Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Hans-Arno Müller.
Microtubule-Associated Serine/Threonine (MAST) kinases represent an evolutionary conserved branch of the AGC protein kinase superfamily in the kinome. Since the discovery of the founding member, MAST2, in 1993, three additional family members have been identified in mammals and found to be broadly expressed across various tissues, including the brain, heart, lung, liver, intestine and kidney.
- protein phosphorylation
- MAST kinase
- cell signaling
1. Introduction
Protein kinases belong to the second-largest protein superfamilies encoded by eukaryotic genomes. The human genome encodes about 518 kinases, roughly half of which are mapped to loci associated with genetically predisposed diseases and over 200 of which are identified as mutated in cancer by the ‘Mutations of Kinases in Cancer’ (MoKCa) database [1,2][1][2]. Protein kinases are key mediators in cellular signaling pathways and many other cellular processes. Their function relies upon the transfer of a phosphoryl group onto proteins to influence the structure, activity, stability, and/or localization of the respective substrates. The catalytic activity of protein kinases resides within a highly conserved kinase domain, which in eukaryotes ranges between 250 and 300 amino acids. The kinase domain catalyzes a phosphorylation reaction by binding a substrate to its active center, which transfers the phosphate in the gamma position of adenosine triphosphate (ATP) to the hydroxyl group of serine/threonine (serine/threonine kinases) or tyrosine (tyrosine kinases) side chains.
Most eukaryotic protein kinases can be grouped into seven superfamilies based on the conservation of functional amino acid motifs and substrate specificities [1,3,4][1][3][4]. The AGC protein kinase superfamily was first defined by Hanks and Hunter in 1995 as proteins containing kinase domains most similar to PKA, PKG and PKC [4,5][4][5]. The superfamily contains more than 10% of over 500 human kinases. A total of 42 of the AGC kinases are modular proteins containing additional protein domains that are involved in regulating the activity and localization of the kinase. The catalytic Ser/Thr kinase domain of AGC kinases is composed of two lobes, which are linked through a hinge region [3,6,7][3][6][7]. All 14 AGC kinases whose structure has been solved adopt this bi-lobal kinase fold, where the beta-strand-based N-lobe and the alpha-helical C-lobe sandwich one molecule of ATP. Interactions between a phosphorylated Ser in the C-terminal tail and positively charged residues in the N-lobe stabilize the active conformation of the kinase domain [7,8][7][8]. The N-lobe and the C-lobe surround the active site cleft, at which substrates interact with the kinase domain [9,10][9][10].
Phospho-regulation through AGC kinases is vital, as exemplified by many disease-associated mutations in genes encoding AGC kinases, pointing to the deregulation of these kinases in various types of cancer and diabetes, as well as other inherited disorders [5]. Members of the AGC kinase family respond to various extracellular signals and are involved in a range of well-characterized signaling pathways in which they phosphorylate distinct protein substrates. Although many AGC kinases have been the focus of molecular cell biology research to reveal their molecular mechanisms in diverse biological processes, some groups within the AGC kinase superfamily, including the microtubule-associated serine/threonine (MAST) kinases, remain poorly understood.
The first member of the MAST kinase family to be identified was MAST2 (originally named MAST205). MAST kinases are modular proteins containing three evolutionary conserved protein domains (Figure 1). The founding member, MAST2, was discovered as a protein associated with the spermatid manchette in mouse testes and has been suggested to play a role in sperm maturation [11,12][11][12]. Although the name suggests that MAST kinases are directly associated with microtubules, a direct interaction of MAST kinases with microtubules has not yet been demonstrated. In the case of the mouse spermatid, the interaction of MAST2 with microtubules depended on microtubule-associated proteins (MAPs) and required the kinase domain of MAST2, as well as a region comprising the PDZ domain [11].
