thus, the use of astaxanthin can significantly reduce the formation of ROS induced by hydrogen peroxide and the apoptosis of RPE cells by activating the PI3K/Akt signaling pathway and enhancing the expression of the transcription factor Nrf2 [165][105]. An increase in Nrf2 activity, as well as the inhibition of NF-kB, showed promise for preventing ROS-induced events in the RPE and photoreceptors in AMD [340,341][185][186]. Lutein and vitamin E reduce UV-induced ROS production and LPO and increase the activity of antioxidant enzyme activities, which reduces apoptosis and increases RPE cells’ viability [342,343][187][188]. Lycopene inhibits NF-κB expression in the RPE, which is largely due to the increased expression of Nrf2 and GSH and the decreased expression of ICAM-1, having an antioxidant effect [171][109].6.2.4. The Activation of Autophagy
The action of autophagy is tightly linked to the regulation of redox homeostasis and seems particularly promising to prevent OS in the RPE. The possibility of modulating RPE redox homeostasis via the pharmacological action of the azapeptide ligand MPE-001 on CD36 (differentiation cluster 36) has been shown. MPE-001 has antioxidant and anti-apoptotic effects on RPE cells as a result of stimulation of the autophagy process, maintaining the viability of RPE cells and retinal photoreceptors [11]. In ARPE-19 cells treated with 5-(N,N-hexamethylene)amiloride (HMA), an inhibitor of pH regulators and Na+/H+ ion exchange, autophagy activation was observed under conditions of moderate OS [42][45].6.2.5. Redox-Sensitive MicroRNA
miRNAs are involved in the regulation of redox homeostasis in the RPE and border tissues, which makes them effective targets in the treatment of OS-associated pathologies [353][189]. Redox-sensitive miRNAs play an important role in the regulation of antioxidant signaling pathways in the RPE. Recent studies have shown that OS enhances the expression of miR-144-3p and mir-144-5p, which block the expression of Nrf2 and controlled target genes for antioxidants Nqo1 and Gclc, reducing the content of GSH in human and mouse RPE and increasing cell death [354,355][190][191]. Nrf2 is a target gene of miR-93; the overexpression of Nrf2 alleviates the high glucose-induced apoptotic effect of ARPE-19 cells and can reverse the pro-apoptotic effect and inflammation of miR-93 [356][192].6.2.6. Synthetic Peptides and Blockers of ROS and Neoangiogenesis
The key role of α1 Na+/K+-ATPase (α1 NKA) in the regulation of OS and the Nrf2 signaling pathway in the retina of mice with oxygen-induced retinopathy has been demonstrated. The α1 NKA targeting strategy using the pNaKtide peptide blocks the formation of α1 NKA/Src/ROS amplification loops and restores physiological ROS values. Strategies to decrease excessive VEGF production have been proposed using α1 NKA as the key target. Unlike other anti-VEGF pharmacological agents, pNaKtide inhibits inflammatory responses and retinal neovascularization [84][48]. The use of antioxidants that inhibit the activity of SMAD2, SMAD3, and VEGFA proteins and stabilize BM [275][193] by reducing the level of oxidized forms of cholesterol (oxysterols) in the RPE is considered a therapeutic approach to blocking uncontrolled angiogenesis, maintaining the stability of RPE cell homeostasis, and preventing cell death [284,359][163][194].6.2.7. Components of the Purinergic Signaling Cascade
In addition to the considered approaches aimed at activating the key components of antioxidant mechanisms in RPE cells, other methods of direct or indirect regulation of OS-dependent signaling pathways continue to be developed. The activation of AMP-activated protein kinase (AMPK) or the inhibition of adenosine kinase can be used to protect the RPE from OS [364][195]. An increase in the level of cytoplasmic cAMP restores the acidic pH of lysosomes as a result of the stimulation of adenosine A2A receptors (A2AR) by the agonist adenosine [365,366][196][197]. Data strongly suggest that cAMP signaling can reduce inflammatory mediators in multiple retinal cell types, which protects the retina against stressors [367][198]. In summary, the elevation of adenosine signaling represents the positive response of RPE cells to AMD. Adenosine stimulates A2AR and reacidifies lysosomes.6.2.8. Genome Editing
Genome editing technologies have revolutionized the gene therapy approach that is commonly used to deliver an exogenous transgene to cure a monogenic disorder. CRISPR/Cas9-mediated gene editing has successfully corrected mutations associated with various ophthalmic pathologies such as AMD, retinitis pigmentosa, and Leber congenital amaurosis [369][199]. Additional possibilities have opened up in the field of gene and cell therapy for retinal degeneration diseases through a combination of the CRISPR system with induced pluripotent stem cells (iPSCs), especially in the late stages with the irreversible loss of the RPE and photoreceptors [370][200].References
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–888.
- Fuhrmann, S.; Zou, C.J.; Levine, E. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150.
- Sinha, T.; Naash, M.I.; Al-Ubaidi, M.R. The Symbiotic Relationship between the Neural Retina and Retinal Pigment Epithelium Is Supported by Utilizing Differential Metabolic Pathways. iScience 2020, 23, 101004.
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharm. 2021, 12, 727870.
- Gupta, S.; Lytvynchuk, L.; Ardan, T.; Studenovska, H.; Faura, G.; Eide, L.; Znaor, L.; Erceg, S.; Stieger, K.; Motlik, J.; et al. Retinal Pigment Epithelium Cell Development: Extrapolating Basic Biology to Stem Cell Research. Biomedicines 2023, 11, 310.
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990.
- Bindoli, A.; Rigobello, M.P. Principles in redox signaling: From chemistry to functional significance. Antioxid. Redox Signal. 2013, 18, 1557–1593.
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748.
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated Autophagy in the RPE Is Associated with Increased Susceptibility to Oxidative Stress and AMD. Autophagy 2014, 10, 1989–2005.
