Polyphenols, as well as volatile compounds responsible for aromatic features, play a critical role in the quality of vegetables and medicinal, and aromatic plants (MAPs). The research conducted has shown that these plants contain biologically active compounds, mainly polyphenols, that relate to the prevention of inflammatory processes, neurodegenerative diseases, cancers, and cardiovascular disorders as well as to antimicrobial, antioxidant, and antiparasitic properties.
- aromatic plants
- bioactive compounds
- consumers
- medicinal plants
- phenolic compounds
- plant breeders
- volatile compounds
- vegetables
1. Plant Bioactive Phenolic Compounds
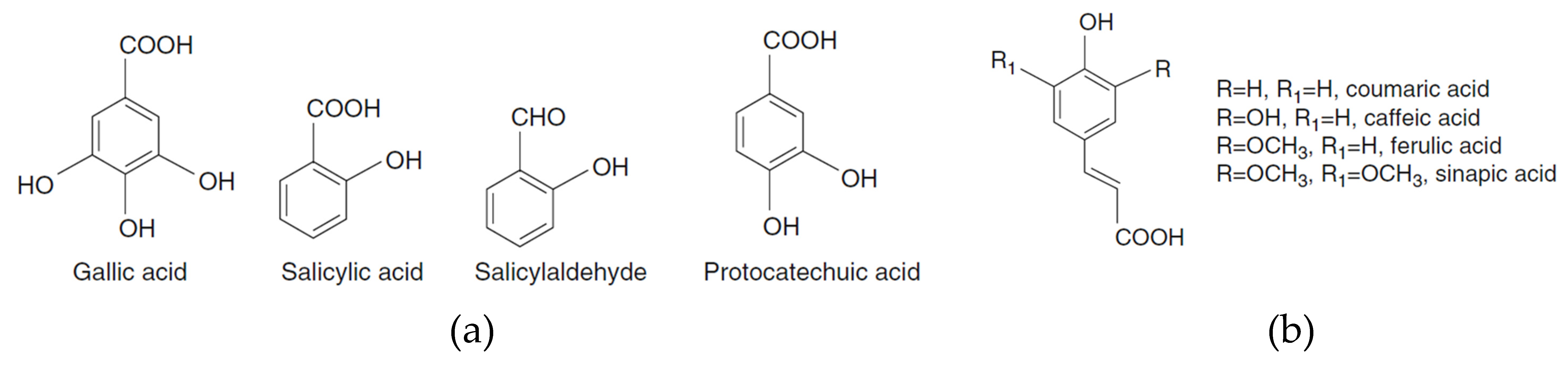
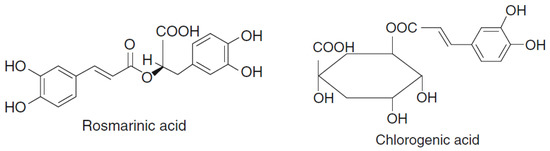
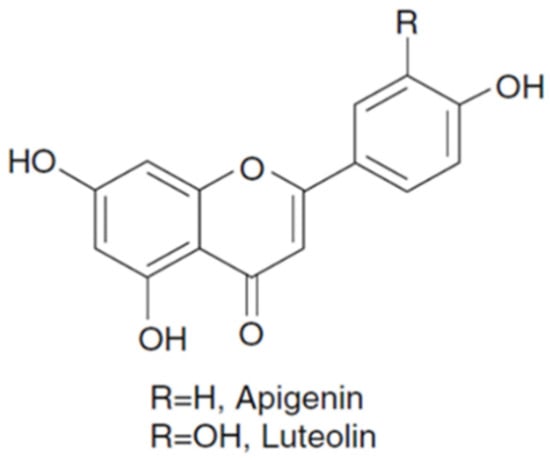
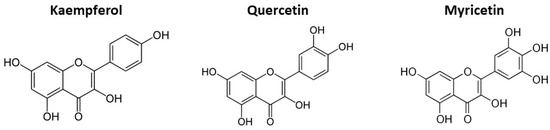
|
Vegetables |
Main Phenolics |
|---|
|
MAP Extracts |
Main Phenolics Identified Bioactivities Pointed |
Ref. |
||||||
|---|---|---|---|---|---|---|---|---|
Bioactivities Pointed |
Ref. |
|||||||
|
Artichoke (Cynara scolymus L.) |
Hydroxytyrosol, verbascoside, apigenin-7-glucoside, oleuropein, quercetin, pinoresinol, and apigenin |
|||||||
|
Fern (Asplenium nidus L.) |
7-O-hexoside and quercetin-7-O-rutinoside |
Anti-inflammatory activities of C. scolymus were found due to the synergistic effect of phenolic compounds. Inhibitory action of artichoke extracts in the inflammatory process such as histamine, bradykinin, and chemokine mediators’ processes was related to the phenolic content. |
Antimicrobial activity against Proteus mirabilis Hauser, Proteus vulgaris Hauser, and Pseudomonas aeruginosa (Schroeter). Migula was shown when fern extracts were applied at different concentrations. | |||||
[ | ][77] |
Broccoli florets (Brassica oleracea L. var. italica) |
||||||
|
Ginkgo leaves ( |
Hydroxybenzoic acid, hydroxycinnamic acid, flavone, polymethoxylated flavone, kaempferol glycosylated and kaempferol derivatives, quercetin-3-O-glucoside and derivatives, isorhamnetin-3-O-rutinoside, isorhamnetin glucoside, and related compounds |
Ginkgo biloba L.) |
Hydroalcoholic extracts were capable of directly reacting with and quenching DPPH and Oxygen (ORAC) radicals. Flavonoids and derivatives showed significant positive correlations to DPPH, and ORAC. |
Quercitin-3-O-glucoside |
[ |
Ginkgo leaf extracts were capable of decreasing sunburn symptoms in UVB-induced skin in vivo models. | ||
[ | ][78] |
Celery (Apium graveolens L.) |
High content of apiin, apigenin, and rutin, 3,7-dihydroxyflavone, cyanidin and diosmetin, and terpenes (α-ionone) |
Antioxidant activity was highly correlated with the presence of apiin, apigenin, and rutin, mainly due to the lower BDE of O–H bonds in their B rings, which enhanced their H atom donating ability. |
||||
|
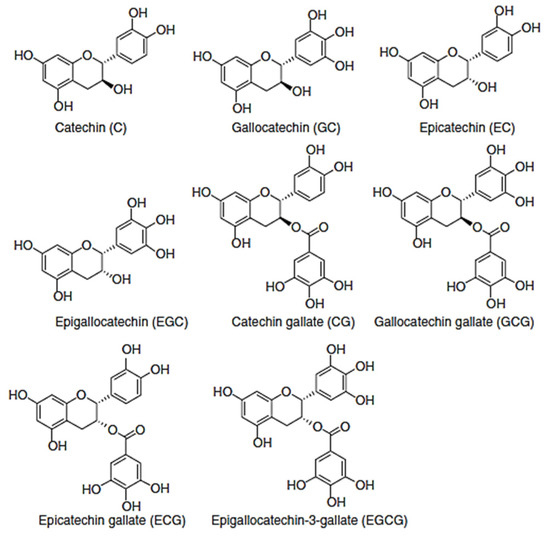
Green tea (Camellia fangchengensis Liang and Zhong) |