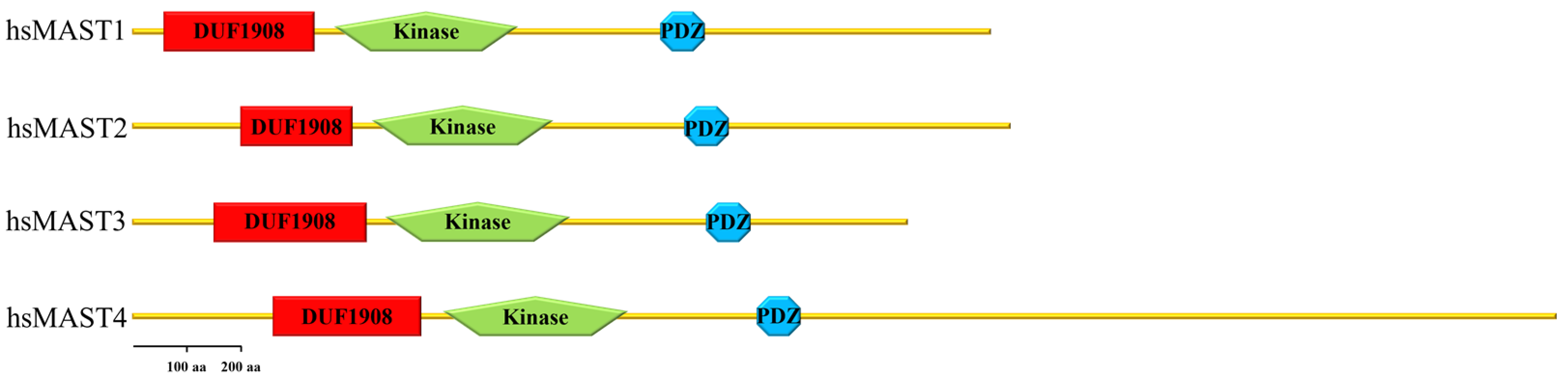
Figure 1. Domain arrangement of human MAST kinases. This cartoon demonstrates the domain composition and arrangement of the four family members of MAST kinases in humans. From N- to C-terminus, the DUF 1908 domain is followed by the serine/threonine kinase domain (drawn without its C-terminal extension) and the PDZ domain in the C-terminal half of the protein.
2. Domain Composition of MAST Kinases
Members of the MAST kinase family are modular proteins characterized by a conserved domain composition and arrangement. The central Ser/Thr kinase domain is flanked towards the C-terminal end by a PDZ domain (post-synaptic density protein, disc large and Zonula occludens-1) and towards the N-terminus by a DUF (domain of unknown function) 1908 domain (Figure 1). The DUF1908 domain is a common feature of all MAST kinases; however, the function of this domain with respect to the activity of MAST kinases remains poorly understood. The DUF1908 domain (PFAM ID PF0826) is about 275 amino acids long, and within the human MAST kinases, this size differs only slightly. The predicted structure of the DUF1908 domain can be divided into an unstructured N-terminal half and a structured C-terminal half containing 8 alpha-helixes (Figure 2). A striking feature of the N-terminal domain of MAST kinases is the fact that their overall sequence is highly enriched serine, tyrosine and threonine residues, which are potential targets for posttranslational modifications including phosphorylation.
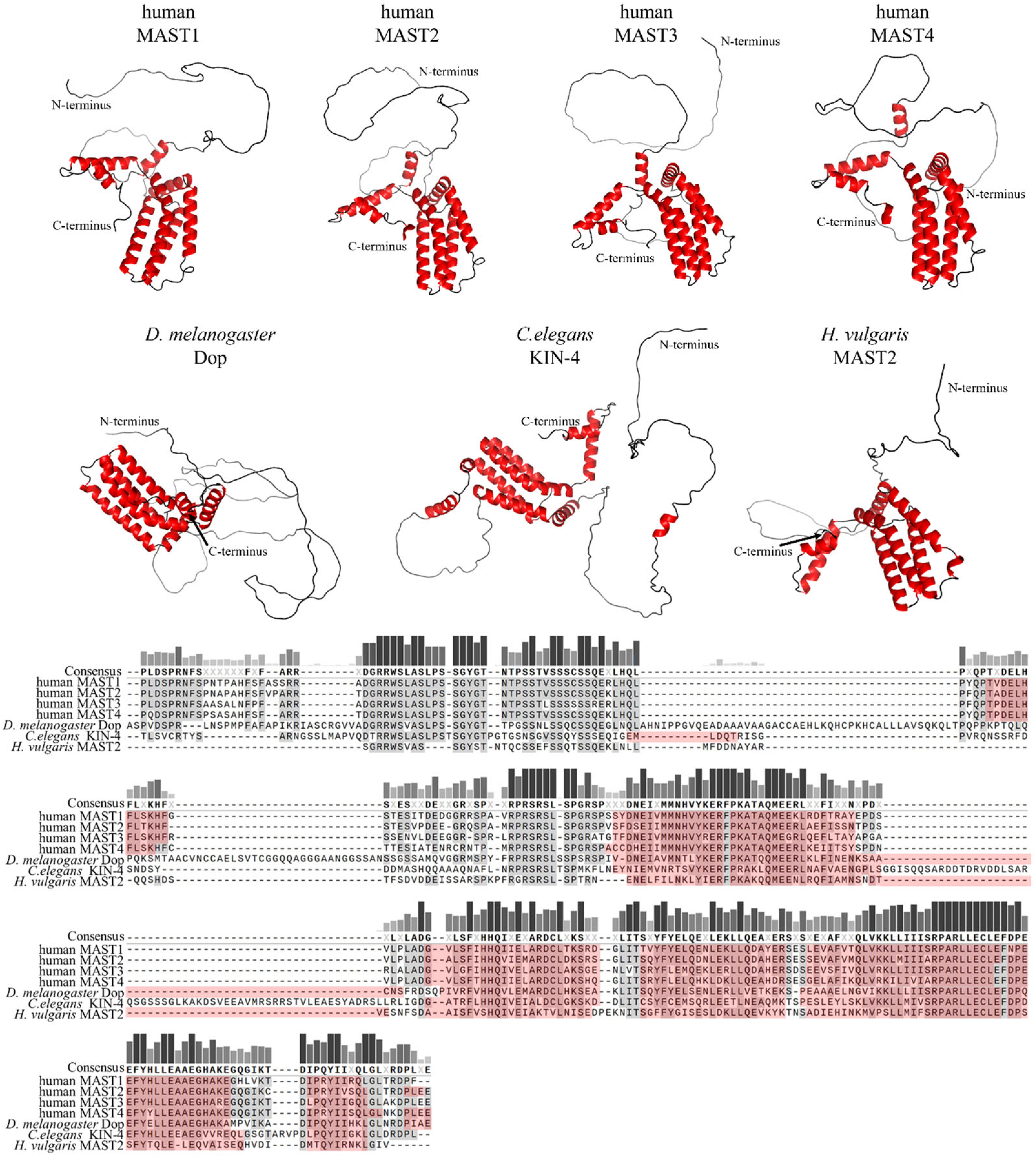

Figure 2. Primary sequence comparison and structural comparison of DUF1908 domains in selected MAST kinases. Top panel: AlphaFold predicted 3D models of DUF1908 of MAST kinases in humans, Drosophila (D.) melanogaster, Caenorhabditis (C.) elegans and Hydra (H.) vulgaris [13,14,15][13][14][15]. Alpha helices are shown in red. The 3D structures were oriented and labeled using the PyMol software (Schrödinger, L.; DeLano, W. PyMOL [Internet]. 