- Kaarniranta, K.; Koskela, A.; Felszeghy, S.; Kivinen, N.; Salminen, A.; Kauppinen, A. Fatty acids and oxidized lipoproteins contribute to autophagy and innate immunity responses upon the degeneration of retinal pigment epithelium and development of age-related macular degeneration. Biochimie 2019, 159, 49–54.
- Dorion, M.-F.; Mulumba, M.; Kasai, S.; Itoh, K.; Lubell, W.D.; Ong, H. The CD36 Ligand-Promoted Autophagy Protects Retinal Pigment Epithelial Cells from Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 6691402.
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543.
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharm. 2011, 153, 175–190.
- Perez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int. J. Mol. Sci. 2017, 18, 2098.
- Mateos, M.V.; Tenconi, P.E.; Giusto, N.M.; Salvador, G.A. Inflammation and Oxidative Stress in Retinal Diseases: The Role of Intracellular Signaling in the Retinal Pigment Epithelium. Int. J. Ophthalmol. Clin. Res. 2015, 4, 1–7.
- Erler, P.; Monaghan, J.R. The link between injury-induced stress and regenerative phenomena: A cellular and genetic synopsis. Biochim. Biophys. Acta 2015, 1849, 454–461.
- Khandhadia, S.; Lotery, A. Oxidation and age-related macular degeneration: Insights from molecular biology. Exp. Rev. Mol. Med. 2010, 12, e34.
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell Longev. 2016, 2016, 3164734.
- Kozhevnikova, O.S.; Korbolina, E.E.; Ershov, N.I.; Kolosova, N.G. Rat retinal transcriptome: Effects of aging and AMD-like retinopathy. Cell Cycle 2013, 12, 1745–1761.
- Beranova-Giorgianni, S.; Giorgianni, F. Proteomics of Human Retinal Pigment Epithelium (RPE) Cells. Proteomes 2018, 6, 22.
- Meyer, J.G.; Garcia, T.Y.; Schilling, B.B.; Gibson Bradford, W.; Lamba, D.A. Proteome and Secretome Dynamics of Human Retinal Pigment Epithelium in Response to Reactive Oxygen Species. Sci. Rep. 2019, 9, 15440.
- O’Hara-Wright, M.; Gonzalez-Cordero, A. Retinal organoids: A window into human retinal development. Development 2020, 147, dev189746.
- Mazzolini, M.; Facchetti, G.; Andolfi, L.; Zaccaria, R.P.; Tuccioc, S.; Treu, J.; Altafini, C.; Di Fabrizio, E.M.; Lazzarino, M.; Rapp, G.; et al. The phototransduction machinery in the rod outer segment has a strong efficacy gradient. Proc. Nat. Acad. Sci. USA 2015, 112, 2715–2724.
- Masutomi, K.; Chen, C.; Nakatani, K.; Koutalos, Y. All-trans retinal mediates light-induced oxidation in single living rod photoreceptors. Photochem. Photobiol. 2012, 88, 1356–1361.
- Pan, W.W.; Wubben, T.J.; Besirli, C.G. Photoreceptor metabolic reprogramming: Current understanding and therapeutic implications. Commun. Biol. 2021, 4, 245.
- Sun, M.; Finnemann, S.C.; Febbraio, M.; Shan, L.; Annangudi, S.P.; Podrez, E.A.; Hoppe, G.; Darrow, R.; Organisciak, D.T.; Salomon, R.G.; et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem. 2006, 281, 4222–4230.
- Adijanto, J.; Banzon, T.; Jalickee, S.; Wang, N.S.; Miller, S.S. CO2-induced ion and fluid transport in human retinal pigment epithelium. J. Gen. Physiol. 2009, 133, 603–622.
- Chen, M.; Rajapakse, D.; Fraczek, M.; Luo, C.; Forrester, J.V.; Xu, H. Retinal pigment epithelial cell multinucleation in the aging eye–a mechanism to repair damage and maintain homoeostasis. Aging Cell 2016, 15, 436–445.
- Amram, B.; Cohen-Tayar, Y.; David, A.; Ashery-Padan, R. The retinal pigment epithelium—From basic developmental biology research to translation approaches. Int. J. Dev. Biol. 2017, 61, 225–234.
- Engel, A.L.; Wang, Y.; Khuu, T.H.; Worrall, E.; Manson, M.A.; Lim, R.R.; Knight, K.; Yanagida, A.; Qi, J.H.; Ramakrishnan, A.; et al. Extracellular matrix dysfunction in Sorsby patient-derived retinal pigment epithelium. Exp. Eye Res. 2022, 215, 108899.
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.M.F.; Bergen, A.A.B. The dynamic nature of Bruch’s membrane. Prog. Ret. Eye Res. 2010, 29, 1–18.
- Guymer, R.; Bird, A.C. Age Changes in Bruch’s Membrane and Related Structures in Retina; Ryan, S.J., Ed.; Mosby: St. Louis, MO, USA, 2006; pp. 1030–1039.
- Sun, K.; Cai, H.; Tezel, T.H.; Paik, D.; Gaillard, E.R.; Del Priore, L.V. Bruch’s membrane aging decreases phagocytosis of outer segments by retinal pigment epithelium. Mol. Vis. 2007, 13, 2310–2319.
- Miceli, M.V.; Liles, M.R.; Newsome, D.A. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp. Cell Res. 1994, 214, 242–249.
- Grimm, C.; Wenzel, A.; Hafezi, F.; Redmond, T.M.; Remé, C.E. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat. Genet. 2000, 25, 63–66.
- Godley, B.F.; Shamsi, F.A.; Liang, F.Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061–21066.
- Yao, J.; Tao, Z.F.; Li, C.P.; Li, X.-M.; Cao, G.-F.; Jiang, Q.; Yan, B. Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell Physiol. Biochem. 2014, 33, 107–116.
