Pectin, a plant-derived polysaccharide, possesses immense technological and biological application value. Several variables influence pectin’s physicochemical aspects, resulting in different fermentations, interactions with receptors, and other functional properties. Some of those variables are molecular weight, degree of methylation and blockiness, and monosaccharide composition. Cancer cell cytotoxicity, important fermentation-related byproducts, immunomodulation, and technological application were found in cell culture, animal models, and preclinical and clinical assessments.
- pectin
- functional properties
- fermentation
- human health
- dietary fiber
1. InIn Vitro Vitro Exploration of Pectin Biological Effects
1.1. Cytotoxicity Effects of Native and Modified Pectins on Cancer Cells
1.2. Gut Microbiota Affects Pectin Fermentation
1.3. Pectin Beneficial Immunomodulation
| Pectin/Fragment Used | In Vitro Model | Main Results Found | Authors |
|---|---|---|---|
| Size-fractioned oligo- and polysaccharides from MCP | HCT116, HT29, and PC3 cell lines | MCP30/10 and 10/3 stimulated cytotoxicity through necroptosis and necrosis on the HCT116 cell line; smaller fragments significantly reduced homotypic aggregation and cell migration | [5] |
| Papaya pectin from different ripening points | HCT116, HT29, and PC3 cell line | Pectins extracted from the third day after harvesting inhibited cancer cell aggregation, migration, and further reduced viability while enhancing cytotoxicity; they also increased pAkt, pErk1/2, p21, and caspase-3 protein expression | [2] |
| Ultrasound-fragmented Sweet Potato pectin with high GalA content and low DM | Oxygen radical absorbance capacity and Ferric reducing antioxidant power assay (ORAC and FRAP) | The most fragmented pectin performed better in both antioxidant assays, starting in lower doses, compared to the other pectins | [3] |
| Enzymatically extracted apple pectin | HCT116 and Caco-2 cell culture | Pectins were able to synergize with Irinotecan increasing colorectal cancer cell cytotoxicity through apoptosis induction compared to drug-only controls; ROS levels increased; reduced IL-6 and COX-2 in LPS-induced HCT116 cells; pectins also reduced E. coli adherence to colorectal cancer cells | [4] |
| Acid and heat-treated pectic extracts from olives | Caco-2 and THP-1 cell culture | Higher doses (3.33 and 10 mg/mL) were able to cease cell proliferation entirely up to seven days; pectin extracts successfully induced caspase-3 activity, compared to MCP and control | [6] |
| Varied potato pectins, citrus polygalacturonic acid, larch AG | HT29, DLD1, HCT116, LoVo; and Caco-2 cell culture | Potato RGI was the most effective in reducing cell proliferation in a dose-response manner; authors detected ICAM-1 downregulation, and the proposed reduction of proliferation was due to cell detachment | [7] |
| Low-molecular-weight citrus pectin | AGS and SW-480 cell culture | LCP lowered cell viability, proliferation, and cell cycle progression; the pectin had comparable values of Cyclin B1 and Bcl-xL downregulation to 5-FU | [10] |
| Papaya pectin from different ripening points | HEK-TLR reporter cell lines | The ripe-fruits pectins activated TLR3, 5, and 9; all pectin samples activated TLR2 and 4; only unripe-fruit pectins inhibited TLR3 and TLR9 | [19] |
| Modified sugar beet pectin fragments | HT29 and DLD1 cell culture | Pectin stimulated apoptosis and detachment of HT29 cells; the galactan fragment was most efficient in lowering cell proliferation | [25] |
| Acid and neutral fractions of papaya pectins from different ripening points | HCT116 and HT29 cell culture, recombinant human Gal-3 hemagglutination | The acid fraction from four days after harvest was able to inhibit Gal-3 hemagglutination from the second-lowest dose onward; cell viability was reduced mostly by full water-soluble fractions or acid fractions | [26] |