2020. Available online: http://www.pymol.org/pymol; accessed on 5 December 2022). Lower panel: Multiple amino acid sequence alignment of DUF1908 of MAST kinases in different species using MAFFT (Snapgen; GSL Biotec, San Diego, CA, USA). The helices are marked in red. Conserved amino acids are highlighted in gray and dark red, respectively. A threshold of 55% was chosen for consensus. The bars above the consensus sequence indicate the degree of conservation; the darker and higher the bar, the higher the conservation. The sequences and domain annotations of the proteins were obtained from InterPro [16].
The PDZ domains, in contrast to DUF1908, are well studied and occur in proteins of a variety of organisms such as bacteria, yeast, plants, and metazoans, including Caenorhabditis elegans, Drosophila melanogaster and Homo sapiens [17]. PDZ domains are often found in multidomain proteins and contain 80–100 amino acids that are arranged in five beta strands and two alpha helices [18,19,20][18][19][20]. Within a given protein, single or several copies of PDZ domains can occur; MAST kinases contain only one PDZ domain in the carboxy-terminal half of the protein (Figure 3). PDZ domains are major hubs for protein-protein interactions, and their binding specificity is often based upon conserved amino acid motifs at the very carboxy-terminus of ligands [19]. PDZ domains can be divided into three classes based on their binding specificities. Class 1 PDZ domains recognize the binding motif X-S/T-X-V/I/L (where X represents any amino acid). MAST2-PDZ is an example of this class, as it recognizes this motif in its ligand PTEN [21]. Class 2 PDZ domains recognize a motif of four amino acids, alternating between a hydrophobic and a variable amino acid, and the third class recognizes the motif X-D/E-X-⌽ [22,23][22][23]. Another mode of binding allows the complexation of PDZ domains with each other, thus causing PDZ-based dimerization, which occurs mainly in proteins that have more than one PDZ domain [24,25][24][25]. Homodimer formation was also observed for proteins with only one PDZ domain; notably, this mode of binding was reported for MAST2-PDZ [21,22][21][22]. This self-association due to PDZ binding suggests an influence of this domain on the regulation of MAST kinases through dimerization.