- Nag, T.C.; Maurya, M.; Roy, T.S. Age-related changes of the human retinal vessels: Possible involvement of lipid peroxidation. Ann. Anat. 2019, 226, 35–47.
- Sparrow, J.R.; Hicks, D.; Hamel, C. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823.
- Alfaar, A.S.; Stürzbecher, L.; Diedrichs-Möhring, M.; Lam, M.; Roubeix, C.; Ritter, J.; Schumann, K.; Annamalai, B.; Pompös, I.-M.; Rohrer, B.; et al. FoxP3 expression by retinal pigment epithelial cells: Transcription factor with potential relevance for the pathology of age-related macular degeneration. J. Neuroinflamm. 2022, 19, 260.
- Dontsov, A.E.; Sakina, N.L.; Ostrovsky, M.A. Light-induced release of A2E photooxidation toxic products from lipofuscin granules of human retinal pigment epithelium. Dokl. Biochem. Biophys. 2009, 425, 98–101.
- Boulton, M.; Rozanowska, M.; Rozanowski, B. The photoreactivity of ocular lipofuscin. Photochem. Photobiol. Sci. 2004, 8, 759–764.
- Terman, A.; Sandberg, S. Proteasome inhibition enhances lipofuscin formation. Ann. N. Y. Acad. Sci. USA 2002, 973, 309–312.
- Sharif, U.; Mahmud, N.M.; Kay, P.; Yang, Y.C.; Harding, S.P.; Grierson, I.; Kamalden, T.A.; Jackson, M.J.; Paraoan, L. Advanced glycation end products-related modulation of cathepsin L and NF-κB signalling effectors in retinal pigment epithelium leads to augmented response to TNFα. J. Cell Mol. Med. 2019, 23, 405–416.
- Altairac, S.; Zeggai, S.; Perani, P.; Torriglia, A. Apoptosis induced by Na+/H+ antiport inhibition activates the LEI/L-DNase II pathway. Cell Death Differ. 2003, 10, 548–557.
- Shivashankar, G.; Lim, J.C.; Acosta, M.L. Proinflammatory Cytokines Trigger the Onset of Retinal Abnormalities and Metabolic Dysregulation in a Hyperglycemic Mouse Model. J. Ophthalmol. 2023, 2023, 7893104.
- Shao, Z.; Chwa, M.; Atilano, S.R.; Park, J.; Karageozian, H.; Karageozian, V.; Kenney, M.C. The Transcriptome Profile of Retinal Pigment Epithelium and Müller Cell Lines Protected by Risuteganib Against Hydrogen Peroxide Stress. J. Ocul. Pharmacol. 2022, 38, 513–526.
- Wang, J.; Wang, X.; Gao, Y.; Lin, Z.; Chen, J.; Gigantelli, J.; Shapiro, J.I.; Xie, Z.; Pierre, S.V. Stress Signal Regulation by Na/K-ATPase As a New Approach to Promote Physiological Revascularization in a Mouse Model of Ischemic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9.
- Fanjul-Moles, M.L.; López-Riquelme, G.O. Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxid. Med. Cell. Longev. 2016, 2016, 7420637.
- Lacy, F.; Gough, D.A.; Schmid-Schonbein, G.W. Role of xanthine oxidase in hydrogen peroxide production. Free Radic. Biol. Med. 1998, 25, 720–727.
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95.
- Reichhart, N.; Figura, A.; Skosyrski, S.; Strauß, O. Control of the retinal local RAS by the RPE: An interface to systemic RAS activity. Exp. Eye Res. 2019, 189, 10838.
- Garza-Lombo, C.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Redox Homeostasis, Oxidative Stress and Mitophagy. Mitochondrion 2020, 51, 105–117.
- Saddala, M.S.; Lennikov, A.; Mukwaya, A.; Huang, H. Transcriptome-Wide Analysis of CXCR5 Deficient Retinal Pigment Epithelial (RPE) Cells Reveals Molecular Signatures of RPE7 Homeostasis. Biomedicines 2020, 8, 147.
- Kinnula, V.L.; Soini, Y.; Kvist-Makela, K.; Savolainen, E.-R.; Koistinen, P. Antioxidant defense mechanisms in human neutrophils. Antioxid. Redox Signal. 2002, 4, 27–34.
- Bazan, N.G. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: Significance in retinal degenerations. Adv. Exp. Med. Biol. 2006, 572, 531–540.
- Guerra, M.H.; Yumnamcha, T.; Singh, L.P.; Ibrahim, A.S. Relative Contribution of Different Mitochondrial Oxidative Phosphorylation Components to the Retinal Pigment Epithelium Barrier Function: Implications for RPE-Related Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 8130.
- Kumar, R.; Amruthanjali, T.; Singothu, S.; Singh, S.B.; Bhandari, V. Uncoupling proteins as a therapeutic target for the development of new era drugs against neurodegenerative disorder. Biomed. Pharmacother. 2022, 147, 112656.
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration. Antioxidants 2021, 10, 661.
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67.
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15.
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201.
- Panchal, K.; Tiwari, A.K. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion 2019, 47, 151–173.
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 2012, 646354.
- Simon, M.V.; Agnolazza, D.L.; German, O.L.; Garelli, A.; Politi, L.E.; Agbaga, M.-P.; Anderson, R.E.; Rotstein, N.P. Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurones protect photoreceptors from oxidative stress. J. Neurochem. 2016, 136, 931–946.
- Boulton, M.; Dontsov, A.; Jarvis-Evans, J.; Ostrovsky, M.; Svistunenko, D. Lipofuscin is a photoinducible free radical generator. J. Photochem. Photobiol. Biol. 1993, 19, 201–204.
- Brennan, A.M.; Suh, S.W.; Won, S.J. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 2009, 12, 857–863.