| Oligosaccharides from jaboticaba, plum, and papaya flours | HCT116 cell culture and recombinant human Gal-3 hemagglutination | Only jaboticaba oligosaccharides were able to inhibit Gal-3 hemagglutination and colorectal cancer cell viability | [27] |
| Lemon pectins with varying DMs | HEK-TLR reporter cell lines | Low-DM pectins inhibited TLR2 activity way more than higher-DM ones; specific TLR2-TLR1 heterodimer inhibition was observed | [28] |
| Lemon pectins with varying DMs | Human pancreatic islets and MIN6 cells | Low-DM pectins showed protective effects on human islets against streptozotocin and IL1b + IFN-y + TNF-a inflammatory stress through a Gal-3 binding-dependent way | [29] |
| High-temperature sunflower pectin and polygalacturonic acid | CT26 colorectal cancer cell line | Pectins were able to upregulate JNK, ERK, and p38 phosphorylation while down-regulating Akt time-dependently; dose-dependent stimulation of apoptosis was also observed in vitro | [30] |
2. InIn Vivo Vivo Exploration of Pectin Biological Effects
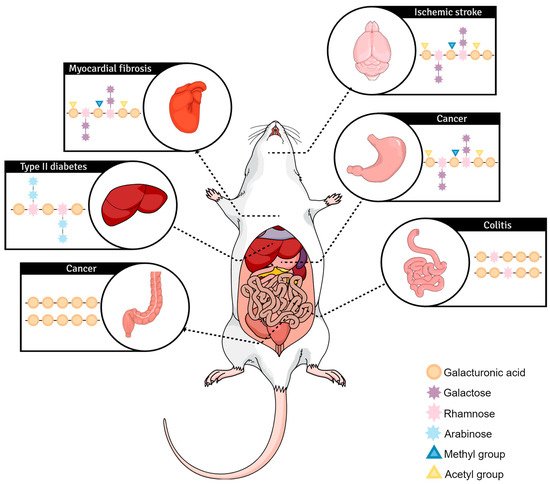
3. Clinical Research and Technological Applications
3. Clinical research and technological applications
There is great interest in the impact of plant-derived dietary fiber on health and disease [51]. As mentioned, beneficial effects could be direct and/or indirect, such as the stimulation of the gastrointestinal immune barrier and the benefits of protecting against cardiovascular diseases [18]. For example, the study by Kumar and Chauhan [52] demonstrated that the formation of a lipase–pectin complex results in lipase inhibition. As a weak acid, pectin resists dissociation in the gastric environment and binds covalently to the active sites of pancreatic lipase. The hypothesis is that the single-bond CO2H groups protonate histidine, and the single-bond OH group of the serine-histidine-aspartic/glutamic acid triad of lipase is what stops the mechanisms to which lipase action is subservient [53][54].
Four studies from the same research group can be cited that used commercially modified pectin (MCP) to investigate the potential for eliminating heavy metals through urine. This specific MCP has been characterized in an earlier in vitro study by one of the same authors as having a smaller size than 15 kDa, under 5% DE, and a content of approximately 10% RG-II. According to the authors of the study, modification is believed to enable preferential transport of short-chain galactans and AG from the small intestinal epithelium into the circulation4. Final Thoughts
Pectins and other types of dietary fiber provide diverse health benefits and reasonable assistance in treating different diseases and maintaining a healthier gastrointestinal environment. Data from human studies are more limited, and the revealed effects are relatively modest [51]. This only highlights the need to expand and further explore the activity and application of those polysaccharides in the human diet and, in the future, pharmacological approaches. The present resviearchw fulfills its purpose by covering a literature gap by thoroughly evaluating pectin at all levels of clinical research. Most of all, it opens space for increasing focus in technological research on pectin applications as molecules of interest regarding effects and stabilizing and carrying properties.
References
- Emran, T.B.; Islam, F.; Mitra, S.; Paul, S.; Nath, N.; Khan, Z.; Das, R.; Chandran, D.; Sharma, R.; Mariana, C.; et al. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules 2022, 27, 7405.
- Do Prado, S.B.R.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-Induced Chemical Modifications of Papaya Pectin Inhibit Cancer Cell Proliferation. Sci. Rep. 2017, 7, 16564.
