Inositols, especially myo-inositol and inositol hexakisphosphate, also known as phytic acid or IP6, with their biological activities received much attention for their role in multiple health beneficial effects. Although their roles in cancer treatment and prevention have been extensively reported, interestingly, they may also have distinctive properties in energy metabolism and metabolic disorders.
- Inositol lipids
- inositols
- inositol phosphates
1. Introduction
Inositol lipids and their derivatives, inositols and inositol phosphates (IPs), are well-known to be important to biology and signaling of eukaryotic cells [1][1]. Myo-inositol (myoIns) and inositol hexakisphosphate (IP6 or InsP6 or phytic acid) are common in biology; these naturally occurring carbohydrates are widely distributed among plants and mammalian cells, with multiple roles [2,3][2][3]. The broad spectrum of their actions has been shown to be related to the energy homeostasis, anti-oxidant and anti-inflammatory activities, and their role as neurotransmitters [4][4]. However, myoIns is only one of several possible structural isomers of inositol (1, 2, 3, 4, 5, 6-cyclohexanehexol) [2,5][2][5].
2. Structure
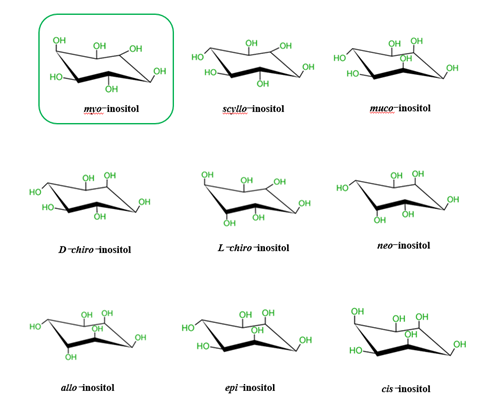
It is known that there are nine possible stereoisomers of inositol (a cyclohexanehexol structure) including cis-, epi-, allo-, myo-, muco-, neo-, (+)-chiro, (−)-chiro-, and scyllo-inositols [7–9][6][7][8]. These are formed through epimerization of its 6 hydroxyl group, and five of them—myo-, scyllo-, muco-, neo- and d-chiro-inositol—occur naturally, while the other four possible isomers (l-chiro-, allo-, epi-, and cis-inositol) are derived from myoIns [7–9][6][7][8]. Here, we illustrate all nine isomers of inositol in Figure 1.
Figure 1. Structures of the 9 stereoisomers of inositol, which exist under 9 stereoisomeric forms through epimerization of its hydroxyl groups. Myo-Inositol (framed) is the most common isomer in plants and animal cells.
References
- Irvine, R.F. A short history of inositol lipids. J. Lipid Res. 2016, 57, 1987–1994.
- Shamsuddin, A.K.; Bose, S. IP6 (Inositol Hexaphosphate) as a Signaling Molecule. Curr. Cancer Rev. 2012, 7, 289–304.
- Biswas, S.; Maity, I.B.; Chakrabarti, S.; Biswas, B.B. Purification and characterization of myo-Inositol hexaphosphate-adenosine diphosphate phosphotransferase from Phaseolus aureus. Arch. Biochem. Biophys. 1978, 185, 557–566.
- Vucenik, I. Anticancer Properties of Inositol Hexaphosphate and Inositol: An Overview. J. Nutr. Sci. Vitam. (Tokyo) 2019, 65, S18–S22.
- Morton, R.K.; Raison, J.K. A complete intracellular unit for incorporation of amino-acid into storage protein utilizing adenosine triphosphate generated from phytate. Nature 1963, 200, 429–433.
- Thomas, M.P.; Mills, S.J.; Potter, B.V. The “Other” Inositols and Their Phosphates: Synthesis, Biology, and Medicine (with Recent Advances in myo-Inositol Chemistry). Angew. Chem. Int. Ed. Engl. 2016, 55, 1614–1650.
- Al-Suod, H.; Ligor, M.; Rațiu, I.-A.; Rafińska, K.; Górecki, R.; Buszewski, B. A window on cyclitols: Characterization and analytics of inositols. Phytochem. Lett. 2017, 20, 507–519.
- Tanaka, K.; Natsume, A.; Ishikawa, S.; Takenaka, S.; Yoshida, K.I. A new-generation of Bacillus subtilis cell factory for further elevated scyllo-inositol production. Microb. Cell Fact 2017, 16, 1–8.