Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Huirong Zhang.
Kaposi’s sarcoma-associated herpesvirus (KSHV) and the Epstein–Barr virus (EBV) are double-stranded DNA oncogenic gammaherpesviruses. These two viruses are associated with multiple human malignancies, including both B and T cell lymphomas, as well as epithelial- and endothelial-derived cancers. KSHV and EBV establish a life-long latent infection in the human host with intermittent periods of lytic replication. Infection with these viruses induce the expression of both viral and host RNA transcripts and activates several RNA sensors including RIG-I-like receptors (RLRs).
- KSHV
- EBV
- antiviral response
1. Introduction
KSHV (also known as human herpesvirus 8, HHV8) and EBV (also known as human herpesvirus 4, HHV4) are members of the gammaherpesvirus family and these viruses are associated with multiple human malignancies. KSHV is the etiological agent of Kaposi’s sarcoma (KS), in addition to two B-cell-derived malignancies: primary effusion lymphoma (PEL) and multicentric Castleman’s disease (MCD) [1,2][1][2]. More recently, KSHV has also been implicated as a causal agent for osteosarcoma [3]. EBV is linked with B cell lymphomas and epithelial cell carcinomas. They include, but are not limited to, Burkitt’s lymphoma (BL), Hodgkin’s lymphoma (HL), non-Hodgkin lymphoma (NHL), nasopharyngeal carcinoma (NPC) and gastric carcinoma (GC). In addition to its association with human tumors, EBV is also linked to several autoimmune diseases such as systemic lupus erythematosus (SLE) and multiple sclerosis (MS) [4,5,6,7,8][4][5][6][7][8].
EBV and KSHV are oncogenic double-stranded DNA (dsDNA) viruses with both viruses exhibiting two distinct phases of their life cycles: latency and lytic replication. During latency, the KSHV genome is replicated as a circular episome by the cellular DNA polymerase and only expresses a limited set of latency-associated proteins and pre-microRNAs [9]. Similarly, EBV also maintains its genome as a latent episome in the nucleus of the host cell and expresses a small group of viral proteins and a number of viral noncoding RNAs including EBV-encoded RNAs (EBERs) and BamHI-A rightward transcripts (BARTs). Furthermore, EBV establishes distinct types of latency programs (III-II-I-0) in different cell types based on specific latent gene expression patterns [8]. Under certain conditions, both viruses are able to reactivate and enter the lytic cycle. During lytic reactivation, all viral genes are transcribed, viral DNA is amplified, progeny virions are produced, and this eventually leads to the death of reactivating cells [10,11][10][11]. As there are more viral proteins and noncoding RNAs induced during lytic replication, the host’s immune responses tend to be more pronounced during lytic infection compared to latency.
The innate immune response triggered by nucleic acid recognition plays an important role during viral infection. This process is initiated by nucleic acid sensors that recognize foreign DNA and RNA, such as viral genomes. Upon sensing the viral nucleic acids, the DNA/RNA sensors and their signaling cascades are activated to produce type I interferons (IFNs) and proinflammatory cytokines, which establish an antiviral state and inhibit viral infection. Several host DNA and RNA sensors have been reported to limit KSHV or EBV infection, including endosomal Toll-like receptors (TLRs), cytosolic DNA and dsRNA sensors cyclic GMP–AMP synthase (cGAS) and retinoic acid-inducible gene I protein (RIG-I)-like receptors (RLRs) [12,13][12][13]. RLRs encompass two major dsRNA sensors of the innate immune system, RIG-I and melanoma differentiation-associated gene 5 (MDA5). Upon binding to their dsRNA ligand, RIG-I/MDA5 are activated and interact with their adaptor protein mitochondrial antiviral signaling (MAVS) to induce the RLR signaling pathway and subsequent type I IFN production [14,15][14][15]. Although both KSHV and EBV are DNA viruses, infection with these viruses has been reported to induce RLR signaling pathways because their infection leads to the production of virus- and host-derived RNAs with double-stranded structures, such as miRNAs, circular RNAs, and long noncoding RNAs. In addition to RLRs, KSHV and EBV infection can also be regulated by other RNA binding proteins involved in innate immunity, such as protein kinase R (PKR) and TLRs 3, 7 and 8 [12].