- Ogutu, F.O.; Mu, T.H. Ultrasonic Degradation of Sweet Potato Pectin and Its Antioxidant Activity. Ultrason. Sonochem. 2017, 38, 726–734.
- Palko-łabuz, A.; Maksymowicz, J.; Sobieszczańska, B.; Wikiera, A.; Skonieczna, M.; Wesołowska, O.; Środa-Pomianek, K. Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity. Cancers 2021, 13, 2952.
- Do Prado, S.B.R.; Shiga, T.M.; Harazono, Y.; Hogan, V.A.; Raz, A.; Carpita, N.C.; Fabi, J.P. Migration and Proliferation of Cancer Cells in Culture Are Differentially Affected by Molecular Size of Modified Citrus Pectin. Carbohydr. Polym. 2019, 211, 141–151.
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Alaiz, M.; Vioque, J.; Girón-Calle, J.; Fernández-Bolaños, J. Pectin-Rich Extracts from Olives Inhibit Proliferation of Caco-2 and THP-1 Cells. Food Funct. 2019, 10, 4844–4853.
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan i Containing Homogalacturonan Inhibits Colon Cancer Cell Proliferation by Decreasing ICAM1 Expression. Carbohydr. Polym. 2015, 132, 546–553.
- Donadio, J.L.S.; do Prado, S.B.R.; Rogero, M.M.; Fabi, J.P. Effects of Pectins on Colorectal Cancer: Targeting Hallmarks as a Support for Future Clinical Trials. Food Funct. 2022, 13, 11438–11454.
- Fabi, J.P.; Cordenunsi, B.R.; Seymour, G.B.; Lajolo, F.M.; do Nascimento, J.R.O. Molecular Cloning and Characterization of a Ripening-Induced Polygalacturonase Related to Papaya Fruit Softening. Plant Physiol. Biochem. 2009, 47, 1075–1081.
- Wang, S.; Li, P.; Lu, S.M.; Ling, Z.Q. Chemoprevention of Low-Molecular-Weight Citrus Pectin (Lcp) in Gastrointestinal Cancer Cells. Int. J. Biol. Sci. 2016, 12, 746–756.
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin–Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415.
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients 2022, 14, 3629.
- Larsen, N.; De Souza, C.B.; Krych, L.; Cahú, T.B.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223.
- Cui, J.; Zhao, C.; Zhao, S.; Tian, G.; Wang, F.; Li, C.; Wang, F.; Zheng, J. Alkali + Cellulase-Extracted Citrus Pectins Exhibit Compact Conformation and Good Fermentation Properties. Food Hydrocoll. 2020, 108, 106079.
- Min, B.; Kyung Koo, O.; Park, S.H.; Jarvis, N.; Ricke, S.C.; Crandall, P.G.; Lee, S.-O. Fermentation Patterns of Various Pectin Sources by Human Fecal Microbiota. Food Nutr. Sci. 2015, 6, 1103–1114.
- Olano-Martin, E.; Gibson, G.R.; Rastall, R.A. Comparison of the in Vitro Bifidogenic Properties of Pectins and Pectic-Oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511.
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T. Pectic Oligosaccharide Structure-Function Relationships: Prebiotics, Inhibitors of Escherichia Coli O157:H7 Adhesion and Reduction of Shiga Toxin Cytotoxicity in HT29 Cells. Food Chem. 2017, 227, 245–254.
- Beukema, M.; Faas, M.M.; de Vos, P. The Effects of Different Dietary Fiber Pectin Structures on the Gastrointestinal Immune Barrier: Impact via Gut Microbiota and Direct Effects on Immune Cells. Exp. Mol. Med. 2020, 52, 1364–1376.
- Prado, S.B.R.; Beukema, M.; Jermendi, E.; Schols, H.A.; de Vos, P.; Fabi, J.P. Pectin Interaction with Immune Receptors Is Modulated by Ripening Process in Papayas. Sci. Rep. 2020, 10, 1629.
- El-zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187.
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066.