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301.
- Pizzolla, A.; Hultqvist, M.; Nilson, B.; Grimm, M.J.; Eneljung, T.; Jonsson, I.-M.; Verdrengh, M.; Kelkka, T.; Gjertsson, I.; Segal, B.H.; et al. Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J. Immunol. 2012, 188, 5003–5011.
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247.
- Lapierre, L.R.; Kumsta, C.; Sandri, M.; Ballabio, A.; Hansen, M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 2015, 11, 867–880.
- Decanini, A.; Nordgaard, C.L.; Feng, X.; Ferrington, D.A.; Olsen, T.W. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 607–615.
- Pan, S.; Liu, M.; Xu, H.; Chuan, J.; Yang, Z. Lipopolysaccharide Activating NF-kB Signaling by Regulates HTRA1 Expression in Human Retinal Pigment Epithelial Cells. Molecules 2023, 28, 2236.
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-related retinopathy in NRF2-deficient mice. PLoS ONE 2011, 6, e19456.
- Gureev, A.P.; Popov, V.N.; Starkov, A.A. Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp. Gerontol. 2020, 328, 113285.
- Winkler, B.S.; DeSantis, N.; Solomon, F. Multiple NADPH-producing pathways control glutathione (GSH) content in retina. Exp. Eye Res. 1986, 43, 829–847.
- Rodríguez-Muela, N.; Koga, H.; García-Ledo, L.; de la Villa, P.; de la Rosa, E.J.; Cuervo, A.M.; Boya, P. Balance between autophagic pathways preserves retinal homeostasis. Aging Cell. 2013, 12, 478–488.
- Han, S.; Chen, J.; Hua, J.; Hu, X.; Jian, S.; Zheng, G.; Wang, J.; Li, H.; Yang, J.; Hejtmancike, J.F.; et al. MITF protects against oxidative damage-induced retinal degeneration by regulating the NRF2 pathway in the retinal pigment epithelium. Redox Biol. 2020, 34, 101537.
- Ma, X.; Li, H.; Chen, Y.; Yang, J.; Chen, H.; Arnheiter, H.; Hou, L. The transcription factor MITF in RPE function and dysfunction. Prog. Retin. Eye Res. 2019, 73, 100766.
- Hua, J.; Chen, H.; Chen, Y.; Zheng, G.; Li, F.; Qu, J.; Ma, X.; Hou, L. MITF acts as an anti-oxidant transcription factor to regulate mitochondrial biogenesis and redox signaling in retinal pigment epithelial cells. Exp. Eye Res. 2018, 170, 138–147.
- Ma, X.; Chen, H.; Jian, S.; He, J.; Liu, Y.; Han, S.; Chang, L.; Li, P.; Chen, Y.A.; Liu, X.; et al. DAPL1 deficiency in mice impairs antioxidant defenses in the RPE and leads to retinal degeneration with AMD-like features. Redox Biol. 2023, 62, 102675.
- Ko, H.; Kim, M.M. H2O2 promotes the aging process of melanogenesis through modulation of MITF and Nrf2. Mol. Biol. Rep. 2019, 46, 2461–2471.
- Seagle, B.L.; Rezai, K.A.; Kobori, Y.; Gasyna, E.M.; Rezaei, K.A.; Norris, J.R., Jr. Melanin photoprotection in the human retinal pigment epithelium and its correlation with light-induced cell apoptosis. Proc. Nat. Acad. Sci. USA 2005, 102, 8978–8983.
- Rozanowski, B.; Burke, J.M.; Boulton, M.E.; Sarna, T.; Różanowska, M. Human RPE melanosomes protect from photosensitized and iron-mediated oxidation but become pro-oxidant in the presence of iron upon photodegradation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2838–2847.
- Ostrovsky, M.A.; Sakina, N.L.; Dontsov, A.E. An antioxidative role of ocular screening pigments. Vis. Res. 1987, 27, 893–899.
- Sarna, T.; Burke, J.M.; Korytowski, W.; Rózanowska, M.; Skumatz, C.M.B.; Zareba, A.; Zareba, M. Loss of melanin from human RPE with aging: Possible role of melanin photooxidation. Exp. Eye Res. 2003, 76, 89–98.
- Castilho, Á.F.; Aveleira, C.A.; Leal, E.C.; Simões, N.F.; Fernandes, C.R.; Meirinhos, R.; Baptista, F.I.; Ambrósio, A.F. Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS ONE 2012, 7, e42428.
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell. Biol. 2015, 33, 8–13.
- Agardh, C.D.; Gustavsson, C.; Hagert, P.; Nilsson, M.; Agardh, E. Expression of antioxidant enzymes in rat retinal ischemia followed by reperfusion. Metabolism 2006, 55, 892898.
- Qi, X.; Lewin, A.S.; Hauswirth, W.W.; Guy, J. Optic neuropathy induced by reductions in mitochondrial superoxide dismutase. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1088–1096.
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidationspecific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 430–440.
- Imamura, Y.; Noda, S.; Hashizume, K.; Shinoda, K.; Yamaguchi, M.; Uchiyama, S.; Shimizu, T.; Mizushima, Y.; Shirasawa, T.; Tsubota, K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proc. Nat. Acad. Sci. USA 2006, 103, 11282–11287.
- Justilien, V.; Pang, J.-J.; Renganathan, K.; Zhan, X.; Crabb, J.W.; Kim, S.R.; Sparrow, J.R.; Hauswirth, W.W.; Lewin, A.S. SOD2 knockdown mouse model of early AMD. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4407–4420.
- Hartong, D.T.; Dange, M.; McGee, T.L.; Berson, E.L.; Dryja, T.P.; Colman, R.F. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat. Genet. 2008, 40, 1230–1234.
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharm. 2014, 5, 196.