2. RIG-I-Like Receptors
2.1. Gammaherpesviruses Activate the RLR Signaling Pathway
RLRs are RNA sensors localized in the cytosol which include RIG-I, MDA5 and the laboratory of genetics and physiology (LGP2). All three RLRs share similar RNA binding domains, including the conserved DExD/H helicase domain and C-terminal domain (CTD) [16]. The N-termini of RIG-I and MDA5 have two additional tandemly linked caspase activation and recruitment domains (CARDs) that mediate the activation of adaptor protein MAVS [14,17,18][14][17][18]. Upon activation, MAVS recruits and activates downstream proteins, TNF receptor-associated factors (TRAFs), IκB kinase (IKK) and TANK-binding kinase 1 (TBK1). These subsequently activate transcription factors, nuclear factor-κB (NF-κB), interferon regulatory factor 3 (IRF3) and IRF7, which induce the expression of Type I IFN genes and proinflammatory cytokine genes (Figure 1) [14]. As LGP2 lacks the CARD domain, it does not directly activate MAVS and is considered a regulator of RIG-I and MDA5 [19]. Although RIG-I and MDA5 induce the same downstream signaling pathways upon RNA binding, they have distinct preferences for the RNA duplex structure they bind to. RIG-I recognizes dsRNA with a triphosphate group at its 5′ end (5′ppp) and RIG-I can also bind to long noncoding RNAs without these ends [20[20][21],21], while MDA5 senses long RNA duplexes (>4 kb) independent of 5′ppp [22].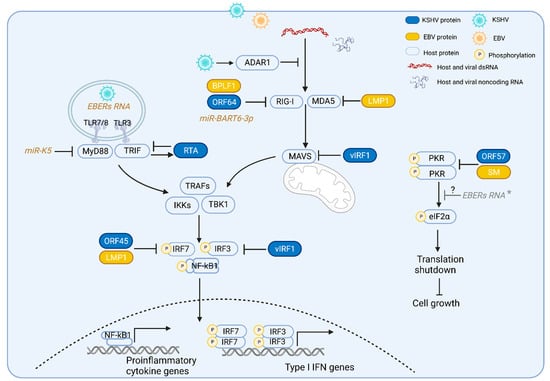
Figure 1. Gammaherpesviruses activate and evade the RNA-induced innate immune pathways. TLRs 3, 7, and 8 detect RNA in the endosome and RLRs (RIG-I and MDA5) detect dsRNA in the cytoplasm. During KSHV and EBV infection, TLRs 3, 7, and 8, and RLRs are activated by host or viral RNAs; activated TLR3 and TLR7/8 recruit and induce their downstream adaptor proteins TRIF and MyD88, respectively. Activated RLR induces the adaptor protein MAVS. These adaptor proteins subsequently recruit and induce common downstream proteins TNF receptor-associated factors (TRAFs) and TANK-binding kinase 1 (TBK1), leading to the phosphorylation and activation of the transcription factors interferon-regulatory factor 3 (IRF3), IRF7 and NF-κB that results in the production of type I interferons and other proinflammatory cytokines. Activation of TLR3 signaling induces KSHV RTA expression, which in turn promotes TRIF degradation. KSHV miRNA K5 blocks MyD88. KSHV ORF64, EBV BPLF1, and EBV miBART-3p inhibit RIG-I activation. EBV LMP1 reduces both RIG-I and MDA5 expression. KSHV vIRF1 inhibits the activation of RLRs’ adaptor protein, MAVS, and TLR3-mediated activation of IRF3. Upon binding to dsRNA, PKR undergoes autophosphorylation and becomes an activated kinase to phosphorylate a key translation initiation factor (eIF2α), inducing the shutdown of global protein synthesis and inhibiting cell growth. KSHV ORF57, EBV SM or EBV noncoding RNA EBERs interact with PKR and inhibit PKR activation. * EBER: the role of EBERs in PKR phosphorylation is unclear due to conflicting reports.