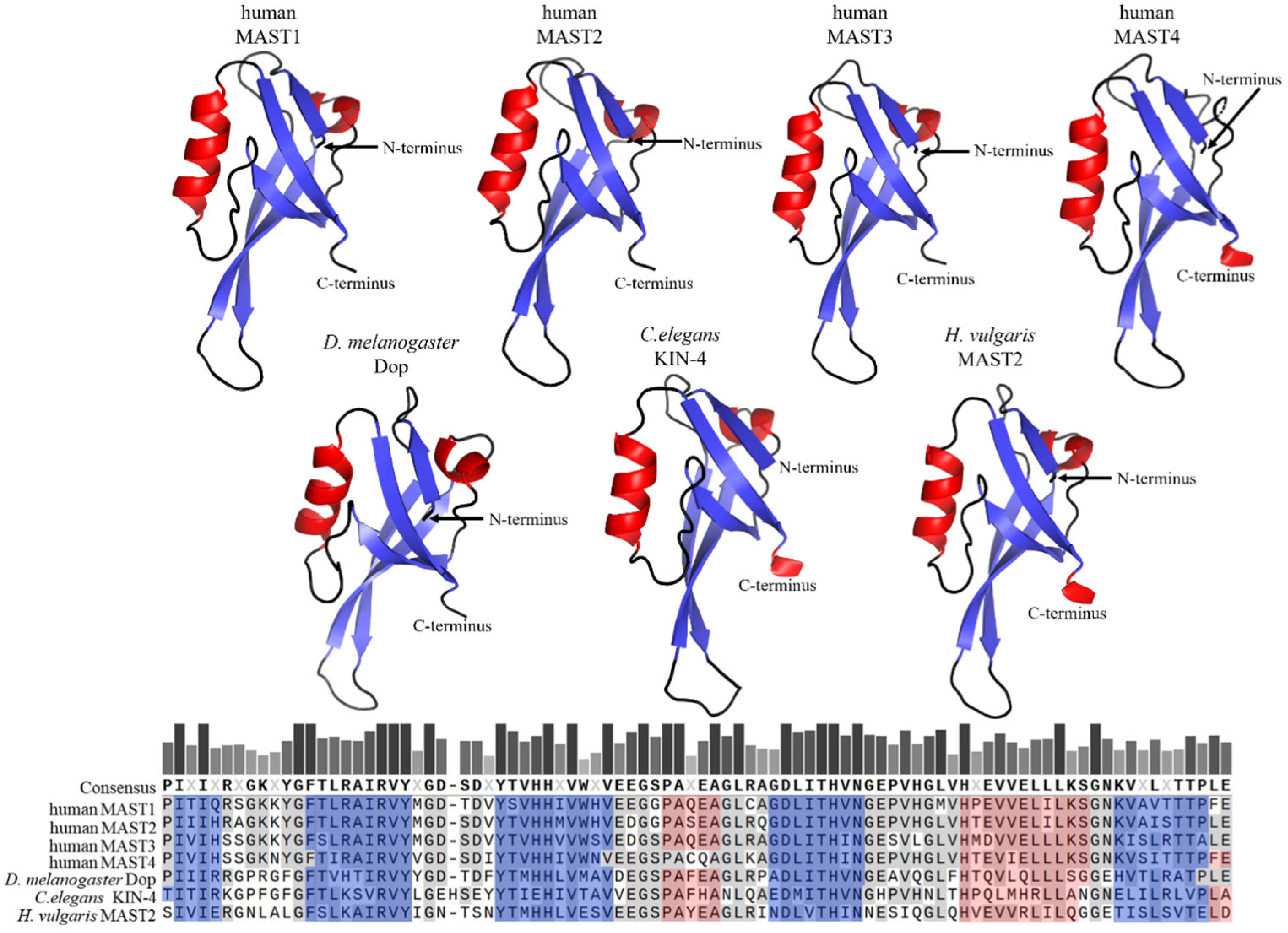

Figure 3. Primary sequence comparison and structural comparison of PDZ domains in selected MAST kinases. AlphaFold-predicted 3D models of PDZ domains of MAST kinases in humans, Drosophila (D.) melanogaster, Caenorhabditis (C.) elegans, and Hydra (H.) vulgaris [13,14,15][13][14][15]. Alpha helices are shown in red, and beta sheets in blue color. The 3D structures were oriented and labeled using the PyMol software (Schrödinger, LLC, New York, NY, USA). The structure of all four human MAST kinases was also solved experimentally (MAST1: https://doi.org/10.2210/pdb3ps4/pdb; MAST2: https://doi.org/10.2210/pdb2kyl/pdb; MAST3: https://doi.org/10.2210/pdb3khf/pdb; MAST4: https://doi.org/10.2210/pdb2w7r/pdb) (accessed on 14 July 2023). The structural models based on AlphaFold and based on experimental data do not exhibit appreciable differences. Lower panel: Multiple protein amino acid sequence alignment of PDZ domains of MAST kinases in different species using MAFFT (Snapgen; GSL Biotec). For further detail see the legend of Figure 2.