Procyanidin B1, B2, B3, procyanidin trimer, fangchengbisflavan A and B, catechin 7-O-β-glucopyranoside, epicatechin, (−)-epicatechin gallate, epigallocatechin, and epicatechin 3-(3-O-methyl) gallate |
Antiradical and antioxidant activity against in vitro studies was shown. |
Garlic (Allium sativum L.) |
|||||
|
Haskap berry (Lonicera caerulea |
The high content of total phenolic content, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, cyanidin-3-(6′-malonyl)-glucoside) |
L.) |
A positive and significant correlation between the content of total phenolic content and antimicrobial and antioxidant activity was found. The highest total phenolics content was significantly correlated with the lowest EC50 values for all the tested antioxidant activity assays. |
Cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, chlorogenic acid, quercitin-3-O-rutinoside, quercitin-3-O-glucoside, and catechin |
[ |
Extracts exhibited comparable anti-inflammatory effects to diclofenac which is a COX inhibitory medicine. | ||
[ | ][80] |
Ginseng leaves (Panax ginseng C. A. Mey.) |
||||||
|
Nutmeg | Gallic acid and galangin |
( Myristica fragrans Houtt) |
30,40,7-trihydroxyflavone The antioxidant capacity in the lipophilic fraction was higher than those in hydrophilic fractions and positive correlations between antioxidant capacity and total phenolic content, gallic acid, and galangin were found. |
Antibacterial activity of nutmeg extracts against the multidrug resistant Gram-negative bacteria Providencia stuartii Ewing and Escherichia coli was observed. | ||||
[ | ][68] |
Leek (Allium porrum L.) |
Rosmarinic acid, quercetin, and apigenin glycosylated forms and respective derivatives |
Extracts showed a favourable antimicrobial activity against | ||||
|
Lavandula (Lavandula pedunculata Mill.) | O-glucuronide, and rosmarinic acid | Staphylococcus aureus, Bacillus subtilis, and Aspergillus niger. Extracts inhibit Hep2c, L2OB, and RD tumor cells in a dose-dependent manner after 48 h treatment period. |
[87][ |
Caffeic acid, luteolin-7-75 |
Exhibited highest anti-inflammatory activity in rat RAW 264.7 macrophages by inhibiting nitric oxide production.] | |||
[ |
Onion (Allium cepa L.) |
|||||||
|
Rosemary (Rosmarinus officinalis L.) |
Quercetin aglycone, quercetin-4′-O-monoglucoside, and quercetin-3,4′-O-diglucoside |
Isorhamnetin-3-O-hexoside, carnosic acid, carnosol, rosmanol, epirosmanol, rosmaridiphenol, rosmarinic acid, and their methoxy derivatives The antioxidant activity of onions was dependent on variation in the contents of quercetin compounds in all onion varieties assessed. Antibacterial activity against Staphylococcus sp. and Escherichia coli was dependent on variation in both phenolic profile and content. |
Antioxidant and antiradical activities were observed. Exerted a direct cytocidal effect via upregulation of nitric oxide (NO) in cancer cells, which in turn acts in a proapoptotic manner and induces cell apoptosis. | |||||
[ |
Watercress (Nasturtium officinale L.) |