- Ueta, T.; Inoue, T.; Furukawa, T.; Tamaki, Y.; Nakagawa, Y.; Imai, H.; Yanagi, Y. Glutathione peroxidase 4 is required for maturation of photoreceptor cells. J. Biol. Chem. 2012, 287, 7675–7682.
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40.
- Baird, S.K.; Kurz, T.; Brunk, U.T. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem. J. 2006, 394 Pt 1, 275–283.
- Kaarniranta, K.; Salminen, A.; Eskelinen, E.-L.; Kopitz, J. Heat shock proteins as gatekeepers of proteolytic pathways-Implications for age-related macular degeneration (AMD). Ageing Res. Rev. 2009, 8, 128–139.
- Ryhanen, T.; Hyttinen, J.M.T.; Kopitz, J.; Rilla, K.; Kuusisto, E.; Mannermaa, E.; Viiri, J.; Holmberg, C.I.; Immonen, I.; Meri, S.; et al. Crosstalk between Hsp70 molecular chaperone, lysosomes and proteasomes in autophagy-mediated proteolysis in human retinal pigment epithelial cells. J. Cell. Mol. Med. 2009, 13, 3616–3631.
- Alge, C.S.; Priglinger, S.G.; Neubauer, A.S.; Kampik, A.; Zillig, M.; Bloemendal, H.; Welge-Lussen, U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3575–3582.
- Whitlock, N.A.; Lindsey, K.; Agarwal, N.; Crosson, C. Heat Shock Protein 27 Delays Ca2+-Induced Cell Death in a Caspase-Dependent and -Independent Manner in Rat Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1085–1091.
- Nahomi, R.B.; Palmer, A.; Green, K.M.; Fort, P.E.; Nagaraj, R.H. Pro-inflammatory cytokines downregulate Hsp27 and cause apoptosis of human retinal capillary endothelial cells. Biochim. Biophys. Acta 2014, 1842, 164–174.
- O’Reilly, A.M.; Currie, R.W.; Clarke, D.B. HspB1 (Hsp 27) expression and neuroprotection in the retina. Mol. Neurobiol. 2010, 42, 124–132.
- Bonilha, V.L. Oxidative Stress Regulation and DJ-1 Function in the Retinal Pigment Epithelium: Implications for AMD. Adv. Exp. Med. Biol. 2018, 1074, 3–9.
- Bergwik, J.; Kristiansson, A.; Allhorn, M.; Gram, M.; Åkerström, B. Structure, Functions, and Physiological Roles of the Lipocalin α 1-Microglobulin (A1M). Front. Physiol. 2021, 12, 645650.
- Yin, J.; Thomas, F.; Lang, J.C.; Chaum, E. Modulation of oxidative stress responses in the human retinal pigment epithelium following treatment with vitamin C. J. Cell Physiol. 2011, 226, 2025–2032.
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307.
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176.
- Mukherjee, P.K.; Marcheselli, V.L.; Barreiro, S.; Hu, J.; Bok, D.; Bazan, N.G. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 13152–13157.
- Bazan, N.G.; Musto, A.E.; Knott, E.J. Endogenous Signaling by Omega-3 Docosahexaenoic Acid-derived Mediators Sustains Homeostatic Synaptic and Circuitry. Integr. Mol. Neurobiol. 2011, 44, 216–222.
- Sanderson, J.; Dartt, D.A.; Trinkaus-Randall, V.; Pintor, J.; Civan, M.M.; Delamere, N.A.; Fletcher, E.L.; Salt, T.E.; Grosche, A.; Mitchell, C.H. Purines in the eye: Recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp. Eye Res. 2014, 127, 270–279.
- Loukovaara, S.; Sahanne, S.; Jalkanen, S.; Yegutkin, G.G. Increased intravitreal adenosine 5′-triphosphate, adenosine 5′-diphosphate and adenosine 5′-monophosphate levels in patients with proliferative diabetic retinopathy. Acta Ophthalmol. 2015, 93, 67–73.
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Rivera-Del, V.N.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374.
- Ma, J.Y.W.; Greferath, U.; Wong, J.H.C.; Fothergill, L.J.; Jobling, A.I.; Vessey, K.A.; Fletcher, E.L. Aging induces cell loss and a decline in phagosome processing in the mouse retinal pigment epithelium. Neurobiol. Aging 2023, 128, 1–16.
- Blasiak, J.; Pawlowska, E.; Szczepanska, J.; Kaarniranta, K. Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 210.
- Davies, K.J. Protein damage and degradation by oxygen radicals. I. General aspects. J. Biol. Chem. 1987, 262, 9895–9901.
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684.
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843.
- Longoni, B.; Demontis, G.C. Polyunsaturated Lipids in the Light-Exposed and Prooxidant Retinal Environment. Antioxidants 2023, 12, 617.
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88.
- Frazer, D.M.; Anderson, G.J. The regulation of iron transport. Biofactors 2014, 40, 206–214.
- Zhang, M.; Jiang, N.; Chu, Y.; Postnikova, O.; Varghese, R.; Horvath, A.; Cheema, A.K.; Golestaneh, N. Dysregulated metabolic pathways in age-related macular degeneration. Sci. Rep. 2020, 10, 2464.
- Cordeiro, J.V.; Jacinto, A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat. Rev. Mol. Cell Biol. 2013, 14, 249–262.
- Mitchell, C.; Lu, W.; Hu, H.; Zhang, X.; Reigada, D.; Zhang, M. The P2X(7) receptor in retinal ganglion cells: A neuronal model of pressure-induced damage and protection by a shifting purinergic balance. Purinergic Signal. 2008, 4, 313–321.
- Wang, K.; Li, H.; Sun, R.; Liu, C.; Luo, Y.; Fu, S.; Ying, Y. Emerging roles of transforming growth factor beta signaling in wet age-related macular degeneration. Acta Biochim. Biophys. Sin. 2019, 51, 1–8.