2.2. Gammaherpesvirues Evade the RLR Signaling Pathway by Utilizing Both Viral and Host Proteins
KSHV and EBV deploy multiple viral proteins to disrupt RLR activation during de novo infection and lytic reactivation in order to efficiently evade the antiviral response and establish their life cycle. The protein homologs BPLF1 and ORF64 are viral deubiquitinating enzymes (DUBs) of EBV and KSHV, respectively, that target the RLR sensor RIG-I [34,35,36][34][35][36]. RIG-I is subject to K63-polyubiquitination by ubiquitin ligases, including tripartite motif protein 25 (TRIM25), Riplet, Mex-RNA binding family member C (MEX3C), TRIM4 and TRIM21 [37,38][37][38]. This K63-polyubiquitination of the RIG-I CARD domain is essential for activating adaptor protein MAVS and recruiting downstream signaling molecules [37,39][37][39]. Both ORF64 and BPLF1 have been shown to decrease RIG-I ubiquitination, leading to reduced RIG-I activation and suppression of downstream innate immune responses [40,41][40][41]. EBV BPLF1 promotes the dimerization and autoubiquitination of TRIM25, which leads to impaired RIG-I ubiquitination [40]. KSHV also utilizes the viral interferon regulatory factor 1 (vIRF1) to target MAVS and block RLR signaling. vIRF1 is recruited to the mitochondria and inhibits MAVS aggregation during virus replication that in turn negatively regulates the MAVS-mediated antiviral responses and promotes KSHV replication [42]. Additionally EBV-encoded latent membrane protein 1 (LMP1) degrades RIG-I and MDA5 by recruiting E3 ubiquitin ligases to induce the proteasomal degradation of RIG-I and MDA5 [43]. Furthermore, EBV-encoded microRNA miBART6-3p targets the 3′ untranslated region (UTR) of the RIG-I mRNA, resulting in the decreased expression of RIG-I-induced interferon and interferon-stimulated genes (ISGs) [44]. In addition, since Pol-III-transcribed EBERs are able to activate the RIG-I sensing pathway as described above, the EBV lytic protein replication and transcription activator (Rta) interacts with Pol III to suppress the expression of EBERs and other immunogenic small RNAs [45]. Thus, gammaherpesviruses not only directly inhibit the activation of proteins involved in the RLR signaling pathway but also decrease the availability of RLR ligands induced during KSHV and EBV reactivation and replication. In addition to utilizing virus-derived proteins and noncoding RNAs, EBV and KSHV also hijack host proteins to evade the RLR signaling pathway. ADARs are RNA-editing enzymes that bind to dsRNA and convert adenosine to inosine in dsRNA. There are three members of the human ADAR family, designated ADAR1 (ADAR), ADAR2 (ADARB1), and ADAR3 (ADARB2), where ADAR1 is responsible for the majority of the A-to-I editing activity in mammalian cells [46]. Widespread A-to-I editing of both the host and viral transcripts has been observed in KSHV-infected cells, and the A-to-I editomes are further expanded during KSHV lytic reactivation [47]. The A-to-I editing of the induced dsRNAs by KSHV infection prevents them from being recognized and detected by RLRs within the cell. Thus, in the absence of ADAR1, these KSHV-induced dsRNAs lacking A-to-I editing are exposed to and recognized by MDA5/RIG-I to stimulate the RLR antiviral signaling pathway, leading to the increased induction of IFNs, and resulting in the inhibition of KSHV lytic replication [48]. A-to-I editing also affects latent EBV viral infection. EBV pri-miR-BART6, targeted by the Dicer enzyme of the mammalian RNA-induced silencing complex (mRISC), modulates the EBV latency state through the control of viral gene expression. A-to-I editing of pri-mi-BART6 suppresses its targeting by Dicer which leads to viral lifecycle transitions to either type III latency or lytic reactivation [49]. In addition to pri-mi-BART6, A-to-I editing has also been found in EBV pri-miR-BART3, pri-miR-BART8 and pri-miR-BART11, as well as the KSHV K12/Kaposin transcript [50,51][50][51]. Furthermore, EBV A-to-I hyperedited OriP transcripts can bind to ADAR1 and promote EBV viral lytic replication [52]. Hence, KSHV and EBV not only utilize their own proteins or RNAs, but also usurp cellular proteins to escape the innate immune response.References
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi sarcoma. Nat. Rev. Dis. Prim. 2019, 5, 9.