Coumaric acid, sinapic acid, caftaric acid, quercetin, and quercetin derivatives were the major phenolic compounds identified |
The radical scavenging activity (RSA) of root, stem, and leaves of watercress methanolic extracts were highly correlated with the variation of phenolics. Watercress leaves had similar antioxidant potential to that of tocopherol. |
|||||
|
Oregano | [ |
( | |||
Origanum vulgare | |||
L.) | |||
Rosmarinic acid, 3,4-dihydroxybenzoic acid | |||
The hydroalcoholic extract shows antioxidant activity in vitro and in vivo models. The oral formulation of oregano preserves antioxidant activity from gastrointestinal digestion. | |||
|
Thymus (Thymus algeriensis Boiss. and Reut) |
Rosmarinic acid, caffeoyl rosmarinic acid, eriodictyol hexoside, kaempferol-O-hexoside, kaempferol-O-hexuronide, luteolin-O-hexuronide, apigenin-C-di-hexoside, and apigenin-O-hexuronide |
Methanolic extracts were found to possess substantial antioxidant and antiacetylcholinesterase activities which were correlated to their phenolic contents; however, significant variations were observed between populations. |
|
|
Sage (Salvia officinalis L.) |
Apigenin, carnosic acid, carnosol, rosmanol, epirosmanol, rosmarinic acid, and their methoxy derivatives |
Antioxidant and antiradical activities were observed. Sage extracts were capable of exerting a direct cytocidal effect via upregulation of nitric oxide (NO) in cancer cells, in a proapoptotic manner which induced cell apoptosis. |
2. Polyphenols as Prebiotics
3. Advances in Phenolic Compounds and Future Research Perspectives
References
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006, 27, 1–93.
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861.
- Sánchez-Moreno, C. Compuestos polifenólicos: Estructura y classificación: Presencia en alimentos y consumo: Biodisponibilidad y metabolismo. Alimentaria 2002, 329, 19–28.
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317.
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. Biomed. Res. Int. 2013.
- Vilela, A.; Pinto, T. Grape Infusions: The flavour of Grapes and Health-Promoting Compounds in Your Tea Cup. Beverages 2019, 5, 48.
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of Phenolic Compounds in the Reduction of Risk Factors of Cardiovascular Diseases. Molecules 2019, 24, 366.
- Llano, D.G.; Liu, H.; Khoo, K.; Moreno-Arribas, M.V.; Bartolomé, B. Some New Findings Regarding the Antiadhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Bacteria. J. Agric. Food Chem. 2019, 67, 2166–2174.
- Robbins, R. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887.
- Giada, M.L.R. Food phenolic compounds: Main classes, sources and their antioxidant power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; González, J.A.M., Ed.; InTech: Rijeka, Croatia, 2013; pp. 87–112.
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985.
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758.