- Shao, X.; Guha, S.; Lu, W.; Campagno, K.E.; Beckel, J.M.; Mills, J.A.; Yang, W.; Mitchell, C.H. Polarized Cytokine Release Triggered by P2X7 Receptor from Retinal Pigmented Epithelial Cells Dependent on Calcium Influx. Cells 2020, 9, 2537.
- Szegezdi, E.; MacDonald, D.C.; Ní Chonghaile, T.; Gupta, S.; Samali, A. Bcl-2family on guard at the ER. Am. J. Physiol. Cell Physiol. 2009, 296, C941–C953.
- Li, Y.; Liu, X.; Zhou, T.; Kelley, M.R.; Edwards, P.; Gao, H.; Qiao, X. Inhibition of APE1/Ref-1 redox activity rescues human retinal pigment epithelial cells from oxidative stress and reduces choroidal neovascularization. Redox Biol. 2014, 2, 485–494.
- Al-Shabrawey, M.; Bartoli, M.; El-Remessy, A.B.; Platt, D.H.; Matragoon, S.; Behzadian, M.A.; Caldwell, R.W.; Caldwell, R.B. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am. J. Pathol. 2005, 167, 599–607.
- Estrago-Franco, M.F.; Moustafa, M.T.; Riazi-Esfahani, M.; Sapkal, A.U.; Piche-Lopez, R.; Patil, A.J.; Sharma, A.; Falatoonzadeh, P.; Chwa, M.; Luczy-Bachman, G.; et al. Effects of Benzo(e)pyrene on Reactive Oxygen/Nitrogen Species and Inflammatory Cytokines Induction in Human RPE Cells and Attenuation by Mitochondrial-involved Mechanism. J. Ophthalmic Vis. Res. 2016, 11, 385–393.
- Wu, L.; Tan, X.; Liang, L.; Zhang, D.; Kijlstra, A.; Yang, P. The Role of Mitochondria-Associated Reactive Oxygen Species in the Amyloid β Induced Production of Angiogenic Factors by ARPE-19. Cells Curr. Mol. Med. 2017, 17, 140–148.
- Pollreisz, A.; Afonyushkin, T.; Oskolkova, O.V.; Gruber, F.; Bochkov, V.N.; Schmidt-Erfurth, U. Retinal pigment epithelium cells produce VEGF in response to oxidized phospholipids through mechanisms involving ATF4 and protein kinase CK2. Exp. Eye Res. 2013, 116, 177–184.
- Yu, A.L.; Fuchsholer, R.; Kook, D.; Kampik, A.; Bloemendal, H.; Welge-Lüssen, U. Subtoxic oxidative stress induces senescence in retinal pigment epithelial cell via TGF-beta release. Investig. Ophthalmol. Vis. Sci. 2009, 50, 926–935.
- Nagineni, C.N.; Samuel, W.; Nagineni, S.; Pardhasaradhi, K.; Wiggert, B.; Detrick, B.; Hooks, J.J. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. J. Cell. Physiol. 2003, 197, 453–462.
- Yamagishi, S.I.; Matsui, T.; Nakamura, K.; Yoshida, T.; Takeuchi, M.; Inoue, H.; Yoshida, Y.; Imaizumi, T. Pigment-epithelium-derived factor suppresses expression of receptor for advanced glycation end products in the eye of diabetic rats. Ophthalmic Res. 2007, 39, 92–97.
- Sun, J.; Xu, Y.; Sun, S.; Sun, Y.; Wang, X. Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: The role of mitochondrial reactive oxygen species. Mol. Cell Biochem. 2010, 343, 27–35.
- Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C.; Semeraro, F. Proliferative Vitreoretinopathy after Eye Injuries: An Overexpression of Growth Factors and Cytokines Leading to a Retinal Keloid. Mediat. Inflamm. 2013, 2013, 269787.
- Mitchell, C.H. Release of ATP by a human retinal pigment epithelial cell line: Potential for autocrine stimulation through subretinal space. J. Physiol. 2001, 534, 193–202.
- Rana, T.M.; Shinde, V.M.; Starr, C.R.; Kruglov, A.A.; Boitet, E.R.; Kotla, P.; Zolotukhin, S.; Gross, A.K.; Gorbatyuk, M.S. An activated unfolded protein response promotes retinal degeneration and triggers an inflammatory response in the mouse retina. Cell Death Dis. 2014, 5, e1578.
- Sauer, T.; Patel, M.; Chan, C.C.; Tuo, J. Unfolding the therapeutic potential of chemical chaperones for age-related macular degeneration. Expert. Rev. Ophthalmol. 2008, 3, 29–42.
- Vembar, S.S.; Brodsky, J.L. One step at a time: Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell. Biol. 2008, 9, 944–957.
- Mammadzada, P.; Corredoira, P.M.; André, H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: A gene therapy perspective. Cell. Mol. Life Sci. 2020, 77, 819–833.
- Arjamaa, O.; Nikinmaa, M.; Salminen, A.; Kaarniranta, K. Regulatory role of HIF-1 in the pathogenesis of age-related macular degeneration (AMD). Ageing Res. Rev. 2009, 8, 349–358.
- Sripathi, S.R.; Hu, M.-W.; Liu, M.M.; Wan, J.; Cheng, J.; Duan, Y.; Mertz, J.L.; Wahlin, K.J.; Maruotti, J.; Berlinicke, C.A.; et al. Transcriptome Landscape of Epithelial to Mesenchymal Transition of Human Stem Cell–Derived RPE. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1–13.
- Wang, M.; Su, S.; Jiang, S.; Sun, X.; Wang, J. Role of amyloid β-peptide in the pathogenesis of age-related macular degeneration. BMJ Open Ophthalmol. 2021, 6, e000774.