- Carbone, A.; Borok, M.; Damania, B.; Gloghini, A.; Polizzotto, M.N.; Jayanthan, R.K.; Fajgenbaum, D.C.; Bower, M. Castleman disease. Nat. Rev. Dis. Prim. 2021, 7, 84.
- Chen, Q.; Chen, J.; Li, Y.; Liu, D.; Zeng, Y.; Tian, Z.; Yunus, A.; Yang, Y.; Lu, J.; Song, X.; et al. Kaposi’s sarcoma herpesvirus is associated with osteosarcoma in Xinjiang populations. Proc. Natl. Acad. Sci. USA 2021, 118, e2016653118.
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191.
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869.
- Soulier, J.; Grollet, L.; Oksenhendler, E.; Cacoub, P.; Cazals-Hatem, D.; Babinet, P.; d’Agay, M.F.; Clauvel, J.P.; Raphael, M.; Degos, L.; et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995, 86, 1276–1280.
- Gewurz, B.; Longnecker, R.; Cohen, J. Epstein-barr virus. In Fields Virology, 7th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2021; pp. 324–389.
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr virus: Biology and clinical disease. Cell 2022, 185, 3652–3670.
- Dittmer, D.P.; Damania, B. Kaposi sarcoma-associated herpesvirus: Immunobiology, oncogenesis, and therapy. J. Clin. Investig. 2016, 126, 3165–3175.
- Broussard, G.; Damania, B. Regulation of KSHV latency and lytic reactivation. Viruses 2020, 12, 1034.
- Sandhu, P.K.; Damania, B. The regulation of KSHV lytic reactivation by viral and cellular factors. Curr. Opin. Virol. 2022, 52, 39–47.
- Lange, P.T.; White, M.C.; Damania, B. Activation and Evasion of Innate Immunity by Gammaherpesviruses. J. Mol. Biol. 2022, 434.
- Broussard, G.; Damania, B. KSHV: Immune Modulation and Immunotherapy. Front. Immunol. 2020, 10, 3084.
- Wu, J.; Chen, Z.J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014, 32, 461–488.
- Hur, S. Double-Stranded RNA Sensors and Modulators in Innate Immunity. Annu. Rev. Immunol. 2019, 37, 349–375.
- Chen, Y.G.; Hur, S. Cellular origins of dsRNA, their recognition and consequences. Nat. Rev. Mol. Cell Biol. 2022, 23, 286–301.
- Jiang, X.; Kinch, L.N.; Brautigam, C.A.; Chen, X.; Du, F.; Grishin, N.V.; Chen, Z.J. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity 2012, 36, 959–973.
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I-and Mda5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988.
- Yoneyama, M.; Kikuchi, M.; Matsumoto, K.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Foy, E.; Loo, Y.M.; Gale, M., Jr.; Akira, S.; et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005, 175, 2851–2858.
- Jiang, M.; Zhang, S.; Yang, Z.; Lin, H.; Zhu, J.; Liu, L.; Wang, W.; Liu, S.; Liu, W.; Ma, Y.; et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell 2018, 173, 906–919.e913.
- Lin, H.; Jiang, M.; Liu, L.; Yang, Z.; Ma, Z.; Liu, S.; Ma, Y.; Zhang, L.; Cao, X. The long noncoding RNA Lnczc3h7a promotes a TRIM25-mediated RIG-I antiviral innate immune response. Nat. Immunol. 2019, 20, 812–823.