- Sircar, D.; Mitra, A. Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota 2: Confirming biosynthetic steps through feeding of inhibitors and precursors. J. Plant. Physiol. 2009, 166, 1370–1380.
- Hur, J.M.; Park, J.G.; Yang, K.H.; Park, J.C.; Park, J.R.; Chun, S.S.; Choi, J.S.; Choi, J.W. Effect of methanol extract of Zanthoxylum piperitum leaves and of its compound, protocatechuic acid, on hepatic drug metabolizing enzymes and lipid peroxidation in rats. Biosci. Biotechnol. Biochem. 2003, 67, 945.
- Kono, Y.; Shibata, H.; Kodama, Y.; Sawa, Y. The suppression of the N-nitrosating reaction by chlorogenic acid. Biochem. J. 1995, 312, 947–953.
- Al-Sereitia, M.R.; Abu-Amerb, K.M.; Sena, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124.
- Zheng, W.; Wang, S. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170.
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641.
- Keating, G.J.; O’Kennedy, R. The chemistry and occurrence of coumarins. In Coumarins: Biology, Applications and Mode of Action; O’Kennedy, R., Thornes, R.D., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1997; pp. 23–66.
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.; Molina, E.; Yordi, E. Coumarins—An Important Class of Phytochemicals. In Phytochemicals—Isolation, characterisation and Role in Human Health; Rao, V., Rao, L., Eds.; InTech: Rijeka, Croatia, 2015; pp. 113–140.
- Gopi, C.; Dhanaraju, M.D. Synthesis, characterisation and anti-microbial action of novel azo dye derived from 4-methyl 7-OH 8 nitro coumarin. J. Pharm. Res. 2011, 4, 1037–1038.
- Raev, L.; Voinov, E.; Ivanov, I.; Popov, D. Antitumor activity of some coumarin derivatives. Pharmazie 1997, 45, 696–697.
- Nofal, Z.L.; El-Zahar, M.; Abd El-Karim, S. Novel coumarin derivatives with expected biological activity. Molecules 2000, 5, 99–113.
- Landete, J.M. Plant, mammalian lignans: A review of source, intake metabolism, intestinal bacteria, health. Food Res. Int. 2012, 46, 410.
- Rothwell, J.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxf.) 2013, bat070.
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571.
- Kumar, S.; Pandey, A.K. Chemistry, and biological activities of flavonoids: An overview. Sci. World J. 2013.
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003.
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561.
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379.
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Romani, A.; Vignolini, P.; Galardi, C.; Mulinacci, N.; Benedettelli, S.; Heimler, D. Germplasm characterisation of Zolfino Landraces (Phaseolus vulgaris L.) by flavonoid content. J. Agric. Food Chem. 2004, 52, 3838–3842.
- Price, K.R.; Colquhoun, I.J.; Barnes, K.A.; Rhodes, M.J.C. Composition, and content of flavonol glycosides in green beans and their fate during processing. J. Agric. Food Chem. 1998, 46, 4898–4903.
- Ewald, C.; Fjelkner-Modig, S.; Johansson, K.; Sjöholm, I.; Ákesson, B. Effect of processing on major flavonoids in processed onoins, green beans, and peas. Food Chem. 1999, 64, 231–235.
- Andlauer, W.; Stumpf, C.; Hubert, M.; Rings, A.; Furst, P. Influence of cooking process on phenolic marker compounds of vegetables. Int. J. Vitam. Nutr. Res. 2003, 73, 152–159.
- Franke, A.A.; Custer, L.J.; Arakaki, C.; Murphy, S.P. Vitamin C and flavonoid levels of fruits and vegetables consumed in Hawaii. J. Food Comp. Anal. 2004, 17, 1–35.
- Hempel, J.; Böhm, H. Quality and quantity of prevailing flavonoid glycosides of yellow and green French beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 1996, 44, 2114–2116.
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavonones in fruits, vegetables, and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110.
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851.
- Krause, M.; Galensa, R. Determination of naringenin and naringenin-chalcone in tomato skins by reversedphase HPLC after solid phase extraction. Z. Lebensm. Unters. Forsch. 1992, 194, 29.
- D’Archivio, M.; Filesi, C.; Benedetto, R.D.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2000, 43, 348.
- Kaur, H.; Kaur, G. A critical appraisal of solubility enhancement techniques of polyphenols. J. Pharm. 2014.
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246.
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075.