- Tan, H.; Chen, W.; Liu, Q.; Yang, G.; Li, K. Pectin Oligosaccharides Ameliorate Colon Cancer by Regulating Oxidative Stress- and Inflammation-Activated Signaling Pathways. Front. Immunol. 2018, 9, 1504.
- Hino, S.; Sonoyama, K.; Bito, H.; Kawagishi, H.; Aoe, S.; Morita, T. Low-Methoxyl Pectin Stimulates Small Intestinal Mucin Secretion Irrespective of Goblet Cell Proliferation and Is Characterized by Jejunum Muc2 Upregulation in Rats. J. Nutr. 2013, 143, 34–40.
- Popov, S.V.; Markov, P.A.; Nikitina, I.R.; Petrishev, S.; Smirnov, V.; Ovodov, Y.S. Preventive Effect of a Peptic Polysaccharide of the Common Cranberry Vaccinium oxycoccos L. on Acetic Acid-Induced Colitis in Mice. World J. Gastroenterol. 2006, 12, 6646–6651.
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified Sugar Beet Pectin Induces Apoptosis of Colon Cancer Cells via an Interaction with the Neutral Sugar Side-Chains. Carbohydr. Polym. 2016, 136, 923–929.
- De Freitas Pedrosa, L.; Lopes, R.G.; Fabi, J.P. The Acid and Neutral Fractions of Pectins Isolated from Ripe and Overripe Papayas Differentially Affect Galectin-3 Inhibition and Colon Cancer Cell Growth. Int. J. Biol. Macromol. 2020, 164, 2681–2690.
- Do Nascimento, R.S.; de Freitas Pedrosa, F.; Diethelm, L.T.H.; Souza, T.; Shiga, T.M.; Fabi, J.P. The Purification of Pectin from Commercial Fruit Flours Results in a Jaboticaba Fraction That Inhibits Galectin-3 and Colon Cancer Cell Growth. Food Res. Int. 2020, 137, 109747.
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary Fiber Pectin Directly Blocks Toll-like Receptor 2-1 and Prevents Doxorubicin-Induced Ileitis. Front. Immunol. 2018, 9, 383.
- Hu, S.; Kuwabara, R.; Beukema, M.; Ferrari, M.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P.; Smink, A.M. Low Methyl-Esterified Pectin Protects Pancreatic β-Cells against Diabetes-Induced Oxidative and Inflammatory Stress via Galectin-3. Carbohydr. Polym. 2020, 249, 116863.
- Guan, Y.; Zhang, Z.; Yu, X.; Yan, J.; Zhou, Y.; Cheng, H.; Tai, G. Components of Heat-Treated Helianthus annuus L. Pectin Inhibit Tumor Growth and Promote Immunity in a Mouse CT26 Tumor Model. J. Funct. Foods 2018, 48, 190–199.
- Pedrosa, L.D.F.; Fabi, J.P. Dietary Fiber as a Wide Pillar of Colorectal Cancer Prevention and Adjuvant Therapy. Crit. Rev. Food Sci. Nutr. 2023, 1–21.
- Cui, Y.; Zhang, N.N.; Wang, D.; Meng, W.H.; Chen, H.S. Modified Citrus Pectin Alleviates Cerebral Ischemia/Reperfusion Injury by Inhibiting NLRP3 Inflammasome Activation via TLR4/NF-ĸB Signaling Pathway in Microglia. J. Inflamm. Res. 2022, 15, 3369–3385.
- Gehlken, C.P.g.; Rogier van der Velde, A.; Meijers, W.C.; Silljé, H.H.W.; Muntendam, P.; Dokter, M.M.; van Gilst, W.H.; Schols, H.A.; de Boer, R.A. Pectins from Various Sources Inhibit Galectin-3-Related Cardiac Fibrosis. Curr. Res. Transl. Med. 2022, 70, 103321.
- Pedrosa, L.D.F.; Raz, A.; Fabi, J.P. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules 2022, 12, 289.
- Zhang, W.; Xu, P.; Zhang, H. Pectin in Cancer Therapy: A Review. Trends Food Sci. Technol. 2015, 44, 258–271.