- Kato, H.; Takeuchi, O.; Mikamo-Satoh, E.; Hirai, R.; Kawai, T.; Matsushita, K.; Hiiragi, A.; Dermody, T.S.; Fujita, T.; Akira, S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008, 205, 1601–1610.
- West, J.A.; Wicks, M.; Gregory, S.M.; Chugh, P.; Jacobs, S.R.; Zhang, Z.; Host, K.M.; Dittmer, D.P.; Damania, B. An important role for mitochondrial antiviral signaling protein in the Kaposi’s sarcoma-associated herpesvirus life cycle. J. Virol. 2014, 88, 5778–5787.
- Zhang, Y.; Dittmer, D.P.; Mieczkowski, P.A.; Host, K.M.; Fusco, W.G.; Duncan, J.A.; Damania, B. RIG-I Detects Kaposi’s Sarcoma-Associated Herpesvirus Transcripts in a RNA Polymerase III-Independent Manner. mBio 2018, 9, e00823-18.
- Zhao, Y.; Ye, X.; Dunker, W.; Song, Y.; Karijolich, J. RIG-I like receptor sensing of host RNAs facilitates the cell-intrinsic immune response to KSHV infection. Nat. Commun. 2018, 9, 4841.
- Burke, J.M.; Kincaid, R.P.; Nottingham, R.M.; Lambowitz, A.M.; Sullivan, C.S. DUSP11 activity on triphosphorylated transcripts promotes Argonaute association with noncanonical viral microRNAs and regulates steady-state levels of cellular noncoding RNAs. Genes Dev. 2016, 30, 2076–2092.
- Burke, J.M.; Sullivan, C.S. DUSP11—An RNA phosphatase that regulates host and viral non-coding RNAs in mammalian cells. RNA Biol. 2017, 14, 1457–1465.
- Glickman, J.N.; Howe, J.G.; Steitz, J.A. Structural analyses of EBER1 and EBER2 ribonucleoprotein particles present in Epstein-Barr virus-infected cells. J. Virol. 1988, 62, 902–911.
- Rosa, M.D.; Gottlieb, E.; Lerner, M.R.; Steitz, J.A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol. Cell Biol. 1981, 1, 785–796.
- Schwemmle, M.; Clemens, M.J.; Hilse, K.; Pfeifer, K.; Tröster, H.; Müller, W.; Bachmann, M. Localization of Epstein-Barr virus-encoded RNAs EBER-1 and EBER-2 in interphase and mitotic Burkitt lymphoma cells. Proc. Natl. Acad. Sci. USA 1992, 89, 10292–10296.
- Ablasser, A.; Bauernfeind, F.; Hartmann, G.; Latz, E.; Fitzgerald, K.A.; Hornung, V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 2009, 10, 1065–1072.
- Samanta, M.; Iwakiri, D.; Kanda, T.; Imaizumi, T.; Takada, K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 2006, 25, 4207–4214.
- Samanta, M.; Iwakiri, D.; Takada, K. Epstein-Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene 2008, 27, 4150–4160.
- Gonzalez, C.M.; Wang, L.; Damania, B. Kaposi’s sarcoma-associated herpesvirus encodes a viral deubiquitinase. J. Virol. 2009, 83, 10224–10233.
- Kattenhorn, L.M.; Korbel, G.A.; Kessler, B.M.; Spooner, E.; Ploegh, H.L. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 2005, 19, 547–557.
- van Gent, M.; Braem, S.G.; de Jong, A.; Delagic, N.; Peeters, J.G.; Boer, I.G.; Moynagh, P.N.; Kremmer, E.; Wiertz, E.J.; Ovaa, H.; et al. Epstein-Barr virus large tegument protein BPLF1 contributes to innate immune evasion through interference with toll-like receptor signaling. PLoS Pathog. 2014, 10, e1003960.
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551.
- Watkinson, R.E.; McEwan, W.A.; Tam, J.C.; Vaysburd, M.; James, L.C. TRIM21 promotes cGAS and RIG-I sensing of viral genomes during infection by antibody-opsonized virus. PLoS Pathog. 2015, 11, e1005253.