- Chang, Q.; Wong, Y.-S. Identification of flavonoids in Hakmeitau beans (Vigna sinensis) by high performance liquid chromatography-electron-spray mass spectrometry (LCESI/MS). J. Agric. Food Chem. 2004, 52, 6694–6699.
- He, J.; Giusti, M.M. Anthocyanins: Natural colourants with health-promoting properties. Ann. Rev. Food Sci. Technol. 2010, 1, 163.
- Tuli, R.T.; Rahman, M.M.; Abdullah, A.T.; Akhtauzzaman, M.; Islam, S.N. Phytochemicals—Tannins in some Leafy Vegetables of Bangladesh. J. Nutr. 2016, 3, 150. Available online: https://www.opensciencepublications.com/fulltextarticles/IJN-2395-2326-3-150.html (accessed on 14 November 2020).
- Fernández-Mar, M.I.; Mateos, R.; Garcıa-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797.
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell Longev. 2015, 837042.
- Chung, M.I.; Teng, C.M.; Cheng, K.L.; Ko, F.N.; Lin, C.N. An antiplatelet principle of Veratrum formosanum. Planta Med. 1992, 58, 274.
- Cichewicz, R.H.; Kouzi, S.A. Biotransformation of resveratrol to piceid by Bacillus Cereus. J. Nat. Prod. 1998, 61, 1313–1314.
- Helsper, J.P.F.G.; Kolodziej, H.; Hoogendijk, J.M.; Vannorel, A. Characterisation and trypsin inhibitor activity of proanthocyanidins from vicia-faba. Phytochemistry 1993, 34, 1255.
- Panche, N.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Rossi, M.; Bosetti, C.; Negri, E.; Lagiou, P.; Vecchia, C. Flavonoids, proanthocyanidins, and cancer risk: A network of case-control studies from Italy. Nutr. Cancer 2010, 62, 871–877.
- Wedick, N.; Pan, A.; Cassidy, A.; Rimm, E.; Sampson, L.; Rosner, B.; Willet, W.; Hu, F.; Sun, Q.; Dam, R. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933.
- Bousova, I.; Skalova, L. Inhibition and induction of glutathione S-transferases by flavonoids: Possible pharmacological and toxicological consequences. Drug Metab. Rev. 2012, 44, 267.
- Wuttke, W.; Jarry, H.; Seidlova-Wuttke, D. Isoflavones—Safe food additives or dangerous drugs? Ageing Res. Rev. 2007, 6, 150–188.
- Williamson, G.; Holst, B. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Brit. J. Nutr. 2008, 99, S55–S58.
- Andres, S.; Abraham, K.; Appel, K.; Lampen, A. Risks and benefits of dietary isoflavones for cancer. Crit. Rev. Toxicol. 2011, 41, 463.
- Del Rio, D.; Bresciani, L. Phenolic compounds as functional ingredients and nutraceuticals: The case of Juice PLUS+. FASEB J. 2018, 31, 646.
- Kaurinovic, B.; Vastag, D. Flavonoids and phenolic acids as potential natural antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/antioxidants/flavonoids-and-phenolic-acids-as-potential-natural-antioxidants (accessed on 17 November 2020).
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nut. Cancer 2009, 62, 1.
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240.
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93.
- Salem, M.B.; Affes, H.; Athmouni, K.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Chemicals compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid Based Complement. Altern. Med. 2017.
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518.
- Dzotam, J.K.; Simo, I.K.; Bitchagno, G.; Celik, I.; Sandjo, L.P.; Tane, P.; Kuete, V. In vitro antibacterial and antibiotic modifying activity of crude extract, fractions and 30, 40, 7-trihydroxyflavone from Myristica fragrans Houtt against MDR Gram-negative enteric bacteria. BMC Complement Altern. Med. 2018, 18.
- Pilar de Torre, M.; Vizmanos, J.L.; Cavero, R.Y.; Calvo, M.I. Improvement of antioxidant activity of oregano (Origanum vulgare L.) with an oral pharmaceutical form. Biomed. Pharm. 2020, 129.
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules 2018, 23, 1139.
- Liu, D.-K.; Xu, C.-C.; Zhang, L.; Ma, H.; Chen, X.-J.; Sui, Y.-C.; Zhang, H.-Z. Evaluation of bioactive components and antioxidant capacity of four celery (Apium graveolens L.) leaves and petioles. Int. J. Food Prop. 2020, 23, 1097.
- Petropoulos, S.; Fernandes, A.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C.F.R. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7.