The core domain of the MAST kinase family is a Ser/Thr kinase domain divided into two subdomains. Since the MAST kinases belong to the AGC kinase family, the amino acid sequences of the human MAST kinase domains show a high degree of conservation compared to AGC kinases, for example, from 34% to 36% sequence identity and 54% to 59% amino acid sequence similarity when comparing PKA, PKC and PKG (Figure 4). Moreover, the catalytic domain of the MAST kinases shares with the AGC kinases all highly conserved motifs such as DFG, APE and HRD, which are important for ATP binding and magnesium transfer and are therefore essential for kinase activity and activation [3,4,5][3][4][5]. However, there is one exception, which is not found in other AGC kinases: the first glycine within the conserved glycine-rich loop (GXGXXG), is replaced by a serine in MAST kinases [11]. This exchange of glycine for a serine allows speculation about possible phosphorylation at this site, which could influence the regulation of the kinase activity. The structure of the modulatory C-terminal tail region of MAST kinases is also well conserved (Figure 5). The comparison of the sequence of the C-terminal tail of the human MAST kinases with PKA and PKG revealed a 42–57% amino acid similarity and 29–43% identity. In summary, the overall structure of the MAST kinase domains exhibits a high degree of structural and sequence conservation with other members of the AGC kinase family.
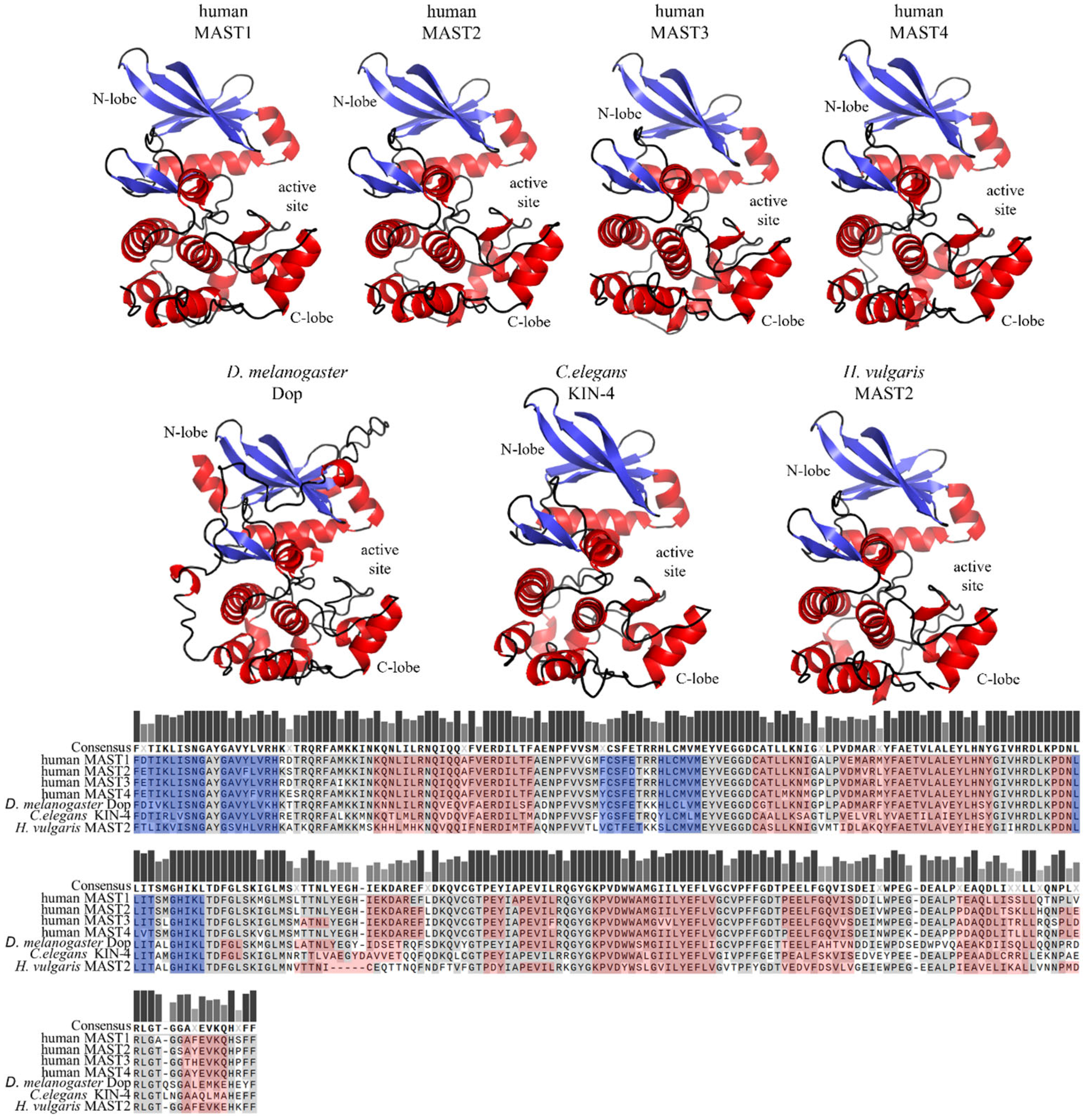

Figure 4. Structure models and sequence comparison of the MAST kinase domains in different species. Top panels: AlphaFold-prediction models of the kinase domain structure in humans, Drosophila (D.) melanogaster, Caenorhabditis (C.) elegans, and Hydra (H.) vulgaris [13,14,15][13][14][15]. The alpha helices are shown in red, and beta sheets in blue. The 3D structures were oriented and labeled using PyMol (Schrödinger, LLC). Lower panel: Multiple protein amino acid sequence alignment of MAST kinase domains of different species. Alignment was performed using MAFFT of the Snapgen software (GSL Biotec). For further detail see the legend of Figure 2.
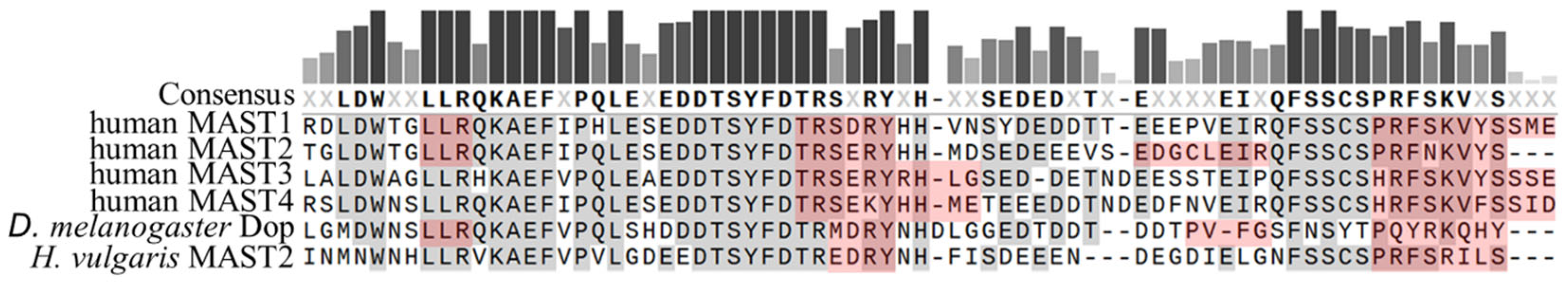

Figure 5. Sequence comparison of the AGC-kinase C-terminal region in MAST kinases of different species. Multiple protein amino acid sequence alignments of AGC-kinase C-terminal regions of MAST kinases in humans, Drosophila (D.) melanogaster and Hydra (H.) vulgaris. Alignment was performed using MAFFT (Snapgene; GSL Biotec). Predicted alpha-helical domains are marked in red and conserved amino acids are marked in grey or dark red.