- Wang, L.; Zhao, L.; Gong, F.; Sun, C.; Du, D.D.; Yang, X.X.; Guo, X. Modified Citrus Pectin Inhibits Breast Cancer Development in Mice by Targeting Tumor-Associated Macrophage Survival and Polarization in Hypoxic Microenvironment. Acta Pharmacol. Sin. 2022, 43, 1556–1567.
- Pienta, K.J.; Nailk, H.; Akhtar, A.; Yamazaki, K.; Replogle, T.S.; Lehr, J.; Donat, T.L.; Tait, L.; Hogan, V.; Raz, A. Inhibition of Spontaneous Metastasis in a Rat Prostate Cancer Model by Oral Administration of Modified Citrus Pectin. J. Natl. Cancer Inst. 1995, 87, 348–353.
- Li, Y.; Liu, L.; Niu, Y.; Feng, J.; Sun, Y.; Kong, X.; Chen, Y.; Chen, X.; Gan, H.; Cao, S.; et al. Modified Apple Polysaccharide Prevents against Tumorigenesis in a Mouse Model of Colitis-Associated Colon Cancer: Role of Galectin-3 and Apoptosis in Cancer Prevention. Eur. J. Nutr. 2012, 51, 107–117.
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal Anti-Inflammatory Effects of Artichoke Pectin and Modified Pectin Fractions in the Dextran Sulfate Sodium Model of Mice Colitis. Artificial Neural Network Modelling of Inflammatory Markers. Food Funct. 2019, 10, 7793–7805.
- Beukema, M.; Ishisono, K.; de Waard, J.; Faas, M.M.; de Vos, P.; Kitaguchi, K. Pectin Limits Epithelial Barrier Disruption by Citrobacter rodentium through Anti-Microbial Effects. Food Funct. 2021, 12, 881–891.
- Ren, T.; Liu, F.; Wang, D.; Li, B.; Jiang, P.; Li, J.; Li, H.; Chen, C.; Wu, W.; Jiao, L. Rhamnogalacturonan-I Enriched Pectin from Steamed Ginseng Ameliorates Lipid Metabolism in Type 2 Diabetic Rats via Gut Microbiota and AMPK Pathway. J. Ethnopharmacol. 2023, 301, 115862.
- Zhu, R.G.; Sun, Y.D.; Li, T.P.; Chen, G.; Peng, X.; Duan, W.B.; Zheng, Z.Z.; Shi, S.L.; Xu, J.G.; Liu, Y.H.; et al. Comparative Effects of Hawthorn (Crataegus pinnatifida Bunge) Pectin and Pectin Hydrolyzates on the Cholesterol Homeostasis of Hamsters Fed High-Cholesterol Diets. Chem. Biol. Interact. 2015, 238, 42–47.
- Ismail, M.F.; Gad, M.Z.; Hamdy, M.A. Study of the Hypolipidemic Properties of Pectin, Garlic and Ginseng in Hypercholesterolemic Rabbits. Pharmacol. Res. 1999, 39, 157–166.
- Munoz-Almagro, N.; Montilla, A.; Villamiel, M. Role of Pectin in the Current Trends towards Low-Glycaemic Food Consumption. Food Res. Int. 2021, 140, 109851.
- Bai, Y.; Wu, P.; Wang, K.; Li, C.; Li, E.; Gilbert, R.G. Food Hydrocolloids Effects of Pectin on Molecular Structural Changes in Starch during Digestion. Food Hydrocoll. 2017, 69, 10–18.
- Dang, G.; Wang, W.; Zhong, R.; Wu, W.; Chen, L.; Zhang, H. Pectin Supplement Alleviates Gut Injury Potentially through Improving Gut Microbiota Community in Piglets. Front. Microbiol. 2022, 13, 1069694.
- Wen, X.; Zhong, R.; Dang, G.; Xia, B.; Wu, W.; Tang, S.; Tang, L.; Liu, L.; Liu, Z.; Chen, L.; et al. Pectin Supplementation Ameliorates Intestinal Epithelial Barrier Function Damage by Modulating Intestinal Microbiota in Lipopolysaccharide-Challenged Piglets. J. Nutr. Biochem. 2022, 109, 109107.