- Phan, A.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical characteristics and antimicrobial activity of Australian grown garlic (Allium sativum L.) cultivars. Foods 2019, 8, 358.
- Deng, G.-F.; Lin, X.; Xu, X.-R.; Gao, L.-L.; Xie, J.-F.; Li, H.-B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260.
- Radovanović, B.; Mladenović, J.; Radovanović, A.; Pavlović, R.; Nikolić, V. Phenolic composition, antioxidant, antimicrobial and cytotoxic activities of Allium porrum L. (Serbia) extracts. J. Food Nutr. Res. 2015, 3, 564.
- Zeb, A. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). SpringerPlus 2015, 4.
- Jarial, R.; Thakur, S.; Sakinah, M.; Zularisam, A.W.; Sharad, A.; Kanwar, S.S.; Singh, L. Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium Nidus. J. King Saud Univ. -Sci. 2018, 30, 185.
- Ozkur, M.K.; Bozkurt, M.S.; Balabanli, B.; Aricioglu, A.; Ilter, N.; Gurer, M.A.; Inaloz, H.S. The effect of EGb 761 on lipid peroxide levels and superoxide dismutase activity in sunburn. Photodermatol. Photoimmunol. Photomed. 2002, 18, 117.
- Meng, X.H.; Liu, C.; Fan, R.; Zhu, L.F.; Yang, S.X.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Antioxidative flavan-3-ol dimers from the leaves of Camellia fangchengensis. J. Agric. Food Chem. 2018, 6, 247.
- Rupasinghe, V.H.P.; Boehm, M.M.A.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A.R. Anti-inflammatory activity of haskap cultivars is polyphenols-dependent. Biomolecules 2015, 5, 1079.
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic composition and bioactivity of Lavandula pedunculata (Mill.) Cav. samples from different geographical origin. Molecules 2018, 23, 1037.
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.; Sayyad, A.; Stosic-Grujicic, N.; Stojanovic, S.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120.
- Jaouadi, R.; Silva, A.; Boussaid, M.; Yahia, I.; Cardoso, S.M.; Zaouali, Y. Differentiation of phenolic composition among tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae) populations: Correlation to bioactive activities. Antioxidants 2019, 8, 515.
- Scalbert, A.; Williamson, G. Dietary intake, and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085.
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325.
- Bian, Y.; Wei, J.; Zhao, C.; Li, G. Natural polyphenols targeting senescence: A novel prevention and therapy strategy for cancer. Int. J. Mol. Sci. 2020, 21, 684.
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884.
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones Francisco, J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422.
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370.
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295.
- Carmody, R.N.; Turnbaugh, P.J. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J. Clin. Investig. 2014, 124, 4173.
- Rastmanesh, R. High polyphenol, low probiotic diet for weight loss because of intestinal microbiota interaction. Chem Biol Interact. 2011, 189, 1.
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol Hepatol. 2019, 16, 605–616.
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernández, E.N.; Putz, P.; Antoine, J.M. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur. J. Nut. 2018, 57, 25–49.
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216.
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169.
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412.
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1.
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, 21.
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224.
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243.
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999.
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374.
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71.
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177.
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Recent Advances in Plant Phenolics. Molecules 2017, 22, 1249.
- Liu, L.; Shen, B.J.; Xie, D.H.; Cai, B.C.; Qin, K.M.; Cai, H. Optimisation of ultra-sound-assisted extraction of phenolic compounds from Cimicifugae rhizoma with response surface methodology. Pharm. Mag. 2015, 11, 682–689.
- Fang, X.; Wang, J.; Hao, J. Simultaneous extraction, identification, and quantification of phenolic compounds in Eclipta prostrata using microwave-assisted extraction combined with HPLC-DAD-ESI-MS/MS. Food Chem. 2015, 188, 527–536.
- Lee, H.S.; Lee, H.J.; Yu, H.J.; Ju, D.W.; Kim, Y.; Kim, C.T.; Suh, H.J. A comparison between high hydrostatic pressure extraction and heat extraction of ginsenosides from ginseng (Panax ginseng CA Meyer). J. Sci. Food Agric. 2011, 91, 1466.
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. J. Food Sci. 2020, 2020.
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Piovesana, S.; Ventura, S. Chromatographic methods coupled to mass spectrometry detection for the determination of phenolic acids in plants and fruits. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 353–370.
- Da Silva, L.A.; Pezzini, B.R.; Soares, L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L. (Lamiaceae) leaves. Pharm. Mag. 2015, 11, 96.