References
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934.
- Richardson, C.J.; Gao, Q.; Mitsopoulous, C.; Zvelebil, M.; Pearl, L.H.; Pearl, F.M. MoKCa database--mutations of kinases in cancer. Nucleic Acids Res. 2009, 37, D824–D831.
- Hanks, S.K.; Quinn, A.M.; Hunter, T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 1988, 241, 42–52.
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596.
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22.
- Hanks, S.K. Genomic analysis of the eukaryotic protein kinase superfamily: A perspective. Genome Biol. 2003, 4, 111.
- Johnson, D.A.; Akamine, P.; Radzio-Andzelm, E.; Madhusudan, M.; Taylor, S.S. Dynamics of cAMP-dependent protein kinase. Chem. Rev. 2001, 101, 2243–2270.
- Kannan, N.; Haste, N.; Taylor, S.S.; Neuwald, A.F. The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc. Natl. Acad. Sci. USA 2007, 104, 1272–1277.
- Knighton, D.R.; Zheng, J.H.; Ten Eyck, L.F.; Xuong, N.H.; Taylor, S.S.; Sowadski, J.M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 1991, 253, 414–420.
- Taylor, S.S.; Soberg, K.; Kobori, E.; Wu, J.; Pautz, S.; Herberg, F.W.; Skalhegg, B.S. The Tails of Protein Kinase A. Mol. Pharmacol. 2022, 101, 219–225.
- Walden, P.D.; Cowan, N.J. A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette. Mol. Cell Biol. 1993, 13, 7625–7635.
- Walden, P.D.; Millette, C.F. Increased activity associated with the MAST205 protein kinase complex during mammalian spermiogenesis. Biol. Reprod. 1996, 55, 1039–1044.
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589.
- Monzon, V.; Paysan-Lafosse, T.; Wood, V.; Bateman, A. Reciprocal best structure hits: Using AlphaFold models to discover distant homologues. Bioinform. Adv. 2022, 2, vbac072.
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444.
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res 2023, 51, D418–D427.
- Liu, X.; Fuentes, E.J. Emerging Themes in PDZ Domain Signaling: Structure, Function, and Inhibition. Int. Rev. Cell Mol. Biol. 2019, 343, 129–218.
- Ponting, C.P.; Phillips, C.; Davies, K.E.; Blake, D.J. PDZ domains: Targeting signalling molecules to sub-membranous sites. Bioessays 1997, 19, 469–479.
- Songyang, Z.; Fanning, A.S.; Fu, C.; Xu, J.; Marfatia, S.M.; Chishti, A.H.; Crompton, A.; Chan, A.C.; Anderson, J.M.; Cantley, L.C. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 1997, 275, 73–77.
- Nourry, C.; Grant, S.G.; Borg, J.P. PDZ domain proteins: Plug and play! Sci. STKE 2003, 2003, RE7.
- Terrien, E.; Chaffotte, A.; Lafage, M.; Khan, Z.; Prehaud, C.; Cordier, F.; Simenel, C.; Delepierre, M.; Buc, H.; Lafon, M.; et al. Interference with the PTEN-MAST2 interaction by a viral protein leads to cellular relocalization of PTEN. Sci. Signal 2012, 5, ra58.
- Lee, H.J.; Zheng, J.J. PDZ domains and their binding partners: Structure, specificity, and modification. Cell Commun. Signal 2010, 8, 8.
- Hung, A.Y.; Sheng, M. PDZ domains: Structural modules for protein complex assembly. J. Biol. Chem. 2002, 277, 5699–5702.
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757–767.
- Chang, B.H.; Gujral, T.S.; Karp, E.S.; BuKhalid, R.; Grantcharova, V.P.; MacBeath, G. A systematic family-wide investigation reveals that ~30% of mammalian PDZ domains engage in PDZ-PDZ interactions. Chem. Biol. 2011, 18, 1143–1152.
More