- Jo, D.H.; Cho, C.S.; Kim, J.H.; Kim, J.H. Intracellular amyloid-β disrupts tight junctions of the retinal pigment epithelium via NF-κB activation. Neurobiol. Aging 2020, 95, 115–122.
- Cano, M.; Datta, S.; Wang, L.; Liu, T.; Flores-Bellver, M.; Sachdeva, M.; Sinha, D.; Handa, J.T. Nrf2 deficiency decreases NADPH from impaired IDH shuttle and pentose phosphate pathway in retinal pigmented epithelial cells to magnify oxidative stress-induced mitochondrial dysfunction. Aging Cell 2021, 20, e13444.
- Du, X.; Matsumura, T.; Edelstein, D.; Rossetti, L.; Zsengellér, Z.; Szabó, C.; Brownlee, M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003, 112, 1049–1057.
- He, Y.; Tombran-Tink, J. Mitochondrial decay and impairment of antioxidant defenses in aging RPE cells. Adv. Exp. Med. Biol. 2010, 664, 165–183.
- Totsukaa, K.; Ueta, T.; Uchida, T.; Roggia, M.F.; Nakagawa, S.; Vavvas, D.G.; Honjo, M.; Aihar, M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019, 181, 316–324.
- Sheu, S.-J.; Liu, N.-C.; Chen, J.-L. Resveratrol Protects Human Retinal Pigment Epithelial Cells from Acrolein-Induced Damage. J. Ocul. Pharmacol. 2010, 26, 231–236.
- Alfarhan, M.; Jafari Eissa Narayanan, S.P. Acrolein: A Potential Mediator of Oxidative Damage in Diabetic Retinopathy. Biomolecules 2020, 10, 1579.
- Zhao, M.; Antunes, F.; Eaton, J.W.; Brunk, U.T. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur. J. Biochem. 2003, 270, 3778–3786.
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118.
- Klettner, A. Oxidative stress induced cellular signaling in RPE cells. Front. Biosci. 2012, 4, 392–411.
- Zetterqvist, A.V.; Blanco, F.; Ohman, J.; Kotova, O.; Berglund, L.M.; de Frutos Garcia, S.; Al-Naemi, R.; Wigren, M.; McGuire, P.G.; Bosc, L.V.G.; et al. Nuclear factor of activated t cells is activated in the endothelium of retinal microvessels in diabetic mice. J. Diabetes Res. 2015, 2015, 428473.
- Wu, W.-C.; Hu, D.-N.; Gao, H.-X.; Chen, M.; Wang, D.; Rosen, R.; McCormick, S.A. Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells. Mol. Vis. 2010, 16, 1864–1873.
- Pennington, K.L.; DeAngelis, M.M. Epigenetic Mechanisms of the Aging Human Retina. J. Exp. Neurosci. 2015, 9, 51–79.
- Barnstable, C.J. Epigenetics and Degenerative Retinal Diseases: Prospects for New Therapeutic Approaches. Asia Pac. J. Ophthalmol. 2022, 11, 328–334.
- Dvoriantchikova, G.; Lypka, K.R.; Ivanov, D. The Potential Role of Epigenetic Mechanisms in the Development of Retinitis Pigmentosa and Related Photoreceptor Dystrophies. Front. Genet. 2022, 13, 827274.
- Blasiak, J.; Sobczuk, P.; Pawlowska, E.; Kaarniranta, K. Interplay between aging and other factors of the pathogenesis of age-related macular degeneration. Ageing Res. Rev. 2022, 81, 101735.
- Sura, A.A.; Chen, L.; Messinger, J.D.; Swain, T.A.; McGwin, G., Jr.; Freund, K.B.; Curcio, C.A. Measuring the Contributions of Basal Laminar Deposit and Bruch’s Membrane in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19.
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593.
- Ferrington, D.A.; Sinha, D.; Kaarniranta, K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog. Retin. Eye Res. 2016, 51, 69–89.
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400.
- Álvarez-Barrios, A.; Álvarez, L.; García, M.; Artime, E.; Pereiro, R.; González-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89.
- Gu, X.; Neric, N.J.; Crabb, J.S.; Crabb, J.W.; Bhattacharya, S.K.; Rayborn, M.E.; Hollyfield, J.G.; Bonilha, V.L. Age-related changes in the retinal pigment epithelium (RPE). PLoS ONE 2012, 7, e38673.
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114.
- Shilovsky, G.A. Lability of the Nrf2/Keap/ARE Cell Defense System in Different Models of Cell Aging and Age-Related Pathologies. Biochem. 2022, 87, 70–85.
- Gredilla, R.; Garm, C.; Stevnsner, T. Nuclear and mitochondrial DNA repair in selected eukaryotic aging model systems. Oxid. Med. Cell Longev. 2012, 2012, 282438.
- Chucair, A.J.; Rotstein, N.P.; Sangiovanni, J.P.; During, A.; Chew, E.Y.; Politi, L.E. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: Relation with docosahexaenoic acid. Investig. Ophtalmol. Vis. Sci. 2007, 48, 5168–5177.
- Hsueh, Y.-J.; Chen, Y.-N.; Tsao, Y.-T.; Cheng, C.-M.; Wu, W.-C.; Chen, H.-C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255.
- Kim, D.H.; Kim, J.-H.; Hwangbo, H.; Kim, S.Y.; Ji, S.Y.; Kim, M.Y.; Cha, H.-J.; Park, C.; Hong, S.H.; Kim, G.-Y.; et al. Spermidine Attenuates Oxidative Stress-Induced Apoptosis via Blocking Ca2+ Overload in Retinal Pigment Epithelial Cells Independently of ROS. Int. J. Mol. Sci. 2021, 22, 1361.
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Nat. Acad. Sci. USA 1998, 95, 11140–11145.
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240.