- Li, J.; Wang, L.; Yang, K.; Zhang, G.; Li, S.; Gong, H.; Liu, M.; Dai, X. Structure Characteristics of Low Molecular Weight Pectic Polysaccharide and Its Anti-Aging Capability by Modulating the Intestinal Homeostasis. Carbohydr. Polym. 2023, 303, 120467.
- Zhang, S.; Mao, Y.; Zhang, Z.; Li, Z.; Kong, C. Theranostics Pectin Supplement Significantly Enhanced the Anti-PD-1 Efficacy in Tumor-Bearing Mice Humanized with Gut Microbiota from Patients with Colorectal Cancer. Theranostics 2021, 11, 4155–4170.
- Yuan, Y.; Liu, Y.; He, Y.; Zhang, B.; Zhao, L.; Tian, S.; Wang, Q. Biomaterials Intestinal-Targeted Nanotubes-in-Microgels Composite Carriers for Capsaicin Delivery and Their Effect for Alleviation of Salmonella Induced Enteritis. Biomaterials 2022, 287, 121613.
- Tanes, C.; Bittinger, K.; Gao, Y.; Bushman, F.D.; Lewis, J.D.; Wu, G.D. Article Role of Dietary Fiber in the Recovery of the Human Gut Microbiome and Its Metabolome Role of Dietary Fiber in the Recovery of the Human Gut Microbiome and Its Metabolome. Cell Host Microbe 2021, 29, 394–407.e5.
- Kumar, A.; Chauhan, G.S. Extraction and Characterization of Pectin from Apple Pomace and Its Evaluation as Lipase (Steapsin) Inhibitor. Carbohydr. Polym. 2010, 82, 454–459.
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922.
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging Concepts in the Nutraceutical and Functional Properties of Pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239.
- Eliaz, I.; Weil, E.; Wilk, B. Integrative Medicine and the Role of Modified Citrus Pectin/Alginates in Heavy Metal Chelation and Detoxification—Five Case Reports. Forsch Komplementärmed 2007, 14, 358–364.
- Eliaz, I.; Raz, A. Pleiotropic E Ff Ects of Modified Citrus Pectin. Nutrients 2019, 11, 2619.
- Eliaz, I.; Hotchkiss, A.T.; Fishman, M.L.; Rode, D. The Effect of Modified Citrus Pectin on Urinary Excretion of Toxic Elements. Phyther. Res. 2006, 864, 859–864.
- Zhao, Z.Y.; Liang, L.; Fan, X.; Yu, Z.; Hotchkiss, A.T.; Wilk, B.J.; Eliaz, I. The Role of Modified Citrus Pectin as an Effective Chelator of Lead in Children Hospitalized with Toxic Lead Levels. Altern. Ther. 2008, 14, 34–38.
- Mercadante, S.; Prestia, G.; Adile, C.; Casuccio, A.; Care, I.; Relief, P.; Care, S.; Maddalena, L. Intranasal Fentanyl Versus Fentanyl Pectin Nasal Spray for the Management of Breakthrough Cancer Pain in Doses Proportional to Basal Opioid Regimen. J. Pain 2014, 15, 602–607.
- Portenoy, R.K.; Burton, A.W.; Gabrail, N.; Taylor, D.; Pectin, F. Fentanyl Pectin Nasal Spray (FPNS) in the Treatment of Breakthrough Cancer Pain. Pain 2010, 151, 617–624.
- Watts, P.; Smith, A. PecSys: In Situ Gelling System for Optimised Nasal Drug Delivery. Expert Opin. Drug Deliv. 2009, 6, 543–552.
- Morello, G.; Quarta, A.; Gaballo, A.; Moroni, L.; Gigli, G.; Polini, A.; Gervaso, F. A Thermo-Sensitive Chitosan/Pectin Hydrogel for Long-Term Tumor Spheroid Culture. Carbohydr. Polym. 2021, 274, 118633.