- Zeng, W.; Sun, L.; Jiang, X.; Chen, X.; Hou, F.; Adhikari, A.; Xu, M.; Chen, Z.J. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 2010, 141, 315–330.
- Gupta, S.; Yla-Anttila, P.; Callegari, S.; Tsai, M.H.; Delecluse, H.J.; Masucci, M.G. Herpesvirus deconjugases inhibit the IFN response by promoting TRIM25 autoubiquitination and functional inactivation of the RIG-I signalosome. PLoS Pathog. 2018, 14, e1006852.
- Inn, K.S.; Lee, S.H.; Rathbun, J.Y.; Wong, L.Y.; Toth, Z.; Machida, K.; Ou, J.H.; Jung, J.U. Inhibition of RIG-I-mediated signaling by Kaposi’s sarcoma-associated herpesvirus-encoded deubiquitinase ORF64. J. Virol. 2011, 85, 10899–10904.
- Hwang, K.Y.; Choi, Y.B. Modulation of Mitochondrial Antiviral Signaling by Human Herpesvirus 8 Interferon Regulatory Factor 1. J. Virol. 2016, 90, 506–520.
- Xu, C.; Sun, L.; Liu, W.; Duan, Z. Latent Membrane Protein 1 of Epstein-Barr Virus Promotes RIG-I Degradation Mediated by Proteasome Pathway. Front. Immunol. 2018, 9, 1446.
- Lu, Y.; Qin, Z.; Wang, J.; Zheng, X.; Lu, J.; Zhang, X.; Wei, L.; Peng, Q.; Zheng, Y.; Ou, C.; et al. Epstein-Barr Virus miR-BART6-3p Inhibits the RIG-I Pathway. J. Innate. Immun. 2017, 9, 574–586.
- Long, X.; Yang, J.; Zhang, X.; Yang, Z.; Li, Y.; Wang, F.; Li, X.; Kuang, E. BRLF1 suppresses RNA Pol III-mediated RIG-I inflammasome activation in the early EBV lytic lifecycle. EMBO Rep. 2021, 22, e50714.
- Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010, 79, 321–349.
- Rajendren, S.; Ye, X.; Dunker, W.; Richardson, A.; Karijolich, J. The cellular and KSHV A-to-I RNA editome in primary effusion lymphoma and its role in the viral lifecycle. Nat. Commun. 2023, 14, 1367.
- Zhang, H.; Ni, G.; Damania, B. ADAR1 Facilitates KSHV Lytic Reactivation by Modulating the RLR-Dependent Signaling Pathway. Cell Rep. 2020, 31, 107564.
- Iizasa, H.; Wulff, B.E.; Alla, N.R.; Maragkakis, M.; Megraw, M.; Hatzigeorgiou, A.; Iwakiri, D.; Takada, K.; Wiedmer, A.; Showe, L.; et al. Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J. Biol. Chem. 2010, 285, 33358–33370.
- Lei, T.; Yuen, K.S.; Tsao, S.W.; Chen, H.; Kok, K.H.; Jin, D.Y. Perturbation of biogenesis and targeting of Epstein-Barr virus-encoded miR-BART3 microRNA by adenosine-to-inosine editing. J. Gen. Virol. 2013, 94, 2739–2744.
- Gandy, S.Z.; Linnstaedt, S.D.; Muralidhar, S.; Cashman, K.A.; Rosenthal, L.J.; Casey, J.L. RNA editing of the human herpesvirus 8 kaposin transcript eliminates its transforming activity and is induced during lytic replication. J. Virol. 2007, 81, 13544–13551.
- Cao, S.; Moss, W.; O’Grady, T.; Concha, M.; Strong, M.J.; Wang, X.; Yu, Y.; Baddoo, M.; Zhang, K.; Fewell, C.; et al. New Noncoding Lytic Transcripts Derived from the Epstein-Barr Virus Latency Origin of Replication, oriP, Are Hyperedited, Bind the Paraspeckle Protein, NONO/p54nrb, and Support Viral Lytic Transcription. J. Virol. 2015, 89, 7120–7132.
More