- Katz, M.L.; Robison, W.G.; Herrmann, R.K.; Groome, A.B.; Bieri, J.G. Lipofuscin accumulation resulting from senescence and vitamin E deficiency: Spectral properties and tissue distribution. Mech. Ageing Dev. 1984, 25, 149–159.
- Chan, C.-M.; Huang, C.-H.; Li, H.-J.; Hsiao, C.-Y.; Su, C.-C.; Lee, P.-L.; Hung, C.-F. Protective Effects of Resveratrol against UVA-Induced Damage in ARPE19 Cells. Int. J. Mol. Sci. 2015, 16, 5789–5802.
- Alaimo, A.; Di Santo, M.C.; Rubio, A.P.D.; Chaufan, G.; Linares, G.G.; Pérez, O.E. Toxic effects of A2E in human ARPE-19 cells were prevented by resveratrol: A potential nutritional bioactive for age-related macular degeneration treatment. Arch. Toxicol. 2020, 94, 553–572.
- Zou, X.; Gao, J.; Zheng, Y.; Wang, X.; Chen, C.; Cao, K.; Xu, J.; Li, Y.; Lu, W.; Liu, J.; et al. Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death. Cell Death Dis. 2014, 5, e1218.
- Zhang, H.; He, S.; Spee, C.; Ishikawa, K.; Hinton, D.R. SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by Resveratrol and its relevance to choroidal neovascularization. Cytokine 2015, 76, 549–552.
- Bai, Y. Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem. Pharm. 2014, 92, 484–493.
- Dridi, S.; Hirano, Y.; Tarallo, V.; Kim, Y.; Fowler, B.J.; Ambati, B.K.; Bogdanovich, S.; Chiodo, V.A.; Hauswirth, W.W.; Kugel, J.F.; et al. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 13781–13786.
- Chan, C.M.; Chang, H.H.; Wang, V.C.; Huang, C.L.; Hung, C.F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRbeta, PI3K/Akt and MAPK pathways. PLoS ONE 2013, 8, e56819.
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.-M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Nat. Acad. Sci. USA 2004, 101, 3381–3386.
- Xiong, W.; Garfinkel, A.E.M.C.; Li, Y.; Benowitz, L.I.; Cepko, C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Investig. 2015, 125, 1433–1445.
- Li, S.Y.; Fu, Z.J.; Ma, H. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investig. Ophthalmol. Vis. Sci. 2009, 50, 836–843.
- Aimjongjun, S.; Sutheerawattananonda, M.; Limpeanchob, N. Silk lutein extract and its combination with vitamin E reduce UVB-mediated oxidative damage to retinal pigment epithelial cells. J. Photochem. Photobiol. B Biol. 2013, 124, 34–41.
- Ciesielska, S.; Slezak-Prochazka, I.; Bil, P.; Rzeszowska-Wolny, J. Micro RNAs in Regulation of Cellular Redox Homeostasis. Int. J. Mol. Sci. 2021, 22, 6022.
- Yam, M.; Engel, A.L.; Wang, Y.; Zhu, S.; Hauer, A.; Zhang, R.; Lohner, D.; Huang, J.; Dinterman, M.; Zhao, C.; et al. Proline Mediates Metabolic Communication Between Retinal Pigment Epithelial Cells and the Retina. J. Biol. Chem. 2019, 294, 10278–10289.
- Jadeja, R.N.; Martin, P.M. Data on the Role of miR-144 in Regulating Fetal Hemoglobin Production in Retinal Pigmented Epithelial Cells. Data Brief 2020, 28, 104874.
- Luo, R.; Jin, H.; Li, L.; Hu, Y.X.; Xiao, F. Long Noncoding RNA MEG3 Inhibits Apoptosis of Retinal Pigment Epithelium Cells Induced by High Glucose via the miR-93/Nrf2 Axis. Am. J. Pathol. 2020, 190, 1813–1822.
- Cho, Y.-K.; Lee, S.-M.; Kang, Y.-J.; Kang, Y.-M.; Jeon, I.-C.; Park, D.-H. The Age-Related Macular Degeneration (AMD)-Preventing Mechanism of Natural Products. Processes 2022, 10, 678.
- Intartaglia, D.; Giamundo, G.; Conte, I. The Impact of miRNAs in Health and Disease of Retinal Pigment Epithelium. Front. Cell. Dev. Biol. 2021, 8, 589985.
- Qin, S.; De Vries, G.W. alpha2 but not alpha1 AMP-activated protein kinase mediates oxidative stress-induced inhibition of retinal pigment epithelium cell phagocytosis of photoreceptor outer segments. J. Biol. Chem. 2008, 283, 6744–6751.
- Liu, J.; Lu, W.; Reigada, D.; Nguyen, J.; Laties, A.M.; Mitchell, C.H. Restoration of Lysosomal pH in RPE Cells from Cultured Human and ABCA4(-/-) Mice: Pharmacologic Approaches and Functional Recovery. Investig. Ophthalmol. Vis. Sci. 2008, 49, 772–780.
- Pavan, B.; Capuzzo, A.; Forlani, G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp. Eye Res. 2014, 120, 50–54.
- Steinle, J.J. Review: Role of cAMP signaling in diabetic retinopathy. Mol. Vis. 2020, 26, 355–358.
- Cho, G.Y.; Abdulla, Y.; Sengillo, J.D.; Justus, S.; Schaefer, K.A.; Bassuk, A.G.; Tsang, S.H.; Mahajan, V.B. CRISPR-mediated Ophthalmic Genome Surgery. Curr. Ophthalmol. Rep. 2017, 5, 199–206.
- Dehghan, S.; Mirshahi, R.; Shoae-Hassani, A.; Naseripour, M. Human-induced pluripotent stem cells-derived retinal pigmented epithelium, a new horizon for cells-based therapies for age-related macular degeneration. Stem Cell Res. 2022, 13, 217.